
Analysis of the methodology of skin cancer incidence registration in German cancer registries
Introduction
Cancer comprises one of the highest burdens of non-communicable diseases globally and according to Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) (1), the most recent estimates for the year 2018 are 18.1 million new cancer cases [17 million excluding non-melanoma skin cancer (NMSC)] and 9.6 million cancer deaths (9.5 million excluding NMSC) across the world. This statement itself highlights the burden of NMSC and yet this is the first time that GLOBOCAN included it [still excluding basal cell cancer (BCC)] in its estimates, and nonetheless, are to be interpreted cautiously (2) as there are extensive differences in the completion of NMSC registration among the national cancer registries. Germany is one of the countries which collects high-quality cancer data and is one of the very few countries which provides data on NMSC (3).
A short history of German cancer registries
The first German cancer registry was established in 1926 in Hamburg (4) and further registries [Joint cancer registry (JCR- 6 registries combined), Saarland, North Rhine-Westphalia (NRW), Rhineland-Palatinate, Schleswig-Holstein, Bavaria, Bremen, Lower Saxony, Hesse, and Baden-Wuerttemberg] developed over decades with a total now of 16 population-based registries. All the registries transmit data to the Robert Koch Institute (RKI-the center of cancer registry data) (5). The Cancer Screening and Registration Act in 2013 (6) directed the federal states to set up clinical cancer registries to collect more detailed information on treatment and progression of cancers and complement the epidemiological registries.
Cancer burden in Germany
Looking at the cancer burden in Germany, The absolute incidence of NMSC (excluding BCC) 2018 exceeds even that of breast cancer (Table 1) and after including BCC, the estimated incidence was as high as 211,600 in 2013 (7). In spite of such huge number of new skin cancer cases, the mortality rate is extremely low-NMSC in Germany (excluding BCC) contributing to 0.3% of total cancer-related deaths and malignant melanoma (MM) to 1.6% (3), in fact, the relative 5-year survival rate of BCC reported is >100% (4).
Table 1
| Type of cancer | Absolute incidence | Absolute mortality | ASIR (World standardized) |
|---|---|---|---|
| NMSC (excluding BCC) | 77,272 | 1,048 | 27.5 |
| Breast | 71,888 | 19,376 | 85.4 |
| Lung | 66,749 | 50,560 | 33.7 |
| MM | 31,432 | 3,641 | 21.6 |
NMSC, non-melanoma skin cancer; BCC, basal cell cancer; MM, malignant melanoma.
Rising trends of skin cancer incidence in Germany
The RKI estimated the incidence rate of MM to have increased by five times in 2014 (4) in both the genders since the 1970s. The study by Rudolph et al., 2015 (8), one of the first to analyze the NMSC incidence rates in Germany, found an increase from 43.1 cases per 100,000 in 1998 to 105.2 in 2010 with 71.6% of NMSCs being BCC, 26% squamous cell carcinoma (SCC) and 2.3% other NMSCs.
In this study, the data could be obtained for the entire period of 1998–2010 barely from 11 out of the 16 cancer registries. One registry provided data only from 2003 to 2010 while two other had data only for some selected regions from 2006 to 2010 and two more could not provide any data at all. This confirms both the delay in NMSC data collection as well as the lack of uniformity across German registries. On the other hand, MM is being registered since the late 60s in Saarland registry and even a separate Central Malignant Melanoma registry (CMMR) was established in Berlin for registering MM cases in 1983 (9). This discrepancy is justified by the out-patient based treatment of NMSC, very low case fatality rate and practical problems in collecting data on such a huge number of cases (7).
Because of the rising incidence of skin cancer in Germany over decades, a national skin cancer screening (SCS) program was introduced in 2008. Under SCS, men and women ≥35 yrs. with compulsory health insurance undergo skin examination every 2 years by a physician, either a dermatologist or a general practitioner (10). This contributed to a sudden surge in skin cancer cases and added to complications in predicting whether there is an actual increase in incidence or not.
Thus, many factors can influence the incidence estimation, quality and reliability of the skin cancer data and as this information is highly valuable for the governments’ decisions over the use of resources, it is necessary to get accurate estimates. It is also important to understand the elements which make the true estimation of NMSC incidence more difficult and different from MM. Therefore, the aim of this study is to analyze the factors influencing the overall skin cancer registration and incidence estimation in the German cancer registries, to study the quality of skin cancer data with a reflection on differences between MM and NMSC and further to suggest improvements.
Methodology
The information on the registration methodology and on the quality assessment of MM and NMSC data by the German cancer registries was collected from their latest annual reports (11-21) available till September 2018 on the website of each cancer registry, from the RKI website (22), and its national cancer reports (4) and the Manual of cancer registration by GEKID (the Association of Population-based cancer registries in Germany) (6). The national skin cancer incidence trends from 1995 to 2015 were obtained from GEKID atlas (23). A questionnaire (attached in Supplementary) was developed based on the queries arising from these annual reports and was sent to all the registries in August 2018. A reminder was sent if no reply was received within 2 weeks and the registries which did not respond after 2 reminders were approached telephonically. In order to suggest improvements, the methodology of the German cancer registries was compared to that of Nordic cancer registries which claim to have complete data for the last 60 years. The methodology followed by the registries of Denmark, Norway, and Sweden was extracted from the Cancer Statistics for Nordic countries (NORDCAN) database (23), the reference article Pukkala et al., 2018 (24), and from information requests sent to these registries. Relevant articles were searched in the PubMed, in references of the annual reports, and the Manual of cancer registration, and the research materials as suggested in the surveys.
Results
Epidemiological and clinical cancer registries in Germany have different historical development and legal bases. The findings in this study are mainly derived from the epidemiological registries.
Factors influencing skin cancer registration and incidence rate
Legislative laws governing the skin cancer data collection
The §65c Social Code Book V (SGB V) (25) for the clinical cancer registries lays down the rules for payment by the Statutory insurance for tumor notifications. Paragraph 4 (25) in it states that if a clinical cancer registry meets the eligibility criteria, then the health insurance pays 119 Euros to the registry for each new tumor reported except for NMSC and its early stages. Paragraph 6 (25) states that for each notification of the clinical data made to the cancer registry, a reporting fee is paid to the service providers by the respective cancer registry for all cancers except for NMSC and its early stages. It is noteworthy that §65c SGB V rules are for clinical cancer registries which are still in a developmental stage and not uniformly running across all federal states. In the GEKID recommendations 2005 (26), the third recommendation states that “all epidemiological registries should comprehensively register other skin tumors (C44)” which by definition includes NMSC.
So, although registration is recommended for NMSC by the GEKID, no reimbursement for NMSC notifications can definitely result in underreporting of these cancers by service providers, especially in those regions where clinical registries are the only source of data collection and hence, lead to underestimation of the incidence rates.
Source of data
Mostly the data is obtained from hospitals but for cancers like NMSC, patients do not always require hospitalization adding to difficulties in data collection. Apart from including dermatologists as data source (4), another method used by the registries to fill in the gap of the cases missed during routine registration is the inclusion of the ‘death certificate notified’ (DCN) cases (6) i.e., the patients diagnosed with cancer only after death. A traceback is run on DCN cases and if successfully investigated, they are referred to as ‘death certificate initiated’ (DCI) cases otherwise recorded as new incident cases from the time of death as ‘death certificate only’ (DCO) cases.
Considering the very low case fatality rate of skin cancer, especially NMSC, death certificates cannot serve the purpose of finding the missed cases. Moreover, the death certificates do not differentiate between the type of NMSC (4) i.e., SCC and BCC, resulting in inaccurate estimation of incidence separately for both cancers.
Coding of skin cancer
The registries use both the German modification of International Classification of Diseases -10th revision (ICD-10-GM) and International Classification of Diseases for Oncology 3rd revision (ICD-O-3) (27) for coding the tumors. Using the same coding system implies uniformity in case-definition across German registries and internationally. While the ICD-10 system is only based on the topography i.e., site of the tumor, the ICD-O-3 system (27) includes additionally the morphology code representing the tumor histology (e.g. basal cell/ squamous cell carcinoma), behavior of the tumor (malignant/benign/carcinoma in situ) and histological grading/differentiation of the tumor. The interconversion between the two systems can be difficult if it requires to collect backdate information specifically for skin cancers and the DCO cases, in which this information might be untraceable (28) due to non-uniform data collection.
Cutaneous MM (29)
The ICD code is C43 and according to the specific location of the tumor such as lip, eyelid including canthus, ear and auditory canal, other unspecified parts of face, scalp, etc., a 4th character (0 to 9) is added to the code and it ranges from C43.0 to C43.9.
NMSC (29)
NMSC is coded as C44 and similar to MM, the code varies from C44.0 to C44.9 depending upon the site involved.
Carcinoma of genital organs’ skin are excluded from the C44 code (29) which underestimates the overall skin cancer incidence rate. Only a few sites of the skin have specific ICD codes while the rest get included in the code for unspecified sites (C43.9 or C44.9), effecting the incidence rate of specific skin sites (30) and tumors with multiple site involvement (31).
Coding rules for multiple tumors
German cancer registries follow “International Rules for Multiple Primary Cancers (ICD-O-3)” International Agency for Research on Cancer (IARC) report 2004 (31) to code multiple tumors according to which, multiple tumors of same histology involving the same organ are to be counted only once. Skin being a single organ, multiple primaries in it are counted only once. While the criteria state to count any involvement of a different site as a separate tumor but in the coding system (ICD) many sites of skin get designated as unspecified tumors which therefore will not be counted as a second primary.
So, this is another important factor leading to underestimation of skin cancer incidence rates, especially NMSC which has high propensity of involving multiple sites and developing again in the same patient (32).
Skin cancer screening program (SCS)
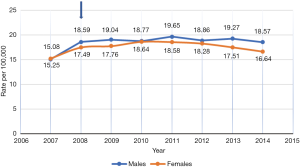
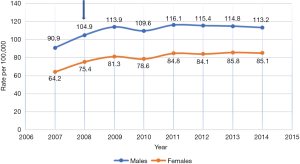
Figures 1 and 2 show the incidence rate trends of MM and NMSC (excluding BCC) respectively in Germany from 2007 to 2014. It is clear that there was a discrete increase in national incidence estimates in 2009 as compared to 2007 and after that, the rate has stabilized. This sudden increase can only be explained by the introduction of SCS in 2008 but the influence of screening on incidence rate is not routinely mentioned in any of the annual reports of the registries. As far as the coding and recoding of tumors detected by screening are concerned, no related information could be traced back, though the occasion of cancer diagnosis like screening is recorded at the time of registration.
Quality of skin cancer data
Quality assessment parameters of cancer data include its completeness, validity, and comparability (6). For German cancer registries, these are assessed regularly by the RKI (22). It is based on the overall quality of registry data that a ‘reference pool’ of registries is defined (4) to which other registries are compared. The differences between the quality of data of MM and NMSC among the registries are recapitulated in Table 2 (detailed criteria for each registry attached in Supplementary). Information for MM data quality was available from all the registries and only from five for NMSC and that too not discretely for SCC and BCC, rather combined. An important fact highlighted by the cancer registry of Bavaria was that in their state, NMSC data are collected only in three (Middle Franconia, Upper Palatinate, and Lower Bavaria) out of seven administrative regions and projecting these regional estimates to the whole of Bavaria does not reflect the true incidence. The various quality parameters are explained in detail below.
Table 2
| Data quality parameter | Recommended standard (6) | Status for MM (data from 16 registries) (11-21) | Status for NMSC (data from only 5 registries) (11,14,16-18) |
|---|---|---|---|
| DCO rate (completeness) | <5% | Ranges from 0.6% to 5.4% | Ranges from 0% to 0.4% |
| M/I index (completeness) | Constant across regions | Ranges from 0.1 to 0.44 | Ranges from 0 to 0.1 |
| Missing stage (completeness) | <20% | Ranges from 9% to 60% | >80% |
| Overall completeness for any cancer | >90% | >90% except Hamburg cancer registry & Joint cancer registry | Not available |
| Histological confirmation (validity) | >85% | >90% ⇒ indicates lack of clinical reports | >92% |
MM, malignant melanoma; NMSC, non-melanoma skin cancer; DCO, death certificate only; M/I, mortality/incidence.
Completeness of data
A high rate of capture for a registry doesn’t necessarily implicate high coverage of each cancer site, for instance, though the overall completeness of Hamburg cancer registry is >90%, it is as low as 73–77% for MM (11) and the probable cause mentioned is either fewer MM cases diagnosed in Hamburg than the rest of Germany or due to a lapse in reporting.
The parameters used to evaluate the completeness of a registry are discussed as follows:
(I) The share of DCO rate for MM and NMSC
The recommended value of DCO rate by IARC for an epidemiological registry is less than 5% (6). For MM, DCO rate varies from 0.6% to 5.4% across the registries with the highest in Schleswig-Holstein (16). For NMSC, the rate is even lower, almost 0%.
Such low DCO rate does not directly imply a proficient registration system, rather reflects the few death certificates received because of the very low mortality rate of NMSC. The reason being that NMSC is common in >70-year age-group (4) in which death is more likely due to age-related diseases rather than oncological complications. So, there is a risk of missing these cases by death registries.
Inference: DCO rate is not at all a reliable parameter to assess the NMSC data quality.
(II) Mortality/incidence index (M/I)
Use of M/I quotient to assess completeness is based on the presumption that the ratio for particular cancer, age-group, gender distribution, and diagnosis year remains constant across various regions in Germany and no major regional differences exist between the survival rate for particular cancer (6). The quotient for MM ranges from 0.1 to 0.44 across registries, and for NMSC, is approx. ‘0’ (Table 2), again indicating the low mortality rate.
Differences in the distribution of tumor stages of MM or NMSC among the federal states and regional variations in the proportion of incidence of a particular site tumor, for e.g., involvement of different sites of the skin, can affect the chances of survival and hence, result in regional deviations in the ‘true’ M/I quotients (6). Also a lot of information on stages is missing (Table 2), more for NMSC than MM and moreover, many sites are coded under unspecified sites (as mentioned in the coding section), so the constancy of M/I ratio cannot be assured.
For all the diagnoses except for thyroid cancer and MM, registries with a degree of capture of at least 90% are considered as complete. But considering the strong instabilities in the M/I ratio of MM, the standard has been lowered to 80% (4). For NMSC, as the M/I index is almost zero, this method is not suitable for evaluating its completeness and hence, the information is missing from all the annual reports. Rhineland-Palatinate registry mentions the estimated completeness for NMSC based on Saarland as >95% (15) but the method used was not clarified.
Inference: The regional variability and extremely low values make M/I ratio an unreliable method to assess the completeness of skin cancer data (both MM and NMSC).
(III) Log-linear model
This approach, carried out centrally by the RKI, is based on the same principle and presumptions as M/I index along with the adjustment of age- and gender-specific polynomial trends to the logarithmic quotients of incidence and mortality for each type of cancer (6). The complex modeling and smoothing procedure lead to stable estimates by taking into account the cancer-specific fluctuations which means the disparities affecting the M/I index can be taken care of by this model. Still, this approach is not considered suitable for NMSC (4) because of large variations in data across the regions.
Inference: Log-linear model is appropriate for MM data completeness assessment but not for NMSC.
(IV) Completeness of information on diagnosis and tumor classification
The information on “the percentage of cases for which tumor classification such as Tumor Node Metastasis (TNM) staging or histopathological grading is defined” (6) is valuable for assessing the survival rates, evaluating stage-specific therapy and screening programs and has the recommended standard of at least 80%.
The proportion of MM cases with missing/incomplete TNM/Union for International Cancer Control (UICC) stage varies across the registries from 8–9% in Saxony (12) and Lower Saxony (19) to around >60% in Schleswig-Holstein (16). For NMSC, this rate is almost double that of MM and ranges from 77–85% across the registries (Table 2).
Inference: There is a huge gap in the completeness of tumor classification for both MM and NMSC.
(V) Completeness of therapy information
The standard for completeness of therapy/treatment information has not been defined so far (6). This was not included in the comparison (Table 2) because the facts for MM and NMSC were available from only one registry: Schleswig-Holstein (16) which elaborates the proportion of cases undergoing various treatments like surgery, chemotherapy, immunotherapy, etc.
Comparability
Defined by Bray and Parkin (34) as “the extent to which coding and classification procedures at a registry, together with the definitions of recording and reporting specific data items, agree with international guidelines”.
The principle of coding tumors and case definitions followed by German registries are similar across all regions as already described in the ‘coding of skin cancer’ and ‘coding rules for multiple tumors’ section. It is to maintain international comparability, that NMSC is not included in the reported incidences of ‘all cancer sites’ (4) and only the first skin tumors are counted.
Validity
The validity of a cancer register is assessed based on two factors:
(I) The proportion of cases with inaccurate/unspecific primary tumors
A high proportion of such cases indicates a low-quality data and should be <10% for an epidemiological cancer registry (6). ICD-10 codes for such tumors are C26.0, C26.9, C39.0, C39.9, C76, and C80.9 (29). This parameter is not specific to any particular cancer but rather an indicator of the overall quality of a cancer registry.
The annual report of JCR (12) specifies the proportion of such cases for MM coded as ICD-O-3 C80 ranging from 1% to 2.5%. The controversial point is that C80 represents the tumors for which the primary site could not be identified, but when the cases (as in the JCR annual report) specify the diagnosis as MM, then the primary site is automatically skin. In case the definite site of skin involvement was not clear, the tumors should instead be coded as C43.9 or C44.9.
Inference: The evidence seems debatable, and no final conclusion can be drawn.
(II) The proportion of Histologically confirmed/Microscopically verified (MV%) cases
These are the cases for which the diagnosis has been confirmed histologically on biopsy/ excision samples by the pathology laboratories and indicate the validity of diagnoses. The standard value of MV share is >85% (6) for all cancers.
It is >90% for both MM and NMSC in all the registries (Table 2) except for NRW (14) which has MV% of approx. 86% for MM. Though high MV% indicates good quality data, a proportion as high as 100% also points out towards only histology reports being the source of notification and lack of clinical reports (6).
Inference: MV% is high for both MM and NMSC, indicating pathological reports as the sole source of notification and scarcity of clinical reports.
Handling missing data
Missing values cannot be completely avoided (6), for e.g., staging in MM based on tumor thickness cannot be completed if the patients do not undergo surgery. To deal with this problem of missing data, one of the methods followed is ‘Imputation’. No information on the use of imputation for skin cancer data could be found but the German registries do always report the proportion of missing data to assess the potential bias.
Response evaluation of survey
All the German cancer registries except for the JCR group replied back to the survey by October 2018 (Table 3). The last survey was received from the Berlin registry in February 2019 and was considered as representative of all the six registries included in the JCR (as per confirmation by the Berlin cancer registry).
Table 3
| Registry | Imputation of missing T stage | Effect of screening included | Multiple tumor counting | M/I index used to calculate incidence | Traceback done for DCN cases |
|---|---|---|---|---|---|
| Hamburg | No | No | No | No | Yes |
| JCR | No | No | No | No | Yes |
| Saarland | No | No | No | No | Yes |
| NRW | No | No | No | No | No |
| Rhineland | No | No | No | No | No |
| Schleswig-Holstein | No | No | No | No | Done once in 2010 |
| Bavaria | No | No | No | No | Done since 2017 |
| Bremen | No | No | No | No | Yes |
| Lower-Saxony | No | No | No | No | Yes |
| Hesse | No | No | No | No | No |
| Baden-Wuerttemberg | No | No | No | No | No |
M/I, mortality/incidence; DCN, death certificate notified; JCR, Joint cancer registry; NRW, North Rhine-Westphalia.
Eleven out of the 16 registries run a regular traceback on DCN cases. Four of the rest five registries don’t run any traceback while Schleswig-Holstein did it just once in 2010 and Bavaria only started in 2017. This can contribute to differences among the registries and further raises questions on the reliability of the DCO rate as a quality indicator. None of the registries use M/I index of the reference registry to calculate the incidence of MM in its region, confirming that all the incidence data is actually collected data and not just estimated. For counting multiple tumors, all the registries confirmed to count only the first tumor of MM and NMSC of one histological type. None of the registries include the effect of screening on the incidence rates. All the registries denied using an imputation method to fill in the missing stage information for skin cancer. All of them confirmed that the RKI annually evaluates the completeness using log-linear model for MM but not for NMSC.
Comparison with Nordic countries
The Nordic cancer registries have an extensive data collection system and an international database called NORDCAN (23) and claim to have complete data for the last 60 years, even for skin cancers (>90%). The GLOBOCAN 2018 estimates of ASIR for NMSC (excluding BCC) and MM in Nordic countries (Table 4) indicate the lower NMSC incidence as compared to Germany. For international comparison, BCC is not included in NMSC by NORDCAN too. The ASIR (World standardized) of BCC in these countries with only the first BCC counted was approx. 90–110 per 100,000 (24) in 2010 compared to the ASIR (European standardized) of 82.2 per 100,000 estimated by Rudolph et al., 2015 (8) in Germany, though these data are not directly comparable because of different standard populations. The methodology in the context of skin cancers in Nordic registries was compared to German registries and the findings as follows.
Table 4
| Country | NMSC (excluding BCC) | Malignant melanoma |
|---|---|---|
| Denmark | 16.8 | 27.6 |
| Norway | 15.2 | 29.6 |
| Sweden | 16.1 | 24.7 |
ASIR, age-standardized incidence rate; NMSC, non-melanoma skin cancer; BCC, basal cell cancer; MM, malignant melanoma.
Differences in recording tumors (24)
One major difference between Nordic and German registries lies in the BCC registration: Nordic countries record BCC in a separate file with much less extensive manual coding or efforts to ensure the quality of data as compared to other cancers in the main database. Denmark and Norway count pre-invasive and invasive lesions as separate entities if there is a time interval of 4 months between them. This information on in-situ tumors and consideration of time factor does not only significantly impact the incidence rate, but also is important to study the progression of lesions from pre-invasive to invasive stage.
Different recording principles of multiple tumors (24)
In Denmark, if the same patient has second skin cancer, then the code is changed to C43.8 or C44.8 “multiple locations” respectively for MM and NMSC. Sweden counts all multiple tumors occurring at the same time as separate entities even if they have the same morphology such as multiple SCC. Norway counts the first incidence of BCC and then all of the following tumors are coded as “second BCC” irrespective of the number, though for calculating the age-standardized incidence rates (ASRs) (World standardized), only first BCC is counted.
Different quality assessment tools
The M/I index of MM and NMSC reported by NORDCAN (23) for 2011-15 show similar low mortality pattern as observed in Germany. Considering its limitations, instead of M/I index, the Danish cancer registry (35) evaluates overall data completeness by capture-recapture method. Larsen et al., 2009 (36) calculated the completeness of Norwegian cancer registry based on 2001-05 data using quantitative (capture-recapture, and flow method) and semi-quantitative methods (historical data method, M/I ratio compared with one minus five-year relative survival, and the number of notifications per case). Using the capture-recapture method, the completeness of NMSC was 99.78% and of MM was 99.76% (it was not clear if BCC was included but considering the separate file recording of BCC in Norway, it is highly likely that it wasn’t). Other methods also proved high overall completeness of the registry, but data specific to skin cancer could not be extracted. The Swedish cancer registry checks the completeness (37) by comparing to the data of Population death register which is considered to have 98% completeness. It does not include DCO cases but instead uses traceback to validate the diagnosis and then adds them as DCI cases. Not using the DCO cases reduces the completeness of Swedish registry, especially for cancers diagnosed in late stages.
Inference: Counting multiple tumors, and in-situ lesions, make the skin cancer data in Nordic countries more comprehensive. Separating BCC from the rest of NMSCs is an important factor leading to high completeness of NMSC data in Norway and can serve the same purpose, if adopted by Germany too. No final inference could still be drawn on the best approach of completeness assessment for NMSC, but inclusion of other methods after taking into account their limitations as by Norwegian registry (36) might provide a solution.
Discussion
It is clear from the results that several factors can result in over- or under-estimation of true skin cancer incidence rates and in spite of 16 population-based registries established in Germany, the data available for skin cancer are still incomplete, for NMSC more than MM.
The study by Stang et al., 2003 (30), in which he compared the ASR of NMSC based on the anatomic site involved, pointing out the importance of registration of the various sites of tumors and coding principles. The analyses were based on the Saarland Cancer Registry data for the period 1995–1999 and the reason why the author evaluated data only post-1995 was because, until 1994, the majority of NMSC cases were coded according to the ICD-9 system or as unspecified skin cancer according to ICD-O or by a histology variable that categorized NMSC into BCC, SCC or other skin cancer. Until 1995, the annual proportion of unspecified skin cancer was >10% in Saarland registry due to the massive number of NMSC reports and shortage of registry staff.
A similar difficulty is with the principal of coding multiple primary tumors for which the European cancer registries follow the IARC report 2004. Weir et al., 2016 (38) analyzed the differences in incidence rates of various cancers when coded according to the Surveillance, Epidemiology and End Results (SEER) Program compared to that by using the IARC rules. The calculations were based on data from 9 population-based registries covering 10% of the US population. Using SEER rules, the incidence rate for MM was found to be 9% higher than that by using IARC rules. From 1975 to 2005-06, the incidence rate for MM increased annually by 5.7% according to SEER and by 2.7–2.9% according to IARC rules. Similarly, from 2005 to 2011, it increased by 1.3% according to SEER though as per IARC, it remained stable. These differences result from counting each subsite of the skin as a separate entity and considering the laterality of tumors and the timing rules by the SEER. The influence of multiple tumor counting was most evident on the incidence rates of urinary bladder tumors which have a tendency of multifocality. In this study, though NMSC was not included, it can be anticipated that a similar increase in incidence rates would be observed.
As was seen in the results, none of the data completeness assessment tools were appropriate for NMSC and even MM, either due to very low mortality rate or incomplete data. The capture-recapture method is an alternative approach (used even by Nordic countries) based on the comparison of the registry data with another independent data source but also faced by the limitation of practical absence of such sources. Schouten et al., 1994 (39) assessed the reliability of the capture-recapture method using ‘pathological’ and ‘discharge’ reports assuming them to be independent sources. The completeness was lowest for skin cancer because most of the data came from ‘pathological reports’ only as patients with skin cancer usually do not need hospitalization. His analysis also could not prove independence of both the sources. The independence of sources was not assured even in the Norwegian study by Larsen et al., 2009 (36). Considering the CMMR in Germany as an independent source, a study (40) has shown that it records only 35–50% MM patients because of lack of participation by the clinical centers covered by this registry. Hence, the best approach for completeness evaluation of skin cancer especially NMSC still remains unclear.
In addition to completeness, comparability, and validity, another factor listed by Bray and Parkin (34) as an important quality characteristic of a cancer registry is ‘timeliness’, defined as “the time gap between the diagnosis and the publication of the data of that diagnostic year”. This time is required for the process of transfer of information, various checks in the database in a registry, follow-up of the DCN cases, etc. Though no international standards are defined, in the SEER program, the North American Cancer Registry must report the incidence within 22 months of the end of a diagnostic year (6). Norwegian cancer registry has decreased this time gap from 525 days in 2001 to 261 in 2005 (36) and reported an increase in incidence rate by 1.3% for MM and a decrease by 0.5% for NMSC for the diagnosis year 2005 in the 2007 report vs. that published in 2006. In Germany too, an increase in the overall cancer estimates by 2.5% and by 6.6% for MM was observed for the diagnosis year 2012 in the 2014 report (4) compared to the previous one (NMSC not mentioned). This was explained by the late registration of cases in the registries and delay in the estimation method itself.
Another issue highlighted was the use of imputation to deal with missing data. A study by Eisemann et al., 2011 (41) evaluated the accuracy of multiple imputations method to predict the missing UICC stage and TNM stage for breast cancer and MM. For MM, 20% of the imputed values for UICC-stage were different from the observed values, but for T stage imputations, the difference was as high as 50%. This was explained by the high percentage of missing values in MM cases. Whether to use imputation for MM and for NMSC, which has an even higher missing stage information, is still a matter of debate and needs to be evaluated further.
As observed in the results, screening can influence the incidence rates reported and also the M/I quotient variability across regions. A study assessing the impact of the pilot screening project of 2003-04 in Schleswig-Holstein on the stage-specific incidence of MM (42), proves a shift towards the earlier stage and a decrease in the incidence rate of advanced stages, although the high missing stage information (>60% for MM and >80% for NMSC-Table 2), questions the reliability of this interpretation. Also, the RKI (4) reports having observed no such decrease in the incidence of advanced stage MM until 2014. So, it is possible that the results observed in Schleswig-Holstein were temporary. The effectiveness of SCS is another controversial topic as even the US Preventive Service Task Force (43) confirms to have not enough evidence in favor of skin cancer screening.
Limitations
The study is faced by many practical limitations like not being able to extract information on the remuneration rules of registries in each state or to find the actual influence of differences in trace-back among the registries on the DCO rate or to study the actual effect of changing coding system/applying SEER rules on skin cancer incidence rates in Germany. The diagnosis year for the annual report from each registry was different, reducing the comparability of the data quality. Although all the registries filled in the survey, very few registries responded back to the follow-up questions and hence many doubts like ‘why Berlin registry has included C80 code in MM cases’ could not be clarified. Additionally, it was beyond the scope of this paper to analyze and compare results of the completeness of data using alternative approaches. The methodology of cancer registries undergoes very frequent changes, for instance, the new ICD-11 code for classification of diseases, changes in legislative rules and the upcoming clinical cancer registries, making this article valid for a limited duration.
Conclusions
There is undoubtedly underreporting of skin cancer and the quality of existing data is poor, and it can be concluded that the registration of NMSC needs to be handled differently from MM and other cancers. The situation would expectantly improve with both clinical and epidemiological registries working together. The effect of SCS on incidence rates needs to be incorporated to prevent the overestimation of national incidence, and moreover, to evaluate its actual benefits so that its continuation is justified. A uniform and thorough data collection for all skin cancers is of utmost importance. Appropriate training of the service providers to properly code the different types of NMSC and orienting them towards the importance of completing stage- and site-specific, and therapy information, will definitely raise the standards of quality.
Supplementary
Acknowledgments
We would like to thank the following staff of German cancer registries for replying back to the survey: Dr. Annika Waldmann, Dr. Alice Nenneke, Ms. Wirtin Hiltraud Kajueter, Dr. Laura Khil, Dr. Silke Hermann, Dr. M. Meyer, Dr. Ron Pritzkuleit, Mr. Joachim Kieschke, Dr. Bernd Holleczek, Dr. Katharina Emrich, Dr. Sabine Luttmann, Dr. Ernst-Alfred Burkhardt and Dr. Heide Wilsdorf-Koehler. A special thanks to Dr. Bjørn Møller, Dr. Hans Storm and Dr. Shiva Ayoubi from Nordic cancer registries for providing the necessary information and to Dr. Amena Almes Ahmad for all the moral support.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2019.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [PubMed]
- Cancer Today [Internet]. International Agency for Research on Cancer. 2019. Available online: https://gco.iarc.fr/today/home. [Accessed 2 January 2019].
- Cancer in Germany 2013/14. Berlin: Robert Koch Institute, 2017 11ed.
- Hundsdörfer G. Epidemiological cancer registries in Germany: history from a legal point of view. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014;57:7-12. [PubMed]
-
Das Manual Der Krebsregistrierung 2019 . - Epidemiology of cancer -Report on cancer events in Germany 2016. Berlin: Robert Koch Institute, 2016.
- Rudolph C, Schnoor M, Eisemann N, et al. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. J Dtsch Dermatol Ges 2015;13:788-97. [PubMed]
- Garbe C, Orfanos CE. Epidemiology of malignant melanoma in central Europe: risk factors and prognostic predictors. Results of the Central Malignant Melanoma Registry of the German Dermatological Society. Pigment Cell Res 1992;285-94. [PubMed]
- Kraywinkel K, Bertz J, Laudi A, et al. Epidemiology and Early Diagnosis of Common Cancers in Germany. Berlin: Robert Koch Institute, 2012.
- Hamburger Krebsdokumentation 2013-15. Hamburg: Freie und Hansestadt Hamburg, 2017.
- Krebsinzidenz und Krebsmortalität 2009-2012 (Jahresbericht). Berlin: Gemeinsames Krebsregister (ed.), Berlin, 1/2015, 2015.
- Krebs in Saarland. Saarbrücken: Epidemiologisches Krebsregister Saarland; 2009.
- Epidemiologisches Krebsregister Nordrhein-Westfalen – Jahresbericht 2015 mit Datenreport 2013. Müenster: Epidemiologisches Krebsregister NRW gGmbH, 2015.
- Bericht Des Krebsregisters Rheinland-Pfalz 2016. Mainz: Krebsregister Rheinland-Pfalz gGmbH, 2016.
- Krebs in Schleswig-Holstein. Luebeck: Institut für Krebsepidemiologie e.V.; 2017.
- Krebs in Bayern in den Jahren 2011 und 2012. Erlangen: Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit (LGL), 2014.
- Krebserkrankungen im Land Bremen 2009 - 2011. Bremen: Registerstelle des Bremer Krebsregisters, 2014.
- Krebs in Niedersachsen 2015. Oldenburg: Registerstelle des Epidemiologischen Krebsregisters Niedersachsen (EKN), 2018.
- Krebs in Hessen 2017. Wiesbaden: Hessisches Ministerium für Soziales und Integration, 2017.
- Krebs in Baden-Württemberg. Heidelberg: Epidemiologisches Krebsregister (EKR), 2017.
- Robert Koch Institute. Assessment of completeness [updated 22 February 2013]. Available online: https://www.krebsdaten.de. [Accessed 2 January 2019].
- NORDCAN [Internet]. Association of Nordic Cancer Registries. 2009. Available online: http://www-dep.iarc.fr/NORDCAN/english/ frame.asp. [Accessed 24 June 2019].
- Pukkala E, Engholm G, Hojsgaard Schmidt LK, et al. Nordic Cancer Registries - an overview of their procedures and data comparability. Acta Oncol 2018;57:440-55. [Crossref] [PubMed]
- Social Code (SGB V) Fifth Book Statutory Health Insurance. §65c SGB V Clinical Cancer Registry [updated 22 March 2019]. Available online: https://www.sozial gesetzbuch-sgb.de/sgbv/65c.html. [Accessed 24 June 2019].
- Association of Population Based Cancer Registries in Germany. GEKID-Empfehlungen zur Krebsregistrierung. Hannover, 2005.
- German Institute of Medical Documentation and Information (DIMDI). ICD-O-3 First Revision [updated 27 February 2014]. Available online: https://www.dimdi.de/dynamic/en/classifications/icd/icd-o-3/. [Accessed 4 January 2019].
- WHO. Cancer Incidence in Five Continents, Vol. X. Forman D, Bray F, Brewster DH, et al, editors. IARC Scientific Publication no. 164. Lyon: International Agency for Research on Cancer, 2014.
- German Institute of Medical Documentation and Information (DIMDI). ICD-10-GM Version 2018 [updated 21 September 2018]. Available online: https://www.dimdi.de/ static/de/klassifikationen/icd /icd-10-gm/. [Accessed 29 September 2018].
- Stang A, Stegmaier C, Jockel KH. Nonmelanoma skin cancer in the Federal State of Saarland, Germany, 1995-1999. Br J Cancer 2003;89:1205-8. [Crossref] [PubMed]
- WHO. International Rules for Multiple Primary Cancers (ICD-O Third Edition). Lyon: IARC, 2004. Report No.: 2004/02.
- Czarnecki D, Sutton T, Czarnecki C, et al. A 10-year prospective study of patients with skin cancer. J Cutan Med Surg 2002;6:427-9. [Crossref] [PubMed]
- Tabellen zum GEKID-Atlas [Internet]. GEKID. 2017. Available online: http://atlas.gekid.de/Tabellen/Tabellen_D.php. [Accessed 2 January 2019].
- Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer 2009;45:747-55. [Crossref] [PubMed]
- Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry--history, content, quality and use. Dan Med Bull 1997;44:535-9. [PubMed]
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218-31. [Crossref] [PubMed]
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27-33. [Crossref] [PubMed]
- Weir HK, Johnson CJ, Ward KC, et al. The effect of multiple primary rules on cancer incidence rates and trends. Cancer Causes Control 2016;27:377-90. [Crossref] [PubMed]
- Schouten LJ, Straatman H, Kiemeney LA, et al. The capture-recapture method for estimation of cancer registry completeness: a useful tool? Int J Epidemiol 1994;23:1111-6. [Crossref] [PubMed]
- Buettner PG, Leiter U, Eigentler TK, et al. Development of prognostic factors and survival in cutaneous melanoma over 25 years: An analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer 2005;103:616-24. [Crossref] [PubMed]
- Eisemann N, Waldmann A, Katalinic A. Imputation of missing values of tumour stage in population-based cancer registration. BMC Med Res Methodol 2011;11:129. [Crossref] [PubMed]
- Eisemann N, Waldmann A, Katalinic A. Incidence of melanoma and changes in stage-specific incidence after implementation of skin cancer screening in Schleswig-Holstein. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014;57:77-83. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316:429-35. [Crossref] [PubMed]
Cite this article as: Gupta S, Reintjes R, Trialonis-Suthakharan N. Analysis of the methodology of skin cancer incidence registration in German cancer registries. Ann Cancer Epidemiol 2019;3:8.


