
Pre-operative planning for reverse shoulder replacement: the surgical benefits and their clinical translation
Introduction
Reverse shoulder arthroplasty (RSA) has seen a significant increase in utilization in the United States in the past several years as it can offer excellent function to patients with a gamut of glenohumeral pathology including rotator cuff tear arthropathy, glenohumeral arthritis with severe glenoid bone loss (1,2), acute and delayed treatment of proximal humerus fractures (3), failed anatomic shoulder arthroplasty, chronic shoulder dislocations (4), massive irreparable rotator cuff tears, and rheumatoid arthritis. The utilization of RSA in the United States has been growing steadily since FDA approval in 2003, and in 2014 its use surpassed utilization of anatomic TSA for the first time (5). RSA use is likely to continue to increase due to the aging population, expanding indications, and increased surgeon experience with the prosthesis. Long term outcomes have been encouraging, with overall survivorship of 91–93% at minimum 10-year follow up and persistently improved outcome scores (6,7).
Importance of glenosphere position in RSA
Glenosphere positioning has a significant impact on outcomes in RSA. The glenosphere determines the center of rotation and biomechanical traits of the new joint. In both Grammont-style prostheses and newer more anatomic designs, glenosphere position can influence clinical outcomes. Malpositioning can lead to an increase risk of a variety of complications including: dislocation, scapular notching, decreased range of motion, and component loosening. Scapular notching is the most well studied complication and occurs when repeated impingement of the humeral polyethylene insert against the scapular neck during shoulder adduction and/or external rotation leads to progressive bone loss along the inferior neck of the scapula. This bone loss is likely due to a combination of repeated mechanical impingement and a potential wear debris response leading to polyethylene wear, osteolysis, and potential implant loosening. Multiple recent studies suggest glenosphere position is directly correlated with the incidence of scapular notching due to bony impingement, and subsequently negatively influenced clinical outcomes (8-17).
Glenosphere position is determined by surgical technique. Reasons for malpositioning of the glenosphere include inaccurate assessment of pathologic anatomy (18), incorrect choice of implant and/or positioning of the implant to correct pathology (19), and inaccurate execution of the preoperative plan at the time of surgery (19,20). All are influenced by surgical exposure and degree of glenoid bone loss. Glenoid exposure is critical in allowing the surgeon to assess the pathologic anatomy and identifying landmarks for accurate glenosphere placement. This can be increasingly challenging with previous surgery, degree of stiffness, and patient’s body habitus. Avoiding bony impingement and scapular notching in patients with minimal glenoid bone loss and a scapular neck of at least 1 cm is straightforward. It becomes difficult in patients with significant glenoid bone loss or reduced scapular neck length (10). In cases of negligible glenoid bone loss, the surgeon may use the surface of the glenoid fossa, “subchondral smile”, or standard guides with built-in tilt as a reference to place the guide pin in the appropriate amount of tilt. However, some studies have shown that these references can be unreliable. Dilisio et al. performed virtual glenoid baseplate preparation using the “subchondral smile” and a guide with built-in inferior tilt and found them to place the baseplate in 8.9 and 2.8 degrees of superior tilt, respectively (21). Moreover, Verborgt et al. found that when using standard instrumentation implants were positioned with an error range of 16° for glenosphere inclination and 12° for version (22).
Role of advanced imaging
The glenoid surface is the surgeon’s main reference for inserting the guide pin at the time of surgery, yet the scapular plane defines glenosphere orientation. As previously stated, intraoperative surface landmarks can be unreliable in assessing glenoid and scapular morphology. Inaccuracy in the assessment of bony pathology can create problems at the time of surgery potentially resulting in glenosphere malposition or altered surgical plan. In order to better understand pathologic anatomy and prevent glenosphere malposition, preoperative advanced imaging can be utilized. Computed tomography (CT), in particular, provides better detail of bony pathology compared to plain radiographs. Two-dimensional (2D) CT is routinely performed, but several studies have proven the superiority of three-dimensional (3D) CT imaging in quantifying glenoid bone loss and guiding surgical decision-making (20,23,24). Two-dimensional CT can be misleading when used to determine the patient’s preoperative glenoid version and inclination. The measurement of glenoid version using 2D CT scans is inaccurate in the presence of as little as 1 degree of out of plane imaging (25). Bokor et al. (26) showed that minor rotation of the scapula of 15 degrees can alter the accuracy of glenoid version by up to 10 degrees. Hoenecke and others (27) compared the measurement of glenoid version and assessment of the point of greatest wear in the glenoid fossa with respect to the scapula plane on both 2D axial CT scans and 3D CT reconstruction models. They showed that the original CT scans were almost never perpendicular to the scapular body. In addition, the authors showed that the point of greatest wear was missed on 2D scans in 52% of cases and that absolute error in version measured on the 2D CT slice passing through the tip of the coracoid was 5.1 degrees, while in 20% of cases, the error was more than 10 degrees. Similarly, Scalise et al. (23) compared the assessment of the zone of glenoid bone loss, glenoid version and glenoid component fit among four experienced shoulder surgeons and showed that the use of 3D CT reconstructions improved inter-rater reliability. Thus, 3D CT allows the surgeon to more accurately identify the planes of the glenoid and scapula and consequently improve precision in assessing preoperative glenoid version and inclination.
Preoperative planning
Preoperative planning with 3D CT imaging allows the surgeon to better appreciate the relationship between the glenoid and scapular planes, subsequently enabling him/her to accurately calculate glenoid version and inclination (Figure 1A,B). In addition, the inner contour of the normal glenoid vault is uniform across the population. A virtual model has been created along with rules to position it within a pathologic glenoid. Once accurate placed, the model can be used to determine the prepathologic glenoid version, inclination, and joint line position in the presence of glenoid bone loss (27-30). Sizing and placing the vault model requires adjustments in all three planes. In the axial and sagittal planes, the vault is aligned with the inner cortical margin of the anterior wall. In the coronal plane, the vault is aligned with the inner cortical margin of the superior wall at the suprascapular notch. If positioned correctly, the vault model facilitates the surgeon to better define the area of bone loss in pathologic glenoids (Figure 1B,C,D).
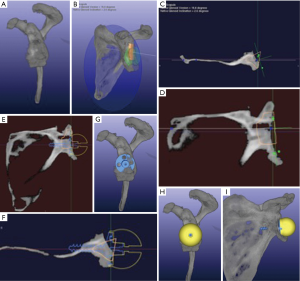
The vault model can be a useful instrument for preoperative planning in RSA, particularly when dealing with bone loss. It can help to address both joint line medialization and pathologic version. It has been shown that glenosphere lateralization and correcting pathologic glenoid version increase impingement-free range of motion (31). This is essential when using a Grammont style implant, where excessive medialization results in decreased arc of motion, subsequent scapular notching and its repercusions. Moreover, by correcting back to pre-morbid anatomy there is an improvement in soft-tissue tensioning that can increase implant stability and potentially improve external rotation strength of any remaining rotator cuff muscles. It is the authors’ practice to place the glenoid baseplate at the patient’s pre-morbid joint line and restore as close to pre-morbid version as possible, as determined by the vault model, while still achieving at least 50% baseplate contact with native glenoid bone (Figure 1E,F,G,H,I). This is supported by Formaini and others who found that glenoid baseplate fixation is no different when when 50% or greater of the baseplate is supported by native glenoid bone (32). Version and medialization can both be corrected by glenoid bone grafting, eccentric reaming, use of an augmented baseplate or lateralized glenosphere, or most commonly a combination of these surgical techniques.
Virtual templating permits the surgeon to place the glenosphere in various positions within the glenoid vault on a 3D CT reconstruction of the patient’s scapula. This enables the surgeon define the ideal implant location and glenosphere size to achieve the desired surgical goals, with awareness that glenosphere size may need to be adjusted intraoperatively in order to address soft tissue tension and stability. Glenosphere size and position is chosen to avoid overhanging glenoid bone anterior, posterior, or inferior to prevent humeral component impingement on range-of-motion (Figure 1H,I).
Once the preoperative plan is established, 3D CT virtual templating can be used to help the surgeon execute the plan in the operating room. The planning software generates the location and orientation of the glenoid guide pin. The surgeon then notes the specific placement of the guide pin relative to the local anatomy of the glenoid fossa, such as a biconcave line if present or small indentations and osteophytes on the periphery, as well as the trajectory of the pin relative to the glenoid surface at that location. The surgeon should be aware that acquired glenoid bone loss, posterior erosion and/or significant osteophyte formation may skew the ideal location of the guide pin on the surface of the glenoid because the center of the glenoid vault does not always correspond to the center of the arthritic glenoid surface. Virtual planning also helps locate the correct guide pin placement when the instrumentation includes a drill guide that is the size and shape of the glenoid baseplate. In this situation, the surgeon should reference an en-face view of the implant on the virtual preoperative plan and note the position of the implant relative to bony landmarks and the rim of the glenoid. The surgeon then places the drill guide in the same location as the implant on the virtual preoperative plan to aid in accurate pin placement.
Another critical step in glenosphere positioning is glenoid reaming, with the goal of creating a uniform contact area and maximizing baseplate seating within native glenoid bone. Some preoperative planning software allows the surgeon to perform simulated reaming. This aids in assessing appropriate reaming depth and location so as to execute preoperative plan during surgery. The surgeon can then confirm that the baseplate is placed sufficiently inferior on the glenoid face to prevent scapular notching.
Eccentric glenosphere and baseplate designs present added versatility in baseplate position. They allow the surgeon to find a location higher within the glenoid vault with adequate screw/peg purchase while still placing the glenosphere in an inferior position and thus maximize impingement-free range of motion. When the vault is too small to achieve adequate fixation in any location, bone grafting may be required. The graft size can be calculated using the preoperative software by measuring the amount of bone loss relative to the vault model and desired baseplate position. The surgeon may also use the alternate glenoid centerline to increase the amount of fixation in bone when advanced bone loss is present. As described by Klein et al. The alternate centerline directs the center screw or peg of the baseplate along the axis of the scapular spine from the center of the glenoid to the junction of the scapular spine as it joins the body of the scapula (2). This has negative impact in terms of impingement free range of motion because it places the implant in approximately 30 degrees of anteversion. This could compromise internal rotation of the arm due to coracoid impingement.
Virtual templating has been shown to improve the accuracy of guide pin position and orientation using standard instrumentation. Iannotti and others first evaluated guide pin position and orientation using bone models. They compared 3D templating to 2D imaging while using standard instrumentation. The authors found an improvement in guide pin accuracy of 4.5±1.0° in version, 3.3±1.3° in inclination, and 0.4±0.2 mm in location with the use of 3D templating (20). This was followed by a randomized clinical trial of 46 patients undergoing total shoulder arthroplasty where they once more demonstrated three-dimensional templating significantly improved the precision of implant placement to within 5° of desired inclination or 10° of version (24).
Patient specific instrumentation
Preoperative virtual templating can be used to translate the preoperative plan into the operating suite in the form of patient specific instrumentation (PSI) and intraoperative navigation. Both methods use computed tomography and preoperative planning software to determine the optimal implant location yet differ in the manner in which they transfer the information to the operating suite. PSI entails generating a custom guide that is able to reference the local anatomy in order to place the guide pin in the desired location, version, and inclination based on the preoperative plan. On the other hand, intraoperative navigation uses an optical tracking system to assess the orientation of the guide pin relative to landmarks on the glenoid and scapula. This requires intraoperative calibration of the tracking system relative to a three-dimensional reconstruction of the patient’s scapula by marking certain anatomical landmarks. Though intraoperative navigation does not require manufacturing a custom guide, it does require specialized tracking equipment and additional steps for calibration that may increase surgical time and cost. Several clinical studies have reported an approximate increase of 30 minutes, and that the current technologies have experienced technical problems that have required aborting the technology in up to 37.5% of cases (33,34).
Commercially available PSI systems
There are two types of commercially available PSI: disposable single-use, and reusable. Single-use patient specific instruments are generated based on the desired glenoid component position established during virtual templating. The information is used to manufacture a guide that references the surface anatomy of the bony glenoid and places the guide pin in the desired location and orientation relative to the plane of the scapula. Several systems also provide a 3D model of the patient’s glenoid in order to assess intraoperatively correct guide fit to the bony anatomy and thus accurately reproduce the preoperative plan.
The second class of PSI uses a reusable instrument that is able to be modified to determine the correct guide pin location and orientation based on native glenoid surface anatomy. First, virtual templating is used to establish desired glenoid component position. Afterwards, the software generates instructions for configuring the guide using a reusable and adjustable base. Alternatively, the guide can be configured by using a printed 3D glenoid model with the planned location and orientation of the guide pin.
Currently, six manufacturers offer patient specific instrumentation for reverse shoulder arthroplasty. Depuy, DJO, Zimmer-Biomet, Tornier and Stryker offer single-use PSI and Arthrex offers patient-specific reusable instrumentation. All offer virtual templating software using a 3D reconstruction of the patient’s scapula. At the time of this publication, single use PSI systems specifically designed for reverse shoulder arthroplasty include: the Depuy TRUMATCH™ Personalized Solutions System, DJO Match PointTM System, the Zimmer/Biomet PSI Shoulder for Trabecular Metal™ Reverse Glenoid System, the Tornier BLUEPRINT™ planning software and PSI, and the Stryker TrueSight™ Personalized Planning System, while the Arthrex Virtual Implant PositioningTM (VIP) System offers a reusable PSI guide.
The DJO-Materialise system uses software where landmarks on the glenoid and scapula are chosen to generate a coordinate system for measuring native glenoid version and inclination. The surgeon is then able to simulate placement of the glenoid guide pin, reaming, and templating of the glenoid baseplate, as well as placement of a bone graft. Once a surgical plan is formulated, the information is used to fabricate a patient specific pin guide that references the surface of the glenoid and base of the coracoid (Figure 2). This guide is used to place the central guide pin or drill hole in the planned location, version, and inclination. A 3D bone model of the patient’s glenoid is also provided to aid in intraoperative patient-specific guide placement.
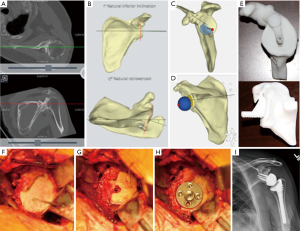
Similarly, the Depuy and Stryker systems use the same Materialise software to create a 3D model based on a patient’s CT scan and a patient specific guide that references off of the coracoid is used intraoperatively to reproduce the preoperative plan.
The Tornier BLUEPRINT™ planning software is unique in that the surgeon creates a 3D reconstruction and can place the implants without the need for a company engineer. Once CT imaging is uploaded, the software automatically calculates native glenoid version and inclination, as well as humeral subluxation, even if the scapula is incompletely imaged. Once the surgeon selects the desired implant size and location, the software determines the ideal guide pin entry point and trajectory, required reaming depth, and percent backside seating. If patient specific instrumentation is to be used, four different points on the edge of the glenoid are selected for the patient-specific guide arms to rest. A 3D printed bone model along with the patient-specific guide is provided for the procedure. It is very important to pay attention to rotational alignment when placing the guide on the glenoid. The company recommends defining the positions of the guide arms in terms of a clock face, with the superior glenoid designated as the 12 o’clock position in order to eliminate any rotational malalignment.
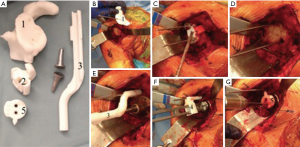
The Zimmer/Biomet system employs a similar software but allows for complete implant templating. The surgeon is able to virtually ream the glenoid, place baseplate screws, and trial different size glenospheres. Once preoperative plan is agreed upon a 3D bone model of the patient’s glenoid is manufactured with multiple patient specific guides. The guides include: a pin guide to direct baseplate location, a ream guide to direct reaming angle and depth, a roll guide to control rotation of the implant, and a drill guide that is placed over the baseplate to control the direction and length of the screws (Figure 3).
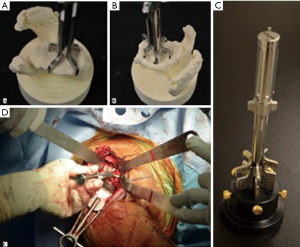
The Arthrex Virtual Implant Positioning™ (VIP) software functions are similar to that of the Zimmer system. The surgeon provides the company with the preoperative CT images, who then generates a preliminary surgical plan predicated upon the surgeon’s implant positioning principles. The surgeon has the ability to then review, modify, and approve the final surgical plan. The company then provides the surgeon with instructions for setting the reusable PSI, named the Glenoid Targeter, from a three-dimensional model of the glenoid or a calibrator device. The Glenoid Targeter is assembled and adjusted intraoperatively to recreate the planned version and inclination (Figure 4).
Published PSI studies
Numerous studies have investigated the precision of pin guide placement using single-use PSI during reverse and anatomic shoulder arthroplasty. Several cadaveric studies have compared the 3D preoperative plan to the final implant position using pre- and postoperative CT and were found to have a mean deviation from plan in inclination and version of less than 5 degrees (37-40). The largest of these was a multi-surgeon cadaveric study by Throckmorton et al. (37) in which 70 shoulders were randomized to PSI or standard instrumentation for either anatomic or reverse TSA. In anatomic TSA, the use of PSI was found to improve mean deviation in version and inclination while in RSA the difference. Though, multivariate analysis for the entire cohort, combining both TSA and RSA, showed statistically significant more accurate final implant position with the use of PSI. Patient specific guides were most beneficial in glenoids with more than ten degrees of retroversion. The authors found no correlation between surgeon’s surgical volume of shoulder arthroplasty and accuracy with the use of standard instrumentation or PSI.
While these cadaveric studies validate the use of patient specific guides as a reliable means of executing the preoperative plan in vitro, it can be more challenging to use these guides in vivo. It is important for the surgeon to recognize the importance of adequate exposure of intraoperative landmarks in order for the patient specific guide to seat properly. Sometimes this exposure can be hampered by body habitus, soft tissue interposition, and joint contractures potentially causing inadequate visualization and/or guide malposition.
In light of this, several clinical studies assessed the results of different types of PSI in the operating room. Hendel et al. (19) performed a randomized controlled clinical trial comparing standard instrumentation and 2D planning to PSI and 3D templating in 31 patients undergoing anatomic total shoulder arthroplasty. In the patient specific instrumentation group there was a reduced mean deviation in version from 6.9° to 4.3°, and in inclination from 11.6° to 2.9°. The greatest benefit was found in patients with glenoid retroversion in excess of 15° where the average deviation in implant position from the preoperative plan for version improved from 10° to 1.2°. No difference was seen in patients with retroversion <7°. This is similar to the above findings by Throckmorton et al. that suggested increased benefit of PSI used in patients with marked glenoid deformity (37). Lastly, there was a substantial increase in selection of the ideal glenoid implant and decreased incidence of implant malposition with PSI and 3D templating. In a similar study, Dallalana et al. (41) demonstrated high accuracy with PSI in vivo in 20 patients who underwent shoulder arthroplasty (10 TSA and 10 RSA) with the aid of a CT based PSI system. Postoperative CT scans showed a mean deviation from plan of 1.8±1.9° in glenoid version and of 1.3±1.0° in inclination. The mean deviation in position on the glenoid face was 0.5±0.3 mm in the anteroposterior plane and 0.8±0.5 mm in the superoinferior plane. Most recently, Villatte and others (42) performed a meta-analysis comprised of 12 studies and 227 participants (patients and cadavers) and found significantly lower deviations in version, inclination, and entry point with the use of PSI compared to standard instrumentation. Range of deviation from preoperative plan with the use of PSI was about 1.88° to 4.96°, depending on the parameter. They also found the number of component outliers were significantly higher with standard instrumentation than with PSI. Of note, heterogeneity was moderate or high for all parameters.
Reusable PSI may be cost and time efficient as it does not require fabrication of a custom guide. In the studies by Iannotti et al. (20,24) they examined the effectiveness of the Intelligent Reusable Instrument (IRI) (Custom Orthopaedic Solutions, Cleveland, OH), a type of reusable PSI now available as the Arthrex VIP™ system, to assist with guide pin placement (Figure 4). In the sawbones study, the authors found a statistically significant improvement of 8.2±0.9° in version, 11.4±1.2° in inclination, and 1.7±0.2 mm in location with the use of the IRI and 3D planning software compared to 2D imaging and standard instrumentation. When compared to standard instrumentation and 3D planning, the use of the IRI and 3-D software improved pin positioning by 3.7±0.9° in version, 8.1±1.2° in inclination, and 1.2±0.2 mm in location (P<0.001 for all). They validated this finding with a randomized controlled trial of 46 patients undergoing anatomic total shoulder arthroplasty randomized to either 3D templating with the IRI or 3D templating with standard instrumentation (24). These groups were compared to a historical control group using 2D imaging with standard instrumentation. All preoperative and postoperative measurements were taken from three dimensional CT reconstructions. In accordance with the sawbone study, the authors found that both 3D templating with PSI and 3D templating with standard instrumentation significantly improved the accuracy of glenoid component placement within 5° of inclination and 10° of version of desired location when compared to the control group. Moreover, the authors found no difference in glenoid component position between patients where 3D templating and the IRI was used and those were 3D templating and standard instrumentation was used. In a follow up study by Iannotti et al. in 173 patients undergoing anatomic TSA, all forms of PSI with preoperative 3D templating showed improved glenoid component position, when compared to 2D glenoid imaging without templating and standard instrumentation. There was a trend towards greater accuracy with the reusable adjustable instrumentation in some comparisons (43).
Technical considerations in the use of PSI
Despite the aforementioned studies demonstrating the increased accuracy of implant positioning with the use of PSI, it is not foolproof. It is important for the surgeon to familiarize themselves with the details of the particular commercially available PSI and preoperative planning software that he or she is using. The surgeon should know if the PSI was created from CT or MRI. If the PSI was created from bony anatomy using a CT scan, the surgeon needs to carefully remove any remaining cartilage, fibrous tissue and labrum from the glenoid fossa at the time of surgery, as is commonly seen on the paleoglenoid in biconcave glenoids. Otherwise, if PSI is made from MRI images these soft tissue structures are vital for adequate guide fit. In addition, osteophytes should be left intact initially, as most PSIs use these as references. This ensures that the PSI rests on the landmarks it was intended to rest on, improving the fit of the device. For PSIs that reference off the base of the coracoid, the labrum and periosteum need to be meticulously removed around the coracoid so that the PSI again rests solely on bone. The surgeon should reference images from the preoperative plan and/or the accompanying glenoid bone model to ensure that that the PSI is in the correct location, thus ensuring that the PSI matches perfectly with the glenoid as intended. Once the PSI is correctly placed and stable, the guide pin is then inserted. Its position and placement should be compared to the pre-operative plan and 3D glenoid model if present, and any adjustments should be made before proceeding with further glenoid instrumentation according to manufacturer recommendations.
After placing the guide pin, the surgeon must still control both the depth of reaming, and also maintain reaming in the trajectory of the guide pin. Bending of the central guide pin with the reamer can occur resulting in the implant being in a different position than the guide pin. The surgeon must also control the roll or rotation of the baseplate. Careful preoperative planning and intraoperative referencing of the plan will assist in this step and subsequently optimize screw length and trajectory. The Zimmer/Biomet PSI system is currently the only system that uses multiple guides to aid in controlling these variables in this part of the procedure.
It is important for the surgeon to remember that patient specific instruments do not define the optimal implant placement, it only provides the ability to accurately translate the virtual preoperative plan to the operating suite. The surgeon must still use judgment in deciding implant type, location, and orientation. These parameters are determined in the preoperative virtual plan. Furthermore, there is still debate about ideal implant position that only long term clinical follow up studies will ultimately resolve.
Intraoperative navigation
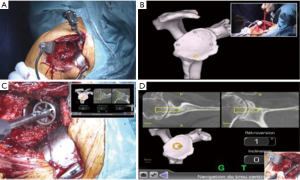
Intraoperative navigation has also been utilized to improve the accuracy of glenoid placement in RSA. It allows for accurate execution of the preoperative plan intraoperatively without the need of a patient specific guide. It requires the surgeon to calibrate the navigation system intraoperatively by marking certain anatomical landmarks. It provides the surgeon with real-time feedback of: guide and reaming retroversion and inclination, reaming and drilling depth, as well as screw placement. The only commercially available system currently is the ExactechGPS™, which is used with the Exactech Equinoxe™ shoulder system (Figure 5). In a study of 14 cadavers there was an improvement glenoid component version of 5.6°, 4.9° of glenoid component tilt, and improved accuracy of screw placement with a CT-based navigation system compared to standard instrumentation (23). Moreover, in a meta-analysis by Sadoghi et al. (44) of 5 studies performing intraoperative navigation in shoulder arthroplasty showed an improvement in weighted mean version of navigated shoulders of 4.4° retroversion versus 10.6° for standard instrumentation. However, despite the improvement in accuracy, studies have raised potential technical difficulties with navigation. In a case series reported by Edwards et al. (33), the accuracy of the navigation system was 2.6° and the additional operative time was initially 20–30 minutes but improved to approximately 10 minutes with experience. In the only prospective randomized clinical trial, Kircher et al. (34) compared standard instrumentation to navigation in TSA in 20 patients and found that standard instrumentation improved glenoid retroversion from 14.4° to 10.9° and navigation improved retroversion from 15.4° to 3.7°. The authors did mention experiencing technical difficulties in 37.5% of cases and an increase of 31.5 minutes in operative time subsequently leading to the discontinuation of navigation.
Patient specific implants
Dealing with complex glenoid deformity is one of the most challenging aspects of reverse shoulder arthroplasty. These deformities can be present secondary to severe primary degenerative or dysplastic pathology and can also be seen secondary to bone loss in the setting of revision surgery. Large defects or cavitary lesions result in a lack of bony support for the glenoid component, limiting treatment options and affecting clinical results. Options included bone grafting, augmented baseplates, or large head hemiarthroplasty as a salvage procedure. More recently, custom made implants have been released as a means of dealing with bone loss in particularly complex cases.
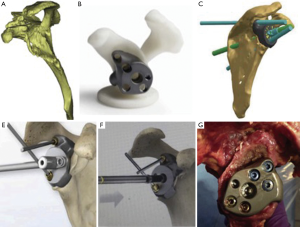
Zimmer/Biomet has released the FDA approved Comprehensive® Vault Reconstruction System, a patient specific reconstruction system for RSA in patients with severe soft-tissue deficiency and glenoid bone loss. The prosthesis is made using CAD/CAM (computer-aided design/computer-aided manufacturing) reconstructions based off the patient’s 3D CT reconstructions (Figure 6). Preoperative planning also allows for the planning, placement, size and trajectory of the implant screws to gain the best glenoid fixation. The implant fills bone voids with a porous plasma spray-coated titanium, which allows for biological fixation and can potentially account for bone loss of up to 50 mm × 50 mm × 35 mm (45). Chammaa et al. (46) reported on another CAD/CAM custom made prosthesis in the United Kingdom in which 37 patients with severe bone loss were treated with the CAD-CAM TSR (Stanmore Implants Worldwide, Elstreee, UK). The prosthesis is custom made utilizing preoperative 3D CT reconstructions and resembles a total hip prosthesis with 5 components including a large hydroxyapatite-coated titanium glenoid shell, with slots for screw fixation into the scapula and titanium fixation screws, followed by a polyethylene liner, and a cobalt-chrome humeral stem with a 28 or 32 mm sized humeral head. At a mean follow up of 5 years, the study showed a significant improvement in mean pain level with activity from 9.2±1.7 to 2.4±2.9 and improvement in the Oxford Shoulder Score from 11±8 points to 27±11 points. Complications requiring reoperation occurred in 9 patients (24%).
3D printed custom implants offer potential solutions in the management of severe glenoid bone loss in the setting of primary or revision shoulder arthroplasty. Additional clinical outcome studies are needed to define their effectiveness and role.
Conclusions
With the increasing use of reverse shoulder arthroplasty and its expanding indications, surgeons today are facing tougher reconstructive challenges while still providing the patient with a good clinical outcome. There are a greater number of primary and revision cases where glenoid vault deformity is encountered. This presents a challenge to the surgeon during glenoid component positioning. He or she must place the implants in a location and orientation that maximizes range of motion and stability while minimizing impingement. In order to address this, surgeons can look to the use of 3D imaging in order to better understand each patient’s pathology. With the use of virtual planning the surgeon has the ability to arrive in the operating room with an established surgical plan in order to better address the deformity present. This can help in determining if glenoid bone grafting, eccentric reaming, or the use of augmented/lateralized components is the best choice in addressing bony deformity and maximizing impingement-free range-of-motion. Furthermore, with the advent of patient specific instrumentation and navigation the surgeon has the means to translate the preoperative plan into the operating room with increased accuracy, thus, decreasing the likelihood of component malposition and its associated complications. In the future, custom implants may grant the surgeon the means to address severe glenoid bone loss that would otherwise not be reconstructable and potentially give the patient improved function.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.12.09). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. VE reports personal fees from DJO Surgical, grants from OREF, outside the submitted work. IJP Iannotti reports personal fees from DJO Surgical, personal fees from Arthrex, Inc., other from Custom Orthopaedic Solutions, personal fees from Depuy Synthes, personal fees from Tornier, personal fees from Wolters Kluwer Health, outside the submitted work. ETR reports personal fees from Depuy Synthes, personal fees from DJO Surgical, personal fees from Journal of Bone and Joint Surgery - American, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mizuno N, Denard PJ, Raiss P, et al. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am 2013;95:1297-304. [Crossref] [PubMed]
- Klein SM, Dunning P, Mulieri P, et al. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am 2010;92:1144-54. [Crossref] [PubMed]
- Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am 2013;95:2050-5. [Crossref] [PubMed]
- Drake GN, O'Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res 2010;468:1526-33. [Crossref] [PubMed]
- Palsis JA, Simpson KN, Matthews JH, et al. Current Trends in the Use of Shoulder Arthroplasty in the United States. Orthopedics 2018;41:e416-23. [Crossref] [PubMed]
- Bacle G, Nove-Josserand L, Garaud P, et al. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J Bone Joint Surg Am 2017;99:454-61. [Crossref] [PubMed]
- Cuff DJ, Pupello DR, Santoni BG, et al. Reverse Shoulder Arthroplasty for the Treatment of Rotator Cuff Deficiency: A Concise Follow-up, at a Minimum of 10 Years, of Previous Reports. J Bone Joint Surg Am 2017;99:1895-9. [Crossref] [PubMed]
- Mollon B, Mahure SA, Roche CP, et al. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017;26:1253-61. [Crossref] [PubMed]
- Simovitch RW, Zumstein MA, Lohri E, et al. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am 2007;89:588-600. [Crossref] [PubMed]
- Paisley KC, Kraeutler MJ, Lazarus MD, et al. Relationship of scapular neck length to scapular notching after reverse total shoulder arthroplasty by use of plain radiographs. J Shoulder Elbow Surg 2014;23:882-7. [Crossref] [PubMed]
- Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524-8. [Crossref] [PubMed]
- Poon PC, Chou J, Young SW, et al. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2014;96:e138 [Crossref] [PubMed]
- Randelli P, Randelli F, Arrigoni P, et al. Optimal glenoid component inclination in reverse shoulder arthroplasty. How to improve implant stability. Musculoskelet Surg 2014;98:15-8. [Crossref] [PubMed]
- Gutiérrez S, Comiskey CA, Luo ZP, et al. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am 2008;90:2606-15. [Crossref] [PubMed]
- Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:807-13. [Crossref] [PubMed]
- Permeswaran VN, Caceres A, Goetz JE, et al. The effect of glenoid component version and humeral polyethylene liner rotation on subluxation and impingement in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1718-25. [Crossref] [PubMed]
- Kolmodin J, Davidson IU, Jun BJ, et al. Scapular Notching After Reverse Total Shoulder Arthroplasty: Prediction Using Patient-Specific Osseous Anatomy, Implant Location, and Shoulder Motion. J Bone Joint Surg Am 2018;100:1095-103. [Crossref] [PubMed]
- Iannotti JP, Greeson C, Downing D, et al. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:48-55. [Crossref] [PubMed]
- Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am 2012;94:2167-75. [Crossref] [PubMed]
- Iannotti J, Baker J, Rodriguez E, et al. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. J Bone Joint Surg Am 2014;96:e71 [Crossref] [PubMed]
- Dilisio MF, Warner JJ, Walch G. Accuracy of the Subchondral Smile and Surface Referencing Techniques in Reverse Shoulder Arthroplasty. Orthopedics 2016;39:e615-20. [Crossref] [PubMed]
- Verborgt O, De Smedt T, Vanhees M, et al. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg 2011;20:21-6. [Crossref] [PubMed]
- Scalise JJ, Codsi MJ, Bryan J, et al. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am 2008;90:2438-45. [Crossref] [PubMed]
- Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am 2015;97:651-8. [Crossref] [PubMed]
- Bryce CD, Davison AC, Lewis GS, et al. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am 2010;92:692-9. [Crossref] [PubMed]
- Bokor DJ, O'Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg 1999;8:595-8. [Crossref] [PubMed]
- Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, et al. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:166-71. [Crossref] [PubMed]
- Scalise JJ, Codsi MJ, Bryan J, et al. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg 2008;17:487-91. [Crossref] [PubMed]
- Codsi MJ, Bennetts C, Gordiev K, et al. Normal glenoid vault anatomy and validation of a novel glenoid implant shape. J Shoulder Elbow Surg 2008;17:471-8. [Crossref] [PubMed]
- Ganapathi A, McCarron JA, Chen X, et al. Predicting normal glenoid version from the pathologic scapula: a comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg 2011;20:234-44. [Crossref] [PubMed]
- Keener JD, Patterson BM, Orvets N, et al. Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: a computer-enhanced range of motion analysis. J Shoulder Elbow Surg 2018;27:339-49. [Crossref] [PubMed]
- Formaini NT, Everding NG, Levy JC, et al. The effect of glenoid bone loss on reverse shoulder arthroplasty baseplate fixation. J Shoulder Elbow Surg 2015;24:e312-9. [Crossref] [PubMed]
- Edwards TB, Gartsman GM, O'Connor DP, et al. Safety and utility of computer-aided shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:503-8. [Crossref] [PubMed]
- Kircher J, Wiedemann M, Magosch P, et al. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg 2009;18:515-20. [Crossref] [PubMed]
- Donohue KW, Ricchetti ET, El-Telbany AS, et al. Patient-Specific Instruments and Implants in Reverse Shoulder Arthroplasty. In: Edwards TB, Editor. Reverse Shoulder Arthroplasty: A Practical Approach. Thieme Publishers: New York. 2017, 173-86.
- Ramkumar PN, Mahylis JM, Ricchetti ET, et al. Patient Specific Instrumentation for Severe Deformity in Reverse Shoulder Arthroplasty. In: Dines J, Dines D, Gulotta L. Editors. Shoulder Surgery: Tricks of the Trade. New York: Thieme Publishers, 2019.
- Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg 2015;24:965-71. [Crossref] [PubMed]
- Levy JC, Everding NG, Frankle MA, et al. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1563-7. [Crossref] [PubMed]
- Walch G, Vezeridis PS, Boileau P, et al. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg 2015;24:302-9. [Crossref] [PubMed]
- Eraly K, Stoffelen D, Vander Sloten J, et al. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg 2016;25:837-45. [Crossref] [PubMed]
- Dallalana RJ, McMahon RA, East B, et al. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg 2016;10:59-66. [Crossref] [PubMed]
- Villatte G, Muller AS, Pereira B, et al. Use of Patient-Specific Instrumentation (PSI) for glenoid component positioning in shoulder arthroplasty. A systematic review and meta-analysis. PLoS One 2018;13:e0201759 [Crossref] [PubMed]
- Iannotti JP, Walker K, Rodriguez E, et al. Accuracy of three-dimensional planning, implant templating and patient-specific instrumentation in anatomic shoulder arthroplasty. J Bone Joint Surg Am 2019; [Epub ahead of print].
- Sadoghi P, Vavken J, Leithner A, et al. Benefit of intraoperative navigation on glenoid component positioning during total shoulder arthroplasty. Arch Orthop Trauma Surg 2015;135:41-7. [Crossref] [PubMed]
- Dines DM, Gulotta L, Craig EV, et al. Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System. Am J Orthop (Belle Mead NJ) 2017;46:104-8. [PubMed]
- Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg 2017;26:101-7. [Crossref] [PubMed]
Cite this article as: Rodríguez JA, Entezari V, Iannotti JP, Ricchetti ET. Pre-operative planning for reverse shoulder replacement: the surgical benefits and their clinical translation. Ann Joint 2019;4:4.


