Morphological and functional reconstruction of the esophago-gastric junction with a double-flap technique after laparoscopic proximal gastrectomy
Introduction
Proximal gastrectomy for gastric cancer at the upper stomach or the esophagogastric junction (EGJ) is a popular procedure for function-preserving gastrectomy. Various attempts have been made to prevent regurgitation during esophagogastric reconstructions after proximal gastrectomy including jejunal interposition, the double-tract method, and esophagogastrostomy.
Esophagogastrostomy is frequently used for reconstructions following proximal gastrectomy because of its simplicity. However, while this procedure is safe and straightforward, it is sometimes accompanied by severe reflux of the gastric contents or bile acid. Several investigators have tried to prevent such regurgitation after esophagogastrostomy, which exhibited the valvular ability for anti-reflux by the intra-gastric pressure (1,2); however, none has achieved a reflux-free esophagogastric anastomosis, and a standard method of reconstruction after proximal gastrectomy has yet to be established.
Kamikawa et al. recently developed a reflux-free reconstruction technique involving two main parts: a hand-sewn esophagogastric anastomosis designed to be soft and flexible, and a double-door valve to prevent the regurgitation of gastric contents (in described Japanese literature). Recently Kuroda et al. reported the paper of the technique applied to the laparoscopic procedure (3). For the present study, we applied this new anastomotic technique for cases requiring laparoscopic proximal gastrectomy (LAPG) with totally intracorporeal anastomosis, and herein present this novel reflux-preventing technique with video.
Patient selection and workup
Esophagogastrostomy using a double-door valve after LAPG was performed on 50 patients [45 patients with epithelial neoplasms including 43 gastric cancer and 2 carcinoids, and 5 patients with gastric submucosal tumor (SMT)] treated by the Department of Gastroenterological Surgery at the Cancer Institute Ariake Hospital, Tokyo, Japan, from January 2013 to December 2014 (Table 1). This valvuloplasty method involving a small incision at the upper abdominal wall was introduced at our institute in 2009 for the treatment of gastric cancer and is only carried out by experienced surgeons. All gastric cancers and carcinoids in this study were classified histologically as adenocarcinomas or neuroendocrine tumors that had invaded only the mucosa or submucosa of the stomach without lymph node metastasis (cT1, cN0). Gastric SMTs were diagnosed as gastrointestinal tumors of 2 cm or more in size.
Table 1
| Characteristics | n (%) |
|---|---|
| Age (years) | 65.2±2.0 [30–89] |
| Gender (male/female) | 33 (66.0)/17 (34.0) |
| BMI (kg/m2) | 24.0±3.3 (18.2–36.2) |
| ASA-PS (1/2/3) | 20/14/0 |
| Previous abdominal operation | 5 (14.7) |
| Tumor type (adenocarcinoma/submucosal tumor) | 45 (90.0)/5 (10.0) |
| Tumor size (mm) | |
| Adenocarcinoma | 29.5±13.4 [10–70] |
| Submucosal tumor | 29.5±13.4 [10–70] |
Means ± SE.
LAPG was indicated if the tumor was located in the upper third of the stomach or if gastric SMT was detected at the EGJ. We evaluated tumor location and the depth of tumor invasion on the basis of endoscopy results, an upper gastrointestinal series, and endoscopic ultrasonography. Distant metastases were evaluated by abdominal ultrasonography and computed tomography. Patients treated by this procedure had early gastric cancer with extra indication of endoscopic resection, such as patients with submucosal cancer or mucosal cancer that was histologically confirmed as a poorly differentiated adenocarcinoma of 2 cm or more in size. Some patients with gastric SMT at the EGJ such as those with tumors <2 cm in size were treated by laparoscopic local resection with preservation of cardiac function.
Pre-operative preparation
- Lesion locations were preoperatively confirmed by endoscopic marking and an upper gastrointestinal series. A negative biopsy was collected at this time to enhance stump safety. Due to the particular difficulty in confirming the location of a lesion in early gastric cancer, the laparoscopic surgery was combined with tattoo marking. Marking was also performed on the oral side in cases where the lesion had spread to the esophageal side.
- LAPG is a technique prone to gastric cancer remnants not being detected, thus lesions on the pyloric side of the remnant stomach were carefully scrutinized.
Equipment preference card
For lymph node dissection, ultrasonic coagulating scissors was preferentially used. During the present procedure, staplers were used only for the division for the stomach and esophagus, however, the anastomosis was made by hand-sewn procedure to keep the softness of the anastomosis site.
Procedure
Anesthesia and position
Epidural anesthesia was combined with general anesthesia for the patients in this study. The position of the body was the same as that used during routine laparoscopic surgery; the patient was placed in the supine position with legs apart and resting on levitators. Five ports on a reverse trapezoid were used; however, because pyloric lymph node dissection was not necessary, the port to the bottom right of the patient was best placed closer to the medial cranial side to better enable dissection of the upper edge of the pancreas and around the cardiac orifice, particularly on the left side.
Lymph node dissection
The range of lymph node dissection was determined in accordance with the “Japanese Gastric Cancer Treatment Guidelines (May 2015 revision “For Doctors”, ver. 4)”, thus lymph node dissection ranges were stipulated for each procedure. A dissection range of D1+: No. 1, 2, 3a, 4sa, 4sb, 7, 8a, 9, 11p is considered valid for LAPG in the case of early gastric cancer (4). Carcinoid tumors were also treated as for epithelial neoplasms, according to the strategy for early gastric cancers. Lymph flow along the posterior gastric artery was considered particularly important in upper gastric cancer, and thus No. 11p was properly dissected along the splenic artery up to the periphery of the posterior gastric arterial root.
The hepatic branch and peripheral pyloric branches of the anterior branch of the vagus nerve were preserved, and if possible, so was the celiac branch, which is the main branch of the posterior vagal trunk.
Division of the distal side of the stomach
After lymph node dissection was complete, the stomach was divided. The dividing line on the distal side was to the oral side of the line demarcating the left and right gastroepiploic arteries and veins. As much remnant stomach as possible was left by checking the preoperatively placed endoscopic markings. The #4sb lymph node was also placed on the resection side, after which the stomach was separated from the greater curvature using a stapler device 2–3 times.
Dissection of the esophagus
In patients without esophageal invasion, dissection was performed on the esophageal side using the EGJ. In patients with esophageal invasion, dissection was performed 2 cm to the oral side of the preoperative markings. Once the portion to be separated was determined, markings were made with blue dye at the dividing position of the esophagus.
Esophagogastrostomy with double-door technique (video-guided) (Figure 1)

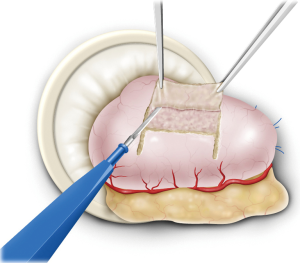
The remnant stomach was withdrawn from the umbilical incision and a 2.5 cm × 3.5 cm seromuscular double-flap was created on the anterior wall of the remnant stomach, leaving a region 1–2 cm from the proximal resection stump (Figure 2). This procedure was performed carefully while cautiously stopping bleeding using an electric scalpel to prevent damage to the flap. The lower edge of the dissected surface was the portion to undergo anastomosis. Afterwards, the remnant stomach was returned to the peritoneal cavity and the peritoneum was re-inflated. Anastomosis was performed under laparoscopic guidance thereafter. First, the upper end of the flap was sutured to the posterior wall of the esophagus, usually with four stitches. The flap was then fixed 5 cm to the oral side of the portion of the esophagus intended for dissection while pulling up the esophagus stump. In doing so, the lower end of the esophagus was ultimately embedded within the stomach wall over a distance of 3–4 cm. Once the esophagus and stomach were secured, subsequent anastomosis became easier. After esophageal stump dissection, 3–4 interrupted stitches were additionally sutured to prevent misalignment of the membrane of the posterior wall of the esophagus and the outer membrano-muscular layer. This step allowed subsequent full-thickness posterior wall anastomosis to be performed safely without missing the outer membrano-muscular layer of the esophagus. Because there is usually a difference in diameter between the esophageal mucosa and gastric anastomosis site, as wide an anastomosis as possible was made to match the diameter of the stomach.
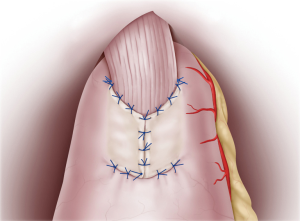
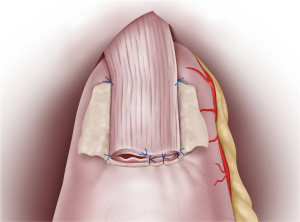
Continuous sutures were applied through all layers of the posterior esophageal wall and the mucosa of the remnant stomach flap detachment surface. Recently, 4-0 barbed sutures have been also used to achieve simultaneously pulling and locking. On the anterior wall, the esophagus and gastric wall at the lower end of the flap detachment surface was anastomosed layer-by-layer using interrupted sutures (Figure 3). Intraluminal anastomosis was also performed safely and easily here by using 4-0 barbed sutures. Once the esophageal-stomach anastomosis was complete, the lumen was endoscopically examined for tucks or other problems. To finish, the flap was positioned so that it covered the anastomosis site in a Y-shape, with the midline first anchored so that the flap covered the widest possible area (Figure 4). Using interrupted stitches and 4-0 barbed sutures, the flap was then secured successively to the left “collar” portion. After securing the right “collar”, the lower end was fixed. The anastomosis site could be widely covered with the flap by suturing the lower end as caudally as possible.
Drain insertion
A closed suction drain with a small diameter was placed below the left diaphragm via the upper right port and via the dorsal side of the anastomosis. This provided information on pancreatic juice leakage at the upper edge of the pancreas and suture failure.
Role of team members
The double-flap technique by the intra-corporeal hand-sewn technique would be difficult to perform for the team which was familiar with the laparoscopic suturing technique. So the team should regularly use the technique for the other laparoscopic surgery. And during operation, we often use the endoscopy for the confirmation of the location of tumor site and EGJ to keep the adequate tumor margin. So the doctor worked outside should constantly prepare the endoscopy. During the procedure, the assistant should offer the good field of vision around EGJ for the success for the procedure. Especially, the traction of the stomach to the caudal side and the traction of the liver are important. It is also important to decide the types of the thread and the length of stitch at the each scene for the preparation by the scrub nurse.
Post-operative management
Table 2 summaries the operative and the post-operative data. The average operation time and estimated blood loss during the described LAPG with double-door technique was 388.9±10.8 min and 89.4±15.3 mL, respectively. No patients required conversion to open surgery, and R0 resection was achieved in all cases.
Table 2
| Variable | |
|---|---|
| Operation time (min) | 388.9±10.8 [244–590] |
| Estimated blood loss (mL) | 89.4±15.3 [10–670] |
| Combined cholecystectomy | 3 (6.0%) |
| Conversion to open surgery | 0 (0%) |
| R0 resection (%) | 50 (100%) |
| Morbidity (CD ≥2) | 1 (2.0%) |
| Anastomotic leakage | 0 (0%) |
| Pancreatic fistula | 1 (2.0%) |
| Mortality, n (%) | 0 (0%) |
| Postoperative hospital stay (days) | 10.7±4.4 [7–31] |
Means ± SE.
With regard to post-operative complications, pancreatic fistula and intra-abdominal bleeding occurred in the same one patient (2%), and a second patient (2%) received a perforated small intestine due to an intra-operative forceps injury (Table 2). No anastomotic leakage occurred in this study, but two patients (4%) who developed stenosis of their esophagogastric anastomosis needed endoscopic balloon dilatation of an anastomotic stricture, 3 and 8 months after surgery, respectively (Table 3). The strictures after surgery had healed by 4 and 6 times of endoscopic balloon dilatation, respectively. Only one patient (2%) developed post-operative reflux-esophagitis of more than grade B based on the Los Angeles Classification, and it was successfully treated by administering a proton pump inhibitor to the patient. There was no mortality in the present study.
Table 3
| Findings | LPG (n=50) |
|---|---|
| Reflux esophagitis (LA ≥ grade B) | 1 (2.0%) |
| Anastomotic stricture (CD ≥ grade IIIa) | 2 (4.0%) |
The endoscopic findings after surgery revealed no esophageal reflux and the anastomosis mimicked normal EGJ formation (Figure 5A,B).
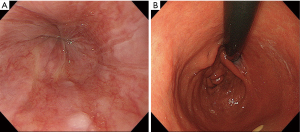
The present procedure was safe with few post-operative complication, so it was unnecessary for the specific post-operative management. However, we should pay the attention for the stenosis of the anastomosis during the mid-term after operation.
Tips, tricks and pitfalls
The present method originally developed by Kamikawa et al. for cases of conventional open proximal gastrectomy has the benefit of strong reflex prevention in the relevant Japanese literature. In this technique, the backflow valve is embedded between the submucosal layer and the seromuscular flap of the stomach, thus preventing backflow when compressed by resistance from intragastric pressure to the side and the flap from the anterior side. We also successfully applied this reconstruction method during total laparoscopic procedures because hand stitching in the profound place near the hiatus is quite difficult for the open procedure while laparoscopic suturing is relative easy in terms of access at this site. Only one patient in the present study developed reflux to the degree of grade B after surgery, and it improved immediately following administration of a proton pump inhibitor. Severe reflux would also be unusual with the present method even in cases undergoing proximal gastrectomy with reconstruction by simple esophagogastrostomy, in which severe esophageal reflux is generally more frequent. Indeed, in such patients, the essential technical component of the present procedure for preventing reflux would be to intentionally fix the lower esophagus between the submucosal layer and the seromuscular flap of the stomach.
Moreover, because the posterior wall of this anastomosis site is composed only of mucosa, there was little constriction during swallowing following the procedure, indicating that the balance between swallowing and antireflux was struck with this post-proximal gastrectomy method of reconstruction. In terms of morphology, the shape of anastomosis was close to that of the original cardiac orifice, which allowed functional and morphological reconstruction of the cardiac orifice. In the present study, two cases developed stricture in the delayed period after surgery, requiring balloon dilatations. Thus, the constructed anastomosis should be made as wide as possible during the procedure to allow the backflow valve to function as intended (i.e., preventing stricture) when compressed by the intragastric pressure from behind the side and the flap on the anterior side.
Another major feature of the present study was the rarity of suture failure because the anastomosis site was covered with a flap, making this procedure extremely safe. While essentially a repetition of simple motions, this procedure is deceptively complex, and the surgeon should be particularly experienced in using a suturing technique under laparoscopy to construct the anastomosis. In addition, without flexibility in hand stitching, the benefits of this procedure could not be reproduced. Reconstruction should therefore be performed patiently without using a stapler after waiting for improvement in the postoperative course, the suturing technique under laparoscopy should be got especially with be particular about the minimally invasive procedure.
There were also limitations to the esophageal dissection carried out as part of the studied procedure. In Japan, the esophagus can be dissected for several centimeters with this procedure using a transhiatal approach (in which case the field of view needs to be ensured via transhiatal left thoracotomy); however, no cases of thoracoscopy have yet been encountered for the present procedure. Nonetheless, indications for esophageal cancer and their utility have already been reported, so we await results involving thoracoscopy (6).
This reconstruction method excelled in striking a balance between swallowing and reflux prevention in a morphologically and functionally successful reformation of the EGJ. Future studies of the procedure should evaluate long-term quality of life.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Committee has approved the study (No.2016-1024). Informed consents were obtained by each patient before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Redo SF, Barnes WA, Ortiz Della Sierra A. Esophagogastromy without reflux utilizing a submuscular tunnel in the stomach. Ann Surg 1960;151:37-46. [PubMed]
- Matsushiro T, Hariu T, Nagashima H, et al. Valvuloplasty plus fundoplasty to prevent esophageal regurgitation in esophagogastrostomy after proximal gastrectomy. Am J Surg 1986;152:314-9. [Crossref] [PubMed]
- Kuroda S, Nishizaki M, Kikuchi S, et al. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg 2016;223:e7-e13. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Nunobe S, Hayami M, Hiki N. The remnant stomach was withdrawn from the umbilical incision and a 2.5 cm × 3.5 cm seromuscular double-flap is created on the anterior wall of the remnant stomach, leaving a region 1–2 cm from the proximal resection stump. Asvide 2017;4:045. Available online: http://www.asvide.com/articles/1351
- Mine S, Nunobe S, Watanabe M. A Novel Technique of Anti-reflux Esophagogastrostomy Following Left Thoracoabdominal Esophagectomy for Carcinoma of the Esophagogastric Junction. World J Surg 2015;39:2359-61. [Crossref] [PubMed]
Cite this article as: Nunobe S, Hayami M, Hiki N. Morphological and functional reconstruction of the esophago-gastric junction with a double-flap technique after laparoscopic proximal gastrectomy. Ann Laparosc Endosc Surg 2017;2:25.