Abstract
Background
Tumor-derived exosomes were considered to be potential candidates for tumor vaccines because they are abundant in immune-regulating proteins, whereas tumor exosomal miRNAs may induce immune tolerance, thereby having an opposite immune function.
Objective
This study was designed to separate exosomal protein and depleted exosomal microRNAs (miRNAs), increasing the immune activity of exosomes for activating dendritic cell/cytokine-induced killer cells (DC/CIKs) against pancreatic cancer (PC).
Methods
PC-derived exosomes (PEs) were extracted from cultured PANC-1 cell supernatants and then ruptured; this was followed by ultrafiltered exosome lysates (UELs). DCs were stimulated with lipopolysaccharide (LPS), PE, and UEL, followed by co-culture with CIKs. The anti-tumor effects of DC/CIKs against PC were evaluated by proliferation and killing rates, tumor necrosis factor-α (TNF-α) and perforin secretion. Exosomal miRNAs were depleted after lysis and ultrafiltration, while 128 proteins were retained, including several immune-activating proteins.
Results
UEL-stimulated DC/CIKs showed a higher killing rate than LPS- and PE-stimulated DC/CIKs.
Conclusions
miRNA-depleted exosome proteins may be promising agonists for specifically activating DC/CIKs against PC.
中文概要
目的
本文通过分离提取无小RNA(miRNA)的外来 体(exosome)刺激树突细胞/细胞因子活化杀伤 细胞(DC/CIKs),激活其对于胰腺癌细胞的免 疫杀伤作用。
创新点
无miRNA 的exosome 超速离心裂解产物可以通 过激活DC/CIKs 细胞增强其对肿瘤细胞的杀伤 作用。
方法
通过收集PANC-1 细胞的上清并超速离心提取其 中的exosome。提取的DC 细胞分别通过脂多糖、 肿瘤来源exosome 及无miRNA 的exosome 刺激 后,与CIK 细胞共培养。通过计算增值与杀伤效 率,肿瘤坏死因子-α(TNF-α)及穿孔素的分泌, 比较各组间CIK 细胞对胰腺癌细胞的杀伤作用。
结论
经无miRNA 的exosome 刺激后的CIK 细胞比其 他两组表现出更高的杀伤效应。实验结果表明无 miRNA 的exosome 蛋白在DC/CIKs 细胞的胰腺 癌治疗中是有相当前景的激动剂。
Similar content being viewed by others
1 Introduction
Exosomes are small extracellular vesicles of about 30–100 nm in diameter, and are secreted by a wide variety of cell types including reticulocytes, epithelial cells, neurons, and various tumor cells (Koga et al., 2005). Tumor-derived exosomes have been identified and considered to be potential candidates for tumor vaccines because they contain multiple tumor antigen epitopes, such as Mart1, gp100, TRP, and HER2 (Wolfers et al., 2001), and are abundant in immune-regulating proteins including HSP70, MHC-I, MHC-II, co-stimulatory molecules, and various adhesion molecules (Pitt et al., 2014). Exosomes may act as “mini-antigen presenting cells,” enabling cancer researchers to use them as novel immunotherapeutic agents. Furthermore, exosomes demonstrate many desirable qualities for industrialized production and can be stored stably for a long time; they have therefore been recognized to be promising as an immune agonist and have been progressed to clinical trials (Tan et al., 2010).
Although previous studies have shown the potential value of exosomes as anti-tumor agonists or vaccines, recently it has been demonstrated that tumor exosomes containing microRNAs (miRNAs) can induce immune tolerance and facilitate cancer invasion and metastasis (Azmi et al., 2013). Exosomes may also act as “messenger carriers,” transferring cellular signals to neighboring or more distant cells, in which miRNAs play important roles by mediating suppression of targeted mRNA in recipient cells. For example, breast cancer-derived miR-105, via exosome-mediated transfer, destroys the vascular endothelial barriers that prevent metastasis by targeting the tight junctions (Zhou W. et al., 2014). Melo et al. (2014) reported that breast cancer cells secrete exosomes with CD43-mediated accumulation of Dicer and capacity for cell-independent miRNA biogenesis, altering the transcriptome of target cells and promoting tumor formation.
Our previous study had demonstrated that pancreatic cancer (PC)-derived exosome can down-regulate the expression of TLR4 in dendritic cells (DCs) via miR-203, inducing immune tolerance (Zhou M. et al., 2014). We hypothesize that PC-derived exosomal miRNAs may down-regulate the anti-tumor activity of DC/cytokine-induced killer cells (CIKs) and that depletion of exosomal miRNAs may enhance the anti-tumor activity of DC/CIKs. This study was designed to study the potential value of miRNA-depleted exosomes for activating DC/CIKs.
2 Materials and methods
2.1 Cell culture and exosome collection
PANC-1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640 (Hyclone, Logan, UT, USA), supplemented with 10% exosome-depleted fetal calf serum (Gibco, Gaithersburg, MD, USA) and 1% (0.01 g/ml) penicillin/streptomycin. The cells were grown in a standard humidified incubator with 5% CO2 at 37 °C and passaged every 4`-7 d using trypsin-ethylene diamine tetraacetic acid (EDTA). Supernatants of PANC-1 cultures were collected, and exosomes were isolated and purified by differential centrifugation. Exosomes were pelleted and washed at 100 000g for 1 h twice and finally resuspended in 1 ml phosphate buffered saline (PBS), followed by electron microscopy to confirm the presence and purity of exosomes. The concentration of exosomes was determined by measuring protein concentration using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA).
2.2 Exosome lysis and ultrafiltration
Ultrafiltered exosome lysates (UELs) were prepared for depletion of the miRNAs of exosomes. The pelleted exosomes were resuspended in 1 ml double-distilled H2O (ddH2O) and shaken vigorously for 1 min. Electron microscopy was performed to validate rupture of the exosomes. The exosome lysate was then transferred to an Amicon Ultra-15 100 kDa centrifugal device (Millipore, Watford, UK) and diluted with ddH2O to a total volume of 15 ml, followed by two lots of centrifugation at 4000g for 30 min. Free miRNAs in the lysate were depleted after ultrafiltration and the exosome proteins were concentrated and purified, which would be confirmed by RNA electrophoresis and proteome assay. A BCA protein assay kit was used to determine the concentration of exosome proteins.
2.3 RNA extraction and electrophoresis
RNA was extracted from exosomes and UEL using an miRNeasy mini kit (Qiagen, Dusseldorf, Germany). One hundred and forty microliters of chloroform were added to the tube containing the pelleted exosome, followed by vigorous shaking for 15 s. After allowing to settle for 2–3 min at room temperature, the exosomes were centrifuged at 12 000g for 15 min at 4 °C. The upper aqueous phase was transferred to a clean column, and 1.5 times volume of 100% ethanol was added, followed by mixing. This column was then centrifuged at 8000g for 15 s at room temperature, the flow-through discarded, and this wash step repeated once. After further wash steps with 700 µl Buffer RWT and 500 µl Buffer RPE, RNA was eluted with 30–50 µl RNA-free water. RNA was extracted from the UEL in a similar manner and both RNA samples were separated by RNA electrophoresis.
2.4 Liquid chromatography-electro spray ionization-mass spectrometry/mass spectrometry (LC-ESI-MS/MS) analysis of proteomics in UEL
Following denaturation of proteins to peptides, the UEL was mixed with buffer A (2% acetonitrile (ACN), 0.1% formic acid (FA)). The supernatant was transferred to the next process, which was performed with EASY nLC1000 nanoUPLC (Thermo Scientific, USA). A 34-min gradient was run at 300 nl/min, starting from 5% to 30% buffer B (80% ACN, 0.1% FA), followed by a 2-min linear gradient to 40% buffer B, then 2 min to 80% buffer B, and maintenance at 80% buffer B for 4 min. The peptides were subjected to a nanospray ionization source followed by tandem MS in Q Exactive (Thermo Scientific, USA) coupled online to the UPLC. Intact peptides and ion fragments were detected in the Orbitrap at a resolution of 70 000 and 17 500, respectively. Peptide and protein identifications were performed using the MASCOT search engine (Ver. 2.3.0, Matrix Science). Tandem MS spectra were searched against the Swiss-Prot human database (http://web.expasy.org/docs/swiss-prot_guideline.html).
2.5 Preparation of DCs pulsed with different stimulants
Twenty healthy volunteers without any inflammatory diseases or drug administration were enrolled in the study. All volunteers gave written consent for their blood samples to be used for scientific research. The research protocol was reviewed and approved by the Research Ethics Committee of Zhejiang University (Hangzhou, China).
Twenty milliliters of peripheral blood were collected into sterile heparin vacutainer tubes from 20 volunteers. Human peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation using Ficoll-Paque™ PREMIUM (GE, USA). Monocytes were sorted from the PBMCs of healthy donors using human anti-CD14 microbeads (Miltenyi Biotec, Germany). The resulting CD14 positive monocyte fraction was cultured in RPMI 1640 containing 40 ng/ml granulocytemacrophage-colony-stimulating factor (GM-CSF) (Sigma, USA) and 20 ng/ml interleukin-4 (IL-4) (Sigma, USA). The cells were incubated for 6 d at 37 °C in a humidified 5% CO2 incubator to induce immature DCs. On the seventh day of culture, after adding lipopolysaccharide (LPS) (1 µg/ml), PC-derived exosome (PE) (1 µg/ml), or UEL (1 µg/ml) to the culture for 24 h, activated DCs were obtained. DCs were counted and verified by both light and fluorescence microscopies, using a monoclonal antibody against CD209 (Dako, Carpinteria, CA, USA), a surface molecule on immature DCs. Flow cytometry was performed to validate the purity of immature DC (iDC) on the sixth day, using anti-HLD-DR and anti-CD 83 monoclonal antibodies (Dako).
2.6 Activation and proliferation of differentially stimulated CIKs
The remaining CD14-negative monocytes were cultured in RPMI 1640 for 2 h. Then the non-adherent lymphocytes were cultured in RPMI 1640 with 10% fetal bovine serum (FBS) and 1000 U/ml interferon-γ (IFN-γ; Peprotech, Heidelberg, Germany) for 24 h. IL-4 (400 U/ml, Peprotech), anti-CD3 and anti-CD28 (both 50 ng/ml; T&L Biotechnology, Beijing, China) monoclonal antibodies were added to the culture media, and the cells were further cultured at 37 °C with 5% CO2 for 7 d to induce CIKs. On the seventh day, the CIKs and stimulant-pulsed DCs were mixed together at a ratio of 4:1 (v/v) and cultured in a CIK culture medium for a further 7 d. Three groups were set up as follows: LPS-DCs-CIKs, stimulated with 1 µg/ml LPS; PE-DCs-CIKs, stimulated with 1 µg/ml PE; UEL-DCs-CIKs, stimulated with 1 µg/ml UEL.
The CIKs were observed with microscopy and verified by flow cytometry on the fourteenth day, using anti-CD3 and anti-CD56 monoclonal antibodies (Dako, Produktionsvej, Denmark).
2.7 Proliferation assay
To generate growth curves, CIKs from the three groups were plated simultaneously into 96-well tissue culture plates at a density of 25 000 cells per well. Six parallel experiments for each sample were used to assess the cell proliferation. PE or UEL (1 µg/ml) was added to the appropriate CIK culture. Cells were incubated for 24 h and then counted using a CCK-8 kit (Beyotime Institute of Biotechnology, Guangdong, China). Absorbance was measured with a microplate reader at test and reference wavelengths of 450 and 650 nm, respectively, and then the number of CIKs in each well was determined according to absorbance and standard curves. The proliferation ratio was calculated as follows: proliferation ratio (%)=(CIK number−25 000)/25 000×100%.
2.8 Analysis of TNF-α and perforin in cell culture medium by ELISA
The concentrations of tumor necrosis factor-α (TNF-α) and perforin were measured by enzyme-linked immuno sorbent assay (ELISA; Abcam, MA, USA). CIK cell culture medium supernatant (100 µl per well) was added to the plate for 90 min at 37 °C. After washing, biotin-labeled antibodies (10 µl per well) were added to the plates and incubated for another hour at 37 °C. Thereafter, unbound antibodies were washed off, and this was followed by the addition of an enzyme. Plates were incubated for 30 min at 37 °C. Finally, color was developed by incubation with chromogenic substrate. The optical density (OD) was read at 450 nm with an ELISA plate reader, and standard curves were established to quantitate the amounts of TNF-α and perforin.
2.9 Analysis of TNF-α and perforin in CIKs by Western blotting
TNF-α and perforin protein expressions in CIKs were also analyzed by Western blotting. Protein was prepared, following cell lysis in a modified cell lysis buffer, for Western blotting and immunoprecipitation. The protein concentration was measured using a BCA protein assay kit. The total protein was loaded onto 10% (0.1 g/ml) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (Beyotime, China), separated by electrophoresis, and electroblotted onto polyvinylidene difluoride membranes. The membranes were then blocked in Western sealing solution followed by incubation overnight with the appropriate primary monoclonal TNF-α and perforin antibody (1 µg/ml, Abcam, USA). The membranes were then washed three times with precast Western wash buffer followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Abcam, USA). Subsequent washes with Western wash buffer were performed prior to detection. Signals were detected by chemiluminescence.
2.10 Cytotoxicity of CIK assay
In the CIK cytotoxicity assay, the CIKs served as effector cells whilst the PANC-1 cells were targets. Stimulated CIKs were cultured with PANC-1 cells (1×104 cells/well) at an effector-to-target cell ratio of 20:1, at 37 °C in 5% CO2 for 24 h. PANC-1 cells alone were used in triplicate as controls. CIK activity was measured by a Cell Counting Kit-8 (CCK-8) as previously described. The killing rate was calculated as follows: killing rate (%)=(Ac−Ae)/(Ac−An)×100%, where Ac, Ae, and An are the absorbances of control, experimental, and normal groups, respectively.
2.11 Statistical analysis
All experiments were carried out independently at least three times. Data (mean±standard error of the mean (SEM)) were analyzed by the Student’s t-test (unpaired, two-tailed), and correlations were analyzed by the Spearman rank correlation test. All P-values less than 0.05 were considered statistically significant.
3 Results
3.1 Exosome isolation and preparation of UEL
To test the potential value of PC-derived exosomes as an agonist for activating DCs and CIKs, supernatants of PANC-1 cultures were collected. Following isolation and resuspension of exosomes in PBS, electron microscopy revealed the presence of cystic vesicles of 30–100 nm in diameter. These vesicles were nearly circular and had a complete capsule, which is consistent with exosomal morphology (Fig. 1a). After lysis and ultrafiltration, the remainder was fine crushed matter without any vesicles visible by electron microscopy, indicating that the exosomes had ruptured after lysis (Fig. 1b).
Because miRNAs contained within exosomes may induce immune tolerance, UEL was prepared to remove the miRNAs from the exosomes. Free miRNAs in UEL were found to be depleted and mRNA was degraded after ultrafiltration (Fig. 2). The miRNA-depleted exosome lysate was then tested for its effect on DC/CIKs.
3.2 Proteomics analysis of UEL
As shown in Table S1, LC-ESI-MS/MS examination of UEL demonstrated that 128 proteins were retained after ultrafiltration, including attractin, complement proteins C3, C4 and C5, integrin, and lactotransferrin. These proteins are known to play roles in lymphocyte activation, cell adhesion, immune regulation, or tumor inhibition (Duke-Cohan et al., 2002; Li et al., 2012).
3.3 Identification of DCs and CIKs
Morphological characterization of DCs was undertaken using an inverted microscope. As shown in Figs. S1a-S1c, no significant differences in morphology were observed between iDCs, PE-DCs, and UEL-DCs. All cells demonstrated characteristics observed in typical immature DCs, in which they were round and in a semi-suspended state. Moreover, their surfaces were rough with irregular burr-like protrusions. The DCs became mature after LPS stimulation, as evidenced by the enlargement and narrowing of the cell body and formation of pseudopodia-like protrusions (Fig. S1d). Immunofluorescence analysis using antibodies specific for CD209, a surface marker found on immature DCs (Neumann et al., 2008), successfully detected immature DCs that exhibited polar distribution (Fig. S1e). HLA-DR-positive cells accounted for 75.5% of iDC, and HLA-DR/CD 83 double positive cells accounted for 68% of iDC (Fig. S1f). These results suggest that DCs were successfully induced.
The morphological differences between LPS-DCs-CIKs, PE-DCs-CIKs, and UEL-DCs-CIKs were also checked (Figs. S2a–S2c). All cells were round with a transparent surface and grew in colonies. Detection of CD3+CD56+ cells using flow cytometry showed that CD3+ cells accounted for 66.4% of all induced CIKs. CD56+ cells accounted for 49.6%, and CD3+CD56+ cells accounted for 25.2%. These results confirmed the successful induction of CIKs (Fig. S2d).
3.4 Determination of CIK proliferation rates after exosome stimulation
To test the potential effect of PC-derived exosomes on CIKs, the stimulant-pulsed DCs were cocultured with CIKs for 7 d. Three groups were set up (Table 1): LPS-DCs-CIKs, without further stimulation; PE-DCs-CIKs, stimulated by PE; UEL-DCs-CIKs, stimulated by UEL. As shown in Table 1, the CIK proliferation rate after stimulation with PE was significantly lower than that observed for CIKs stimulated with LPS and UEL (10.29% versus 24.14% and 29.55%, respectively). No significant differences between the UEL- and LPS-DCs-CIKs proliferation rates were observed.
3.5 TNF-α and perforin expressions in CIKs
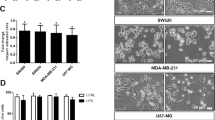
To determine the effect of exosome stimulation on the function of CIKs, Western blot analysis of TNF-α and perforin in the three CIK groups was undertaken. As shown in Fig. 3, TNF-α and perforin expressions varied between the three groups. Higher expressions of both proteins were observed in LPS- and UEL-DCs-CIKs, but only moderate expression was detected in the PE-DCs-CIKs group.
Western blot of TNF-α (a) and perforin (b) in LPS-DCs-CIKs, PE-DCs-CIKs and UEL-DCs-CIKs
L: LPS-DCs-CIKs; P: PE-DCs-CIKs; U: UEL-DCs-CIKs. TNF-α and perforin were observed in the three CIK groups. Strong expressions of both proteins were observed in LPS- and UEL-DCs-CIKs, while moderate expression was detected in PE-DCs-CIKs
TNF-α and perforin levels were also determined in the CIK culture medium. Following 24-h culture of 1×106 cells of each group, the mean concentrations of TNF-α in the culture media were 174.43, 143.10, and 184.06 ng/ml in the LPS-, PE-, and UEL-DCs-CIKs groups, respectively. The mean concentrations of perforin were 2.99, 2.17, and 3.06 ng/ml, respectively. TNF-α and perforin secreted by the PE-DCs-CIKs were significantly lower than those in the LPS- and UEL-DCs-CIKs (P<0.05). However, there were no significant differences in the amount of TNF-α and perforin proteins secreted by the UEL- and LPS-DCs-CIKs (Table 2).
3.6 Cytotoxicity assay of different CIKs targeting PANC-1 cells
To further determine the effect of exosome stimulation on the tumor cell-killing capacity of CIKs, cytotoxicity of the differentially stimulated CIK groups against PANC-1 cells was tested. CIKs are grown in suspension and there was a washing step to ensure that only PANC-1 cells that were attached to the tissue culture dish were assayed. As showed in Table 3, a significantly higher cytotoxicity rate was observed in the UEL-DCs-CIKs, as compared to the other two groups. In addition, the killing rate of PE-DCs-CIKs was significantly lower than that of LPS-DCs-CIKs (Table 3), indicating that UEL enhanced the anti-tumor activity of DCs-CIKs.
4 Discussion
PC is one of the most malignant cancers, with an overall five-year survival rate of 3%–6% (Vincent et al., 2011). The prognosis for PC remains extremely poor; only a few patients can be cured by surgery and half of patients will die within one year of diagnosis. Although adjuvant chemotherapy, radiotherapy, and interventional treatments are also widely used and increase the survival time to some extent (Pinese et al., 2014; Thota et al., 2014), the overall therapeutic outcomes of PC are modest (Hartwig et al., 2013). DC-based adoptive immunotherapy is receiving increasing attention from physicians because it is relatively safe, with fewer side effects, and it can fully mobilize the immune system in vivo (Hunyadi et al., 2014). Therefore, it is considered to be the preferred method of next-generation anti-tumor adoptive cell therapy. Adoptive immunotherapy for PC has progressed to clinical trials and has shown encouraging results in patients with gemcitabine-refractory advanced PC (Chung et al., 2014). Our study suggests that the tumor-killing capacity of DC/CIKs can be significantly elevated when stimulated by UEL, which may represent a promising immune agonist.
In vitro cell-mediated cytotoxicity assays and animal tumor suppression experiments have shown that tumor antigen-induced DC/CIK therapy has satisfactory anti-tumor effects with non-MHC-restricted advantages (Wang et al., 2014). However, it is still difficult to purify multiple tumor antigen epitopes from tumor cells or clinical samples. Tumor-derived exosomes may be a promising immune agonist for DC/CIK activation because they contain multiple tumor antigen epitopes and activating proteins (Wolfers et al., 2001). Exosome proteins, such as CD29, CD51, CD44v7/8, and the cytokine receptor CCR6, can activate monocytes to secret cytokines such as TNF-α, therefore inhibiting tumor growth (Baj-Krzyworzeka et al., 2007). Our study also found that PANC-1-derived exosomes contain several proteins (attractin, complement C3, C4 and C5, integrin, lactotransferrin) which have been confirmed to correlate with lymphocyte activation, cell adhesion, immune regulation, or tumor inhibition (Duke-Cohan et al., 2002; Li et al., 2012). In addition, exosomes can be produced in large quantities and stably preserved, making this an excellent potential immune agonist.
However, tumor-derived exosomes can transfer miRNA to immune cells, which may inhibit the immune response and promote tumor growth. Our previous study has demonstrated that tumor-derived exosomal miRNAs inhibit the anti-tumor activity of DC/CIKs in vitro (Chen et al., 2013). In the current study, we have shown that PC-derived exosomes contain many miRNAs relevant to this disease, for example miR-212-3P, which have been demonstrated to be involved in PC invasion and metastasis by targeting Rb1 (Park et al., 2011) and patch-1 (Ma et al., 2014). Compared with LPS-induced DC/CIKs, PE-stimulated CIKs showed lower proliferation and killing rates against PANC-1 cells. TNF-α and perforin were also inhibited. We believe that PC-related miRNAs may be the primary cause of the decline in killing DC/CIK-mediated tumor cells. Our previous study suggested that PEs can down-regulate the expression of TLR4 in dendritic cells via miR-203, inducing immune tolerance (Zhou M. et al., 2014). So depletion of miRNA can improve the immune activity of PC-derived exosomes.
We prepared the miRNA-depleted exosome proteins by lysis and ultrafiltration. Exosomes were ruptured by the low osmotic pressure of ddH2O; and the contents, including immune-active proteins, miRNAs, and mRNA, were released into the solution. After ultrafiltration, small molecule components such as miRNAs were filtered and exosome proteins were reserved. The results suggested that miRNAs can be removed from exosome by lysis and ultrafiltration, without protein elimination. UEL-stimulated DC/CIKs showed a higher killing rate, similar proliferation rate, and cytotoxic cytokine secretion than LPS-stimulated DC/CIKs, which demonstrated that stimulation with UEL enhanced the specific antitumor cytotoxicity of DC/CIKs. Thus, stimulating CIKs with miRNA-depleted exosome proteins is feasible and can significantly increase tumor-specific cytotoxicity.
Currently, studies on the method of isolating exosomes from plasmas have testified to the feasibility and stability of making progress to achieve the goal of translational medicine. Muller et al. (2014) suggested the use of ultrafiltration to isolate exosomes and preserve the bio-function of immune cells, plasma-free proteins and nucleic acids. However, Kalra et al. (2013) indicated that OptiPrep™ density gradient separation is advanced compared with other methods, but is laborious and cannot be used effectively in clinical settings. Hong et al. (2014) testified that immunoaffinity pull-down has the potential to become a practical method. A variety of studies have testified that the method of isolation of plasma exosome is feasible for realizing the goal of translational medicine. And we will try to explore further this direction and to discover its clinical value.
A limitation of the current study is that we have not investigated mRNA expression in exosomes; thus the influence and mechanisms exerted on DC/CIKs by exosomal mRNA remain unclear. However, the results of RNA electrophoresis suggested that mRNA tended to degrade after ultrafiltration, suggesting that mRNA in the UEL will have little influence on DC differentiation and function. Of course, further studies are required to identify and characterize the detailed regulation mechanisms of all RNA components.
5 Conclusions
Our study indicated that exosomal miRNAs can be removed by lysis and ultrafiltration, without protein elimination. The specific tumor-killing capacity of DC/CIKs was elevated following stimulation with UEL, indicating that miRNA-depleted exosome proteins may represent a promising immune agonist.
Compliance with ethics guidelines
Ri-sheng QUE, Cheng LIN, Guo-ping DING, Zheng-rong WU, and Li-ping CAO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
List of electronic supplementary materials
Table S1 Proteomics identification of pancreatic cancer-derived ultrafiltered exosome lysates
Fig. S1 Morphological and immunofluorescence characterization of DCs
Fig. S2 Characterization of CIKs
References
Azmi, A.S., Bao, B., Sarkar, F.H., 2013. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev., 32(3–4):623–642. http://dx.doi.org/10.1007/s10555-013-9441-9
Baj-Krzyworzeka, M., Szatanek, R., Weglarczyk, K., et al., 2007. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol. Lett., 113(2):76–82. http://dx.doi.org/10.1016/j.imlet.2007.07.014
Chen, J., Ding, G., Chen, W., 2013. The anti-tumor activity of DC/CTL induced by cholangiocarcinoma derived exosome and its ultrafiltered lysate. Chin. J. Exp. Surg., 30: 2258–2261.
Chung, M.J., Park, J.Y., Bang, S., et al., 2014. Phase IIclinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol. Immunother., 63(9):939–946. http://dx.doi.org/10.1007/s00262-014-1566-3
Duke-Cohan, J.S., Tang, W., Schlossman, S.F., 2002. Attractin: a cub-family protease involved in T cell-monocyte/macrophage interactions. In: Langner, J., Ansorge, S. (Eds.), Cellular Peptidases in Immune Functions and Diseases 2. Springer US, p.173–185. http://dx.doi.org/10.1007/0-306-46826-3_20
Hartwig, W., Werner, J., Jäger, D., et al., 2013. Improvement of surgical results for pancreatic cancer. Lancet Oncol., 14(11):e476–e485. http://dx.doi.org/10.1016/S1470-2045(13)70172-4
Hong, C.S., Muller, L., Boyiadzis, M., et al., 2014. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS ONE, 9(8):e103310. http://dx.doi.org/10.1371/journal.pone.0103310
Hunyadi, J., András, C., Szabó, I., et al., 2014. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-a phase I-II study. Pathol. Oncol. Res., 20(2):357–365. http://dx.doi.org/10.1007/s12253-013-9704-3
Kalra, H., Adda, C.G., Liem, M., et al., 2013. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics, 13(22):3354–3364. http://dx.doi.org/10.1002/pmic.201300282
Koga, K., Matsumoto, K., Akiyoshi, T., et al., 2005. Purification, characterization and biological significance of tumorderived exosomes. Anticancer Res., 25:3703–3707.
Li, G.H., Arora, P.D., Chen, Y., et al., 2012. Multifunctional roles of gelsolin in health and diseases. Med. Res. Rev., 32(5):999–1025. http://dx.doi.org/10.1002/med.20231
Ma, C., Nong, K., Wu, B., et al., 2014. miR-212 promotes pancreatic cancer cell growth and invasion by targeting the hedgehog signaling pathway receptor patched-1. J. Exp. Clin. Cancer Res., 33:54.
Melo, S.A., Sugimoto, H., O’Connell, J.T., et al., 2014. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell, 26(5): 707–721. http://dx.doi.org/10.1016/j.ccell.2014.09.005
Muller, L., Hong, C.S., Stolz, D.B., et al., 2014. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods, 411:55–65. http://dx.doi.org/10.1016/j.jim.2014.06.007
Neumann, A.K., Thompson, N.L., Jacobson, K., 2008. Distribution and lateral mobility of DC-SIGN on immature dendritic cells-implications for pathogen uptake. J. Cell Sci., 121(5):634–643. http://dx.doi.org/10.1242/jcs.022418
Park, J.K., Henry, J.C., Jiang, J., et al., 2011. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem. Biophys. Res. Commun., 406(4):518–523. http://dx.doi.org/10.1016/j.bbrc.2011.02.065
Pitt, J.M., Charrier, M., Viaud, S., et al., 2014. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J. Immunol., 193(3):1006–1011. http://dx.doi.org/10.4049/jimmunol.1400703
Tan, A., De La Peña, H., Seifalian, A.M., 2010. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed., 5(1):889–900.
Thota, R., Pauff, J.M., Berlin, J.D., 2014. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology, 28:70–74.
Vincent, A., Herman, J., Schulick, R., et al., 2011. Pancreatic cancer. Lancet, 378(9791):607–620. http://dx.doi.org/10.1016/S0140-6736(10)62307-0
Wang, X., Yu, W., Li, H., et al., 2014. Can the dual-functional capability of CIK cells be used to improve antitumor effects? Cell. Immunol., 287(1):18–22. http://dx.doi.org/10.1016/j.cellimm.2013.11.009
Wolfers, J., Lozier, A., Raposo, G., et al., 2001. Tumorderived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med., 7(3):297–303. http://dx.doi.org/10.1038/85438
Zhou, M., Chen, J., Zhou, L., et al., 2014. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol., 292(1–2):65–69. http://dx.doi.org/10.1016/j.cellimm.2014.09.004
Zhou, W., Fong, M.Y., Min, Y., et al., 2014. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25(4):501–515. http://dx.doi.org/10.1016/j.ccr.2014.03.007
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Natural Science Foundation of China (No. 81272671) and the Health Bureau of Zhejiang Province (No. WKJ2013-2-018), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1500305) contains supplementary materials, which are available to authorized users
ORCID: Li-ping CAO, http://orcid.org/0000-0003-0399-1228
Rights and permissions
About this article
Cite this article
Que, Rs., Lin, C., Ding, Gp. et al. Increasing the immune activity of exosomes: the effect of miRNA-depleted exosome proteins on activating dendritic cell/cytokine-induced killer cells against pancreatic cancer. J. Zhejiang Univ. Sci. B 17, 352–360 (2016). https://doi.org/10.1631/jzus.B1500305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500305