Abstract
Intramuscular fat (IMF) is a crucial factor in the quality of chicken meat. The genetic basis underlying it is complex. Follicle-stimulating hormone (FSH), well-known as an effector in reproductive tissues, was recently discovered to stimulate abdominal fat accumulation in chicken. The effect of FSH on IMF accumulation and the underlying molecular regulatory mechanisms controlling both IMF and abdominal fat deposition in vivo are largely unknown. In this study, two groups of chickens were treated with chicken FSH or a placebo. The lipid content of breast muscle, abdominal fat volume, and serum concentrations of FSH were examined. Related genes implicated in breast muscle and abdominal fat accumulation were also investigated. Compared to the control group, the triglyceride (TG) content of breast muscle and the percentage of abdominal fat in FSH-treated chickens were significantly increased by 64.9% and 56.5% (P<0.01), respectively. The FSH content in the serum of FSH-treated chickens was 2.1 times than that of control chickens (P<0.01). Results from quantitative real-time polymerase chain reaction (qRT-PCR) assays showed that relative expression levels of fatty acid synthase (FAS), lipoprotein lipase (LPL), diacylglycerol acyltransferase 2 (DGAT2), adipocyte fatty acid binding protein (A-FABP), and peroxisome proliferator-activated receptor γ (PPARγ) were significantly upregulated in breast muscle following FSH treatment (P<0.01). Treatment with FSH also significantly increased relative expression levels of FAS, LPL, DGAT2, A-FABP, and PPARγ in abdominal fat tissue (P<0.05). The results of principal component analysis (PCA) for gene expression (breast muscle and abdominal fat) showed that the control and FSH treatment groups were well separated, which indicated the reliability of the data. This study demonstrates that FSH plays an important role in IMF accumulation in female chickens, which likely involves the regulation of biosynthesis genes related to lipid metabolism.
概要
目 的
肌内脂肪 (IMF) 是衡量鸡肉品质的一个重要指标, 其遗传机理是复杂的。探讨卵泡刺激素 (FSH) 对 IMF 的调控作用, 有利于更好地理解鸡肉中 IMF 沉积的分子机制。
创新点
在鸡上证明 FSH 可促进胸肌中 IMF 的沉积, 且此作用与脂质合成基因表达上调有关。
方 法
将 7 日龄北京油鸡母鸡分为对照组 (皮下注射生理盐水) 和处理组 (皮下注射 4 mIU 鸡FSH)。 连续注射 7 天后采集血清和组织, 采用氯仿-甲醇法抽提脂肪, 测定胸肌甘油三酯含量; 采用鸡特异性酶联免疫吸附测定 (ELISA) 试剂盒检测血清 FSH 含量; 使用实时定量聚合酶链反应 (qRT-PCR) 检测胸肌和腹脂组织中 FAS、 LPL、 DGAT2、 A-FABP 和 PPARγ 基因的表达水平, 并对基因表达数据做主成份分析 (PCA) 以检测样本的重复性。
结 论
本实验结果显示外源 FSH 注射能显著提高鸡的胸肌甘油三酯含量和腹脂含量 (图 1); 同时能显著上调胸肌和腹脂组织中 FAS、 LPL、 DGAT2、 A-FABP 和 PPARγ 基因的表达 (图 2)。 本研究结果表明 FSH 可能通过调控脂类合成基因的表达来促进母鸡 IMF 沉积。
Similar content being viewed by others
1 Introduction
As a crucial factor affecting meat quality, intramuscular fat (IMF) has always been a focus of attention in meat-type poultry breeding. A certain amount of lipid in IMF can enhance meat flavor, juiciness, water holding capacity, and tenderness (Ruiz et al., 2001; Jeremiah et al., 2003; Webb and O’Neill, 2008). IMF accumulation during growth results not only from an increase in the number of adipocytes within muscles but also from an increase in adipocyte volume. The genetic basis underlying fat accumulation is extremely complex.
Follicle-stimulating hormone (FSH) is a major glycoprotein hormone of the hypothalamic-pituitary-gonadal axis, as demonstrated by the cooperation between the actions of FSH and estrogen (E2) in follicular development (Heckert and Griswold, 2002) and the broad functions of E2 in lipid metabolism (Clegg, 2012; Malnick et al., 2013). In addition to its well-known role in reproductive tissues, FSH is believed to perform direct actions in a variety of nongonadal tissues (Sowers et al., 2006; Oshima et al., 2007). Of particular interest, an effect of FSH on lipid accumulation was recently discovered, in which FSH stimulated lipid biosynthesis in chicken abdominal fat (Cui et al., 2012). However, the effect of FSH on IMF and the molecular regulatory mechanisms underlying FSH actions in both IMF and abdominal fat remain largely unknown. Therefore, in this study we examined expression of lipid metabolism-related genes in breast muscle and abdominal fat treated with or without exogenous FSH.
Lipid deposition is a complicated process. Adipogenesis is a well-regulated process controlled by the highly coordinated activation of various enzymes and transcription factors (Saez et al., 2009; Fu et al., 2014). As with lipogenic genes, the enzyme fatty acid synthase (FAS) is key to the process of fatty acid synthesis, catalyzing the synthesis of long-chain fatty acids through the condensation of acetyl-coenzyme A (CoA) and malonyl-CoA in a complex seven-step reaction (Albalat et al., 2007). Lipoprotein lipase (LPL) is an endothelial enzyme that catalyzes the hydrolysis of circulating triglyceride (TG) (Gondret et al., 2001; Andre et al., 2007). The major enzyme known to catalyze TG synthesis is the diacylglycerol acyltransferase 2 (DGAT2) (Stone et al., 2004), which catalyzes the covalent addition of fatty acyl chains to TG. Lipid deposition in muscle is related to the intracellular transport of long-chain fatty acids via the adipocyte fatty acid-binding protein (A-FABP), which is also considered an indicator of total intramuscular adipocyte number (Jurie et al., 2007). The peroxisome proliferator-activated receptor γ (PPARγ) is the master regulator of adipose cell differentiation, playing a critical role in systemic lipid metabolism (Szanto and Nagy, 2005; Liu et al., 2015).
The Beijing-You (BJY) is one of the most famous Chinese local chicken breeds with superior meat quality and acceptability (Jiang et al., 2011). In the present study, using female BJY chickens, we assessed the effects of exogenous FSH on TG content in chicken breast muscle, abdominal fat percentage, serum concentrations of FSH and E2, and mRNA expression of lipid metabolism-related genes in chicken breast muscle and abdominal fat. The aim of this study was thus to contribute to a better understanding of the mechanisms of lipid metabolism related to IMF deposition in chickens.
2 Materials and methods
2.1 Animals and treatment
One-day-old female hatchlings were obtained from the Institute of Animal Science, Chinese Academy of Agricultural Sciences (Beijing, China). Individual birds had the same genetic background. Birds of similar weight entered the experiment at seven days of age and were randomly distributed into FSH treatment and control groups for a total of 15 BJY birds per group. Birds were raised in an environmentally controlled room in three-story step cages. The brooding temperature was maintained at 35 °C for the first two days, then gradually decreased to 21 °C (45% relative humidity). Artificial lighting was continuous. The birds were fed basal diets for the entire experiment from 1 to 14 d of age. The basal diets were formulated based on the National Resource Council (1994) requirements and the Feeding Standard of Chicken established by the Ministry of Agriculture, Beijing, China (2004). Feed and water were provided for ad libitum consumption.
The protocol of FSH administration was performed as previously described (Cui et al., 2012). Seven-day-old female BJY chickens in the treated group were injected subcutaneously (dorsal neck) once daily for seven days with 0.5 ml physiological saline containing 4 mIU chicken FSH (Zhonghao Biological Technology Co., Ltd., Beijing, China). Control animals received saline-only injections daily for seven days.
2.2 Sample collection
On the last day of the experiment, the 15 birds in each group were fasted for 12 h with free access to water before euthanization. The chickens were euthanized on Day 14 of the experiment 24 h after receiving the last injection. Individual blood samples were taken, the serum was separated and samples of serum were frozen at −80 °C for subsequent detection of FSH and E2 concentrations. Samples of breast muscle (pectoralis major) and abdominal fat from one side were snap-frozen in liquid nitrogen and then stored at −80 °C for subsequent extraction of total RNA. The breast muscle was removed and stored at −20 °C for the analysis of TG content. All chickens were weighed at the start of the experiment, and abdominal fat was collected after euthanasia. Live weight (LW) and abdominal fat weight (AFW) were measured, and the percentage of abdominal fat (AFP) was calculated according to the following equation: AFP=AFW×100%/LW.
2.3 TG content detection in breast muscle
After obvious reticular tissue had been eliminated, 2.0 g of each sample was homogenized using chloroform:methanol (2:1, v/v) as the solvent. A commercially available kit (Lideman Biological Technology Co., Ltd., Beijing, China) was used to measure the TG content in 10 µl of breast muscle homogenate according to the manufacturer’s protocol.
2.4 FSH and E2 detection in serum
Serum samples were separated by standard procedures and stored at −80 °C for subsequent analysis. Serum concentrations of FSH were measured using a chicken-specific FSH ELISA kit (Zhonghao Biological Technology Co., Ltd., Beijing, China) with a previously described method (Cui et al., 2012). The assay was performed according to the manufacturer’s instructions after dilution to optimize accuracy. Serum concentrations of E2 were determined by radioimmunoassay (RIA) using an RIA kit (Beijing Huaying Biotechnology Research Institute, Beijing, China) with a previously described method (Joanne et al., 2002).
2.5 RNA extraction and reverse transcription
Total RNA was extracted from 100 mg of frozen abdominal fat, breast muscle, and liver tissue using commercially available kits (Tiangen Biological Technology Co., Ltd., Beijing, China). Genomic DNA contamination was removed by DNase I digestion (Tiangen Biological Technology). The purity and yield of RNA were determined by spectrophotometric measurement using a NanoDrop ND-1000 (NanoDrop Technologies, Montchanin, DE, USA) for RNA quantification. RNA integrity was examined by electrophoresis on a 1.2% (0.012 g/ml) agarose gel. RNA was dissolved in RNase-free water and stored at −80 °C for subsequent use in reverse transcription.
Total RNA (5 µg) was used for reverse transcription. First-strand cDNA synthesis was performed using M-MLV Reverse Transcriptase and an oligo (dT) primer according to the manufacturer’s instructions (Promega, USA). The complementary DNA (cDNA) was stored at −20 °C for subsequent quantitative real-time polymerase chain reaction (qRT-PCR).
2.6 qRT-PCR
qRT-PCR was performed using a Thermal Cycler Dice Real Time System (ABI 7500, Applied Biosystems, Foster, CA, USA) with an SYBR Prime-Script RT-PCR kit (TaKaRa, Dalian, China) according to manufacturer’s specifications. Gene-specific primers (Table 1) were designed based on sequences published in GenBank using Primer Premier 5.0 software (Premier Biosoft International., Palo Alto, CA, USA). After initial denaturation for 30 s at 95 °C, amplification was performed for 40 cycles (95 °C for 5 s and 60 °C for 32 s). Samples were assayed in triplicate to derive standard curves. PCR efficiency for the five genes and β-actin was consistent. To determine fold-changes in gene expression, the comparative CT method was used (Livak and Schmittgen, 2001), with fold-change calculated as \({2^{- \Delta \Delta {C_{\rm{T}}}}}\). The results are expressed as the mean fold-change in gene expression from triplicate analyses, using control group samples as the calibrator (arbitrarily assigned an expression level of 1 for each gene). For all transcripts, cDNA from 14-d-old chicken liver served as a standard control. Negative control reactions (without samples) were included in each assay.
2.7 Statistical analysis
Statistical analysis was performed using Student’s unpaired t-test in SPSS Version 16.0 (SPSS Inc., Chicago, IL, USA). Values are reported as mean± standard error of the mean (SEM). Differences were considered statistically significant at P<0.01 or P<0.05. Principal component analysis (PCA) is a widely used tool for data analysis that reduces complex multidimensional data to a few specified dimensions so that it can be effectively visualized, making it possible to identify the most important directions of variability in a multivariate data matrix and to present the results in a graphical plot (Destefanis et al., 2000). PCA was performed using the software RStudio Version 0.99.473 (RStudio, Boston, MA, USA) and using data of genes from mRNA expression analysis.
3 Results
3.1 Effects of FSH on TG content in breast muscle, AFW, and AFP
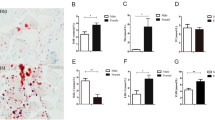
FSH treatment in chickens increased the TG content of breast muscle and enhanced abdominal fat accumulation. Results showed that, compared to the control group, TG content of breast muscle in FSH-treated chickens (Fig. 1a) was significantly increased by 64.9% (P=0.008), and AFW and AFP in FSH-treated chickens (Figs. 1b and 1c) were significantly increased by 59.3% (P=0.002) and 56.5% (P=0.003), respectively.
Effects of FSH on TG content in breast muscle (a), AFW (b), and AFP (c)
Chickens received 4 mIU follicle-stimulating hormone (FSH) daily from Days 7 to 13. Breast muscle and abdominal fat were collected on Day 14, 24 h after the last injection. (a) Triglyceride (TG) content of breast muscle was determined using biochemical methods to examine 10 µl of homogenate. (b) Abdominal fat weight (AFW). (c) Abdominal fat as a percentage of live weight (AFP). Results are expressed as mean± SEM (n=15). ** P<0.01
3.2 Effect of FSH on hormone serum concentrations
The experiment showed that the average serum concentration of FSH in 14-d-old female BJY chickens was 4.10 mIU/ml after 7 d of daily subcutaneous injections of 4 mIU chicken FSH, which was significantly higher (P=0.009) than the concentration found in control chickens (2.00 mIU/ml). However, the concentrations of E2 in serum showed no significant difference between the FSH treatment and control groups (Table 2).
3.3 Effects of FSH on mRNA expression of genes in breast muscle and abdominal fat
qRT-PCR revealed that FSH treatment increased the mRNA abundance of lipid-related genes in chicken breast muscle and abdominal fat (Fig. 2). The mRNA expression levels of the genes FAS, LPL, DGAT2, A-FABP, and PPARγ in the FSH-treated group were significantly higher than those in the control group in both chicken breast muscle (P<0.01) and abdominal fat (P<0.05).
Expression of lipid-related genes assessed by qRT-PCR in 14-d-old female BJY chickens treated with FSH
mRNA expression levels of fatty acid synthase (FAS), lipoprotein lipase (LPL), diacylglycerol acyltransferase 2 (DGAT2), adipocyte fatty acid binding protein (A-FABP), and peroxisome proliferator-activated receptor γ (PPAARγ) were quantified in breast muscle (a) and in abdominal fat (b). Results are expressed as mean±SEM (n=8). P<0.05, P<0.01
To assess individual variability among the four groups, we carried out PCA using the gene expression in abdominal fat and breast muscle from the control and FSH-treated chickens. There was a high correlation between the five variables (r>0.68, P<0.01), including FAS, LPL, DGAT2, A-FABP, and PPARγ. In Fig. 3, these variables placed to the right in the loading plot were close together and, therefore, positively correlated. A clear separation between control and FSH treatment profiles in breast muscle and abdominal fat was apparent from the PCA (Fig. 4).
PCA-plot for gene expression analysis
To visualise the variability of individual gene expression between the control and FSH treatment (breast muscle and abdominal fat), a PCA-analysis of gene expression of 32 samples was performed. Breast muscle and abdominal fat of FSH-treated chickens were clearly distinguished from the closely related control
4 Discussion
IMF content plays a key role in multiple quality traits of chicken meat and is an important index for superior-quality chicken breeding (Hocquette et al., 2010; Ye et al., 2010). IMF can only be measured after slaughtering and must be predicted indirectly using pedigree information, which limits the accuracy of selection and increases the cost of breeding. Therefore, understanding the molecular mechanisms underlying such complex trait formation combined with functional gene discovery may provide valuable information for the breeder. FSH is a glycoprotein hormone mainly secreted by the pituitary gland to regulate gonadal development and function after delivery through the bloodstream (Meduri et al., 2002; Patsoula et al., 2003; Cui et al., 2009). Researchers have found that FSH can increase lipid accumulation in chicken abdominal fat by about 50% (Cui et al., 2012). Thus, it would be of interest to also know its effect on IMF.
In our study, we examined serum hormone concentrations resulting from exogenous FSH (4 mIU daily from Days 7 to 13) and found significantly higher FSH concentrations after treatment of female chickens compared to controls, which demonstrated that exogenous FSH did in fact enter the blood circulation. High levels of FSH, rather than decreased levels of sex hormones, are possibly responsible for fat accumulation and redistribution in aging populations (Liu et al., 2015). Consistent with the report by Cui et al. (2012), AFW and AFP in FSH-treated chickens were significantly increased by 59.29% and 56.49%, respectively. More importantly, we found that FSH treatment increased the TG content in chicken breast muscle by 64.90%, which suggested that FSH promoted IMF deposition in chickens. The data also indicated that the effect of FSH was similar on both breast muscle and abdominal fat accumulation.
The expression profiles of lipid-related genes in breast muscle and abdominal fat from female BJY chickens were further investigated. The results of PCA showed that the control and FSH treatment groups were well separated, which indicated the reliability of the data. The abundance of mRNA from lipid biosynthesis genes (FAS, LPL, DGAT2, A-FABP, and PPARγ) in both breast muscle and abdominal fat was significantly higher in FSH-treated animals compared to controls. FAS, a downstream mediator of adipo-regulatory genes previously discussed (Yu et al., 2014), is critical for lipogenesis, and the expression of FAS was increased by FSH treatment in our study. Similar effects of FSH on FAS expression in 3T3-L1 preadipocytes and adipocytes from mice were reported by Liu et al. (2015). The FAS results suggest that the ability to synthesize lipids in situ contributes partially to lipid deposition in chicken breast muscle and abdominal fat following FSH treatment. LPL is the gatekeeper of fat storage, and when upregulated, acts on lipoproteins to generate increased fatty acids that are transported into tissues (Lafontan, 2014). The uptake of fatty acids by muscle LPL is generally higher in muscles with a high fat content (Chartrin et al., 2006). Thus, the capacity for lipid uptake by muscle tissue is a major determinant controlling IMF levels in chicken. Our results are consistent with previous reports in chicken adipocytes (Cui et al., 2012) and mice adipocytes (Liu et al., 2015) that LPL mRNA was upregulated by FSH treatment. DGAT2 is the main enzyme that catalyzes the final step in the synthesis of TG, which involves esterification of fatty acids for conversion into TG before final storage in various adipose tissues (Krag et al., 2007; Yen et al., 2008). The results of our study showed that the FSH treatment group exhibited higher DGAT2 gene expression in chicken breast muscle and abdominal fat compared to controls, in agreement with similar results on DGAT2 gene expression by FSH stimulation in vitro (Cui et al., 2012). In addition, A-FABP is a marker of lipogenesis, particularly in adipocytes where it is specifically expressed (Hertzel et al., 2006), and our study showed that A-FABP mRNA levels in chicken breast muscle and abdominal fat were upregulated by FSH treatment. PPARγ is the master regulator of adipogenesis (Hocquette et al., 2010), and previous studies found that PPARγ may target genes directly involved in the lipogenic pathway and induce an array of genes related to lipid metabolism, including LPL and FAS; thus, PPARγ may function like a switch to control lipogenic gene expression (Rosen et al., 2002; Hummasti et al., 2008; Hausman et al., 2009). The expression of PPARγ was increased by FSH treatment in our study. Liu et al. (2015) also showed that PPARγ mRNA was upregulated by FSH stimulation in murine adipose tissue.
Taken together, our findings conclude that FSH stimulates IMF accumulation in breast muscle by inducing the expression of lipid biosynthesis genes. With regard to abdominal fat, our in vivo findings on gene regulatory mechanisms are consistent with the in vitro results of Cui et al. (2012).
5 Conclusions
Treatment with exogenous chicken FSH significantly increased the IMF content of breast muscle and upregulated the expression of FAS, LPL, DGAT2, A-FABP, and PPARγ. This study demonstrates that FSH plays an important role in IMF accumulation in female chickens mediated by regulation of biosynthesis genes related to lipid metabolism.
Compliance with ethics guidelines
Xiao-yan CUI, Ying-ying LI, Ran-ran LIU, Gui-ping ZHAO, Mai-qing ZHENG, Qing-he LI, and Jie WEN declare that they have no conflict of interest.
The methods of this study were conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). All experimental protocols were approved by the Science Research Department (in charge of animal welfare issues) of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (CAAS) (Beijing, China).
References
Albalat A., Saera-Vila A., Capilla E., et al., 2007. Insulin regulation of lipoprotein lipase (LPL) activity and expression in gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. B Biochem. Mol. Biol., 148(2):151–159. http://dx.doi.org/10.1016/j.cbpb.2007.05.004
Andre J.M., Guy G., Gontier-Latonnelle K., et al., 2007. Influence of lipoprotein-lipase activity on plasma triacylglycerol concentration and lipid storage in three genotypes of ducks. Comp. Biochem. Physiol. A Mol. Integr. Physiol., 148(4):899–902. http://dx.doi.org/10.1016/j.cbpa.2007.09.006
Chartrin P., Bernadet M.D., Guy G., et al., 2006. Does overfeeding enhance genotype effects on energy metabolism and lipid deposition in breast muscle of ducks? Comp. Biochem. Physiol. A Mol. Integr. Physiol., 145(4): 413–418. http://dx.doi.org/10.1016/j.cbpa.2006.07.024
Clegg D.J., 2012. Minireview: the year in review of estrogen regulation of metabolism. Mol. Endocrinol., 26(12): 1957–1960. http://dx.doi.org/10.1210/me.2012-1284
Cui H.X., Zhao S.M., Cheng M.L., et al., 2009. Cloning and expression levels of genes relating to the ovulation rate of the Yunling black goat. Biol. Reprod., 80(2):219–226. http://dx.doi.org/10.1095/biolreprod.108.069021
Cui H.X., Zhao G.P., Liu R.R., et al., 2012. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. Lipid Res., 53(5):909–917. http://dx.doi.org/10.1194/jlr.M025403
Destefanis G., Barge M., Brugiapaglia A., et al., 2000. The use of principal component analysis (PCA) to characterize beef. Meat Sci., 56(3):255–259. http://dx.doi.org/10.1016/S0309-1740(00)00050-4
Fu R.Q., Liu R.R., Zhao G.P., et al., 2014. Expression profiles of key transcription factors involved in lipid metabolism in Beijing-You chickens. Gene, 537(1):120–125. http://dx.doi.org/10.1016/j.gene.2013.07.109
Gondret F., Ferré P., Dugail I., 2001. ADD-1/SREBP-1 is a major determinant of tissue differential lipogenic capacity in mammalian and avian species. J. Lipid Res., 42(1): 106–113.
Hausman G.J., Dodson M.V., Ajuwon K., et al., 2009. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci., 87(4):1218–1246. http://dx.doi.org/10.2527/jas.2008-1427
Heckert L.L., Griswold M.D., 2002. The expression of the follicle-stimulating hormone receptor in spermatogenesis. Recent Prog. Horm. Res., 57:129–148.
Hertzel A.V., Smith L.A., Berg A.H., et al., 2006. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am. J. Physiol. Endocrinol. Metab., 290(5):E814–E823. http://dx.doi.org/10.1152/ajpendo.00465.2005
Hocquette J.F., Gondret F., Baeza E., et al., 2010. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal, 4(2):303–319. http://dx.doi.org/10.1017/s1751731109991091
Hummasti S., Hong C., Bensinger S.J., et al., 2008. HRASLS3 is a PPAR?-selective target gene that promotes adipocyte differentiation. J. Lipid Res., 49(12):2535–2544. http://dx.doi.org/10.1194/jlr.M800269-JLR200
Jeremiah L.E., Dugan M.E., Aalhus J.L., et al., 2003. Assessment of the relationship between chemical components and palatability of major beef muscles and muscle groups. Meat Sci., 65(3):1013–1019. http://dx.doi.org/10.1016/s0309-1740(02)00309-1
Jiang R., Zhao G., Chen J., et al., 2011. Effect of dietary supplemental nicotinic acid on growth performance, carcass characteristics and meat quality in three genotypes of chicken. J. Anim. Physiol. Anim. Nutr., 95(2):137–145. http://dx.doi.org/10.1111/j.1439-0396.2010.01031.x
Joanne F.D., Thomas R.F., Robert P.M., et al., 2002. Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids, 67(3):151–158. http://dx.doi.org/10.1016/S0039-128X(01)00147-7
Jurie C., Cassar-Malek I., Bonnet M., et al., 2007. Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. J. Anim. Sci., 85(10):2660–2669. http://dx.doi.org/10.2527/jas.2006-837
Krag M.B., Gormsen L.C., Guo Z., et al., 2007. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am. J. Physiol. Endocrinol. Metab., 292(3):E920–E927. http://dx.doi.org/10.1152/ajpendo.00374.2006
Lafontan M., 2014. Adipose tissue and adipocyte dysregulation. Diabetes Metab., 40(1):16–28. http://dx.doi.org/10.1016/j.diabet.2013.08.002
Liu X.M., Chan H.C., Ding G.L., et al., 2015. FSH regulates fat accumulation and redistribution in aging through the Gai/Ca2+/CREB pathway. Aging Cell, 14(3):409–420. http://dx.doi.org/10.1111/acel.12331
Livak K.J., Schmittgen T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 CT method. Methods, 25(4):402–408.
Malnick S., Somin M., Goland S., 2013. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med., 369(25):2455–2457. http://dx.doi.org/10.1056/NEJMc1313169#SA4
Meduri G., Charnaux N., Driancourt M.A., et al., 2002. Follicle-stimulating hormone receptors in oocytes. J. Clin. Endocrinol. Metab., 87(5):2266–2276. http://dx.doi.org/10.1210/jcem.87.5.8502
Oshima Y., Matsuda K.I., Yoshida A., et al., 2007. Localization of estrogen receptors a and ß in the articular surface of the rat femur. Acta Histochem. Cytochem., 40(1):27–34. http://dx.doi.org/10.1267/ahc.06015
Patsoula E., Loutradis D., Drakakis P., et al., 2003. Messenger RNA expression for the follicle-stimulating hormone receptor and luteinizing hormone receptor in human oocytes and preimplantation-stage embryos. Fertil. Steril., 79(5):1187–1193. http://dx.doi.org/10.1016/S0015-0282(03)00071-2
Rosen E.D., Hsu C.H., Wang X., et al., 2002. C/EBPα induces adipogenesis through PPAR?: a unified pathway. Genes Dev., 16(1):22–26. http://dx.doi.org/10.1101/gad.948702
Ruiz J.A., Guerrero L., Arnau J., et al., 2001. Descriptive sensory analysis of meat from broilers fed diets containing vitamin e or; ß-carotene as antioxidants and different supplemental fats. Poult. Sci., 80(7):976–982. http://dx.doi.org/10.1093/ps/80.7.976
Saez G., Davail S., Gentes G., et al., 2009. Gene expression and protein content in relation to intramuscular fat content in Muscovy and Pekin ducks. Poult. Sci., 88(11):2382–2391. http://dx.doi.org/10.3382/ps.2009-00208
Sowers M.R., McConnell D., Jannausch M., et al., 2006. Estradiol and its metabolites and their association with knee osteoarthritis. Arthritis Rheumatol., 54(8):2481–2487. http://dx.doi.org/10.1002/art.22005
Stone S.J., Myers H.M., Watkins S.M., et al., 2004. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem., 279(12):11767–11776. http://dx.doi.org/10.1074/jbc.M311000200
Szanto A., Nagy L., 2005. Retinoids potentiate peroxisome proliferator-activated receptor ? action in differentiation, gene expression, and lipid metabolic processes in developing myeloid cells. Mol. Pharmacol., 67(6):1935–1943. http://dx.doi.org/10.1124/mol.104.006445
Webb E.C., O’Neill H.A., 2008. The animal fat paradox and meat quality. Meat Sci., 80(1):28–36. http://dx.doi.org/10.1016/j.meatsci.2008.05.029
Ye M.H., Chen J.L., Zhao G.P., et al., 2010. Associations of A-FABP and H-FABP markers with the content of intramuscular fat in Beijing-You chicken. Anim. Biotechnol., 21(1):14–24. http://dx.doi.org/10.1080/10495390903328116
Yen C.L.E., Stone S.J., Koliwad S., et al., 2008. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res., 49(11):2283–2301. http://dx.doi.org/10.1194/jlr.R800018-JLR200
Yu X., Liu R., Zhao G., et al., 2014. Folate supplementation modifies CCAAT/enhancer-binding protein a methylation to mediate differentiation of preadipocytes in chickens. Poult. Sci., 93(10):2596–2603. http://dx.doi.org/10.3382/ps.2014-04027Poult.
Author information
Authors and Affiliations
Corresponding author
Additional information
The two authors contributed equally to this work
Project supported by the National Natural Science Foundation of China (No. 31372305), the Agricultural Science and Technology Innovation Program (No. ASTIP-IAS04), and the Project of State Key Laboratory of Animal Nutrition (No. 2004DA125184G1101), China
ORCID: Xiao-yan CUI, http://orcid.org/0000-0003-0669-5790
Rights and permissions
About this article
Cite this article
Cui, Xy., Li, Yy., Liu, Rr. et al. Follicle-stimulating hormone increases the intramuscular fat content and expression of lipid biosynthesis genes in chicken breast muscle. J. Zhejiang Univ. Sci. B 17, 303–310 (2016). https://doi.org/10.1631/jzus.B1500139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500139