Starting from scratch: implementing outcome measurement in clinical practice
Introduction
Outcome measurement is becoming increasingly important in palliative care, not only in research but also in clinical care (1). Often, structure and process measures are seen as most relevant for measuring quality of care, but outcomes are essential as they matter to patients. Ideally, outcomes are reported by patients as patient reported outcome measures (PROMs) which are defined as ‘standardised, validated questionnaires that are completed by patients to measure their perceptions of their own functional status and wellbeing’ (2). There are limitations to PROMs in palliative care when patients become too ill or are dying. Proxy assessment by professionals or relatives can help to fill the gap, at least partially and with its own limitations. To take account of the person centeredness of outcome measurement, the term patient centred outcome measurement (PCOM) was suggested (3). Based on a systematic review, Etkind et al. described the advantages of PCOM feedback in palliative care populations in improving awareness of unmet need and allowing professionals to act to address patients’ needs (3). Regular ongoing assessments in palliative care clinical practice have the potential to enable consistent monitoring of disease status and prognosis, the effectiveness of interventions, accurate symptom assessment to ensure appropriate clinical management, and better quality discussions around the concerns and priorities as defined by the patient (4). Policy makers or relevant scientific bodies of palliative care increasingly demand the use of outcome measurement although it has not been widely translated into every day work. Recommendations have been developed for the implementation of PROMs in clinical practice in palliative care (5,6). Nevertheless, implementing routine outcome measurement into clinical practice remains a challenge. Therefore, the aim of this article is to describe the process of implementing routine outcome measurement into daily clinical work in a university palliative care unit.
Methods
According to the recommendations of Antunes for implementing outcome measurement into clinical care, these steps were followed in the first instance (5):
- selection of outcomes of interest;
- selection of outcome measure(s);
- education about the measure and use of results;
- selection of one coordinator/facilitator;
- who applies the measure and its periodicity.
Setting
Outcome measurement was implemented in a 10-bedded palliative care unit in the Department of Palliative Medicine at Munich University Hospital. The Department also runs a hospital support team and a specialist palliative home care team. The palliative care unit focusses on symptom management and support for psychosocial and spiritual concerns of patients and also support for informal carers. Patients are mainly suffering from advanced cancer, advanced lung and heart disease as well as neurological disease. Average length of stay on the palliative care unit is 10 days and about 40% of patients are discharged from the unit either home or to an inpatient hospice (for long-term care of terminally ill patients). The multi-professional team consists of doctors (consultant and registrars), nurses, a social worker, a psychologist, a breathing therapist, a physiotherapist and two chaplains.
Results
Since 2014, a process of implementing outcome measures in routine clinical care has started in the Department of Palliative Medicine.
Selection of outcomes of interest
The head of department and the clinical leads agreed to implement outcome measurement into the daily clinical routine of the palliative care unit. The focus of outcome measurement and respective tools should be on most prevalent symptoms of patients and their psychological, practical and spiritual concerns as well as the functional status of patients. Because of the holistic nature of palliative care, burden of relatives should also be part of the assessment.
Selection of outcome measure(s)
Based on previous activities of the first author (1,6-8), international developments and collaboration with the Cicely Saunders Institute at King’s College London and especially the Outcome Assessment and Complexity Collaborative (OACC), internationally comparable outcome measures were chosen for routine clinical use. Other measures, such as the Problem Checklist of the German National Palliative Care register (9), the Minimal Documentation System (MIDOS) (10), the Edmonton Symptom Assessment Scale (ESAS) or the ECOG Performance Status were considered. However, they deemed not to fulfil the criteria mentioned above for outcomes of interest, especially the inclusion of psychological, practical and spiritual concerns. The ECOG Performance Status was not chosen as it is mainly used in oncology but not in patients with non-malignant disease. Measures had to have demonstrated reliability and validity for a palliative care population as well as responsiveness to change.
- The Integrated Palliative Care Outcome Scale (IPOS) is a multidimensional scale measuring symptoms and other concerns of patients with advanced disease (11). Seventeen questions focus on patients’ main concerns, common symptoms, patient and family distress, existential well-being, sharing feelings with family, information received and practical concerns. Answer options range from 0 (not at all) to 4 (overwhelmingly) with verbal explanations on a Likert scale. The IPOS is primarily patient reported but a version for professionals also exists. It takes professionals about 2–5 minutes to complete the IPOS. For patients, it takes about 8 minutes. The time to complete the IPOS decreases with repeated assessments;
- ‘Phase of illness’ is a concept developed in context of the Australian Case Mix Classification which describes the stage of a patient’s illness in five distinct, clinically meaningful phases—stable, unstable, deteriorating, terminal, bereavement (12,13). Both the patient’s and families’ situation are included in the assessment. Phase of illness can be used as an indicator of acuity and to reflect complexity in conjunction with other tools (4). Palliative care phases provide a clinical picture of a patient trajectory including a distinction between expected and unexpected changes in the type of care required (4). As care ends with the discharge or death of a patient on the palliative care unit, bereavement was omitted as phase in everyday clinical documentation. Phase of illness is judged by professionals;
- The Australian Karnofsky Performance Status (AKPS) was chosen to describe the functional status of the patients (14). It is a useful modification of the Karnofsky Perfomance Status (KPS) but is more appropriate for clinical settings that include multiple venues of care such as palliative care (14). The AKPS is also rated by professionals.
Education about the measure and use of results
Before introducing the above-mentioned measures in the daily clinical routine of the unit, the team was introduced to outcome measurement in several team meetings including introduction of outcome measurement in palliative care in general, rationale and explanation of the chosen measures and role plays on how to use the measures. Education sessions were provided by members of the clinical and research team with specific experience in outcome measurement and also in providing training for outcome measurement in palliative care. To deepen the understanding and experience, a weekend team retreat focussed on outcome measurement in palliative care. Again, team members learned to use outcome measures, especially IPOS, in a 15-minute role play encounter between a professional and a patient with one observer, with time for feedback to the “professional” and discussion afterwards. In a second role play, a team meeting was simulated with 4–6 professionals discussing a patient’s problems of the last days and measures for management based on IPOS, AKPS and phase of illness. Recommended steps for completing the IPOS together with the patient were (I) knowledge of the questionnaire, (II) opening of the conversation (introduction, explanation why IPOS is used, content and answering options, time for completion), (III) use of IPOS (building up rapport, unjudgmental interviewing of the patient reading out the IPOS questions and answer options, non-verbal cues), and (IV) ending the conversation (reassurance that IPOS answers are used confidentially and for clinical use only, what happens next, checking that all answers are filled in correctly); (V) after the interview, answers are checked and acted upon accordingly; for IPOS scores 0–1: checking with patient whether and what support is necessary; for IPOS scores 2, 3 or 4 indicating a more severe problem or symptom burden, checking the management plan and discussing it in the multi-professional team. Questionnaires should be included in the patient record or entered in the electronical database.
Selection of one coordinator/facilitator
The consultant responsible for the palliative care unit was chosen as coordinator for outcome measurement on the ward. The team members, who also provided the training sessions served as facilitators on the ward.
Who applies the measure and its periodicity
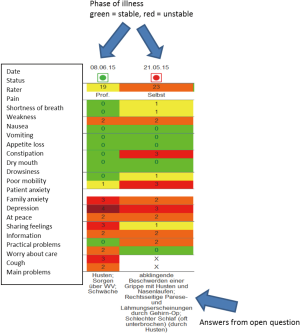
At the same time of implementing outcome measurement on the ward, an electronic patient record was introduced in the Department of Palliative Medicine. IPOS, phase of illness and AKPS were included in the online system. The IPOS answer options were colour-coded with 0= green to 4= red to demonstrate the score in a more visual way.
On admission to the palliative care unit, a baseline assessment is completed by the responsible physician for every patient including phase of illness, IPOS and AKPS. When patients are well enough they receive the IPOS for filling in. The completed IPOS is then collected at the ward round on the next day.
In the daily multi-professional morning round, professionals reassess jointly the phase of illness of every patient. If a change in phase of illness is agreed upon, the IPOS will be completed on the same day during the ward round. If the phase of illness has changed, the online systems demands that the IPOS to be filled in.
Once a week, either patients are asked to complete the IPOS on the day before the weekly multi-professional team meeting or the IPOS is rated as proxy-assessment by professionals. These IPOS scores and phase of illness judgements are then used in the team meeting where every patient is discussed in more detail. Scores are demonstrated on a computer screen visible to all participants (see Figure 1). Every team member then briefly reports their views on the patient either supporting the IPOS score or commenting on diverting views. On this basis, a management plan is agreed on for the following week. Ideally, the IPOS is again completed about 48 hours before discharge.
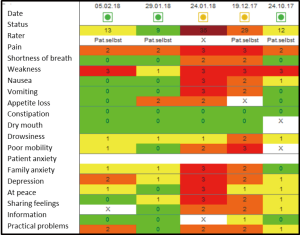
Case study
Mrs. W, 52 years old, ovarian cancer. She was first seen in the outpatient clinic before Christmas for pain management.
On the 24th January, she was admitted to the palliative care unit because of incomplete gastrointestinal obstruction with nausea, vomiting and abdominal pain. She received antiemetics and analgesics via continuous subcutaneous infusion. After 3 days when the symptoms were settled, the drug regimen was changed back to oral application. Patient is rather anxious and suffering from her increasing weakness. She was discharged home with good symptom control and referred to the specialist palliative home care team.
The IPOS scores shown in Figure 2 demonstrate her main problems pain and weakness on the 24th October (column right side). At the second visit in the outpatient clinic, problems have increased with moderate levels of breathlessness, nausea and vomiting and severely affected by pain, weakness and reduced mobility. On admission to the palliative care unit on the 24th January, the patient was also severely affected by vomiting, own and the relatives’ anxiety and worry as well as feeling depressed and only occasionally feeling in peace. After 5 days on the ward, most physical symptoms and psychological and spiritual concerns have been resolved.
Analyses of outcome measures over time
Completion of outcome measures has improved over 2 years as demonstrated in the heat maps in Figure 3. In 2015, the first year of implementation of outcome measures, the proportion of missing values was as high as 70% with higher rates of missing values on IPOS questions on psychosocial and family issues. AKPS and phase of illness showed missing values of <10%. In the second year, 2016, missing values decreased to O% for most months for phase of illness and AKPS. Missing values for IPOS scores also decreased but there was still a substantial proportion of missing values.
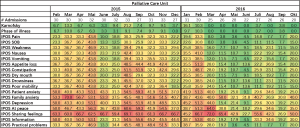
Figure 4 demonstrates frequencies of IPOS scores in relation to phase of illness of 574 patients admitted to the palliative care unit. This helps the team to better understand the needs of the population they serve.

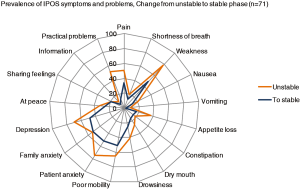
To illustrate the change in symptom scores over time, e.g., in relation to phase of illness, data can be presented in form of spider maps (see Figure 5) to show the team effects of care but also potential areas for improvement.
Barriers and facilitators
Facilitators that were helpful for the implementation of routine outcome measurement in clinical care were the necessity to conduct a structured assessment of patients and the need to use the assessment in weekly team meetings for funding of palliative care units. It was also helpful that the clinical leads and the head of department had experience in outcome measurement and that one member in the research team had both clinical and research experience in outcome measurement and especially with IPOS. She also produced the figures and statistical analyses of the measures to demonstrate to the team. Finally, patient reported outcome measurement is one of the key recommendations in the German Guideline for palliative care for patients with incurable cancer and reduction in pain and breathlessness are part of quality indicators (15). Therefore, outcome measurement is increasingly demanded to be implemented by services.
There were also barriers for the implementation, e.g., prejudices in the team questioning the benefit of outcome measurement in everyday life or the attitude that the description of patients with the outcome measure does not reflect their real situation. Some team members also complained about the amount of documentation already necessary or felt that their duty is patient care but not assisting research.
Discussion
Implementation of outcome measurement in clinical routine is both challenging and exciting. As others have shown, we also think that it is feasible when following a structured process (16). According to Greenalgh’s taxonomy, use of outcome measurement in clinical care can serve both the individual and the group. In our example, outcome measurement is used for monitoring patients’ symptoms, palliative care needs and functional status and on the group level for facilitation of communication with the multi-professional team and as decision aid (17). Ideally, outcome measurement combines clinical documentation, online assessment, visualisation for team meetings, quality assurance and data collection for research in one system and outcome measure. Not many measures serve these requirements, but we felt that the IPOS is a very good measure as it is valid and reliable in a palliative care population, short and easy to use and sensitive to change. The use of IPOS, AKPS and phase of illness helps to foster and structure information and communication on relevant outcomes within the clinical team and especially team meetings. In the future, it is planned to implement a feedback loop and have reports on outcomes with IPOS scores, also in relation to phase of illness, twice a year. This will hopefully help us to identity areas for change and improvement as for example demonstrated in the completeness of data as shown in the heat maps.
Implementation of outcome measurement in routine clinical care touches on principles of change management. It has been recommended that for successful change management other factors than those relating to individual professionals may be important and that more systematic use of theories in planning and evaluating quality-improvement interventions in clinical practice theories may help to explain whether change is possible (18). We have not based our implementation process or the educational components on such a theory which might have been helpful and should be considered for future projects.
Facilitators play an important role in the implementation process to improve the likelihood of success. Facilitators provide support to help people change their attitudes, habits, skills, ways of thinking, and working (19). Kitson distinguishes between internal belonging to the team and external facilitators coming from outside of the team (19). The facilitators in our implementation process almost had a dual role as they were not members of the care team but researchers from the department known to most members of the care team. Having experience with outcome measurement and with training staff in outcome measurement was an important factor for choosing facilitators to support the implementation process as the whole concept of outcome measurement is still quite new in Germany.
Criteria for funding of palliative care services in the Diagnose Related Groups (DRG)-system include structured assessments of patients at baseline and a care plan discussed in weekly team meetings. To be acknowledged as a palliative care service, it has to be demonstrated that valid measures are used. As funding is related to individual patients, it will be checked whether the assessment has been undertaken on the individual level and if these assessments are missing, extra funds for palliative care might not be granted. However, outcome measurement as such with repeated measurements is not demanded yet for funding. There is also no benchmarking system for palliative care in Germany and no adjustment for complexity to compare services based on potential differences between services rather than between patients as this is done in Australia. However, patient reported outcome measurement using validated tools is recommended in the German guideline ‘Palliative care for patients with incurable cancer’ and the IPOS is one of the recommended tools (15). Five out of ten quality indicators are on reduction of symptoms (breathlessness and pain, restlessness in the dying phase, general assessment in the dying phase) or on screening for palliative care needs. It is recommended to show this reduction on validated tools. These quality indicators are now used for regular audits for Comprehensive Cancer Centres and palliative care services in these centres have to demonstrate a reduction.
As suggested by Antunes we tried to follow a structured process for the implementation (5). The educational component proved to be crucial but remains challenging with staff changes and new professionals joining the team. If resources allow it, regular workshops on outcome measurement with role plays and general information on how to use outcome measures would be beneficial. Also, having a dedicated person on the team with extra time for the implementation process or ideally a quality improvement facilitator as in the Palliative Care Outcome Collaborative (PCOC) (20) would be a major support for the team.
Implementing outcome measurement in clinical care improves person-centred care through supporting clinicians with relevant information on patients’ needs and functional status, and also facilitates quality improvement of a service and benchmarking. Nevertheless, implementation is a time consuming and long-lasting process needing continuous attention and never seems to be completed. However, the benefits outweigh the burden of implementation and it is a task worthwhile undertaking.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bausewein C, Daveson BA, Currow DC, et al. EAPC White Paper on outcome measurement in palliative care: Improving practice, attaining outcomes and delivering quality services - Recommendations from the European Association for Palliative Care (EAPC) Task Force on Outcome Measurement. Palliat Med 2016;30:6-22. [Crossref] [PubMed]
- Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ 2010;340:c186. [Crossref] [PubMed]
- Etkind SN, Daveson BA, Kwok W, et al. Capture, Transfer, and Feedback of Patient-Centered Outcomes Data in Palliative Care Populations: Does It Make a Difference? A Systematic Review. J Pain Symptom Manage 2015;49:611-24. [Crossref] [PubMed]
- Rawlings D, Hendry K, Mylne S, et al. Using Palliative Care Assessment Tools to Influence and Enhance Clinical Practice. Home Healthc Nurse 2011;29:139-45. [Crossref] [PubMed]
- Antunes B, Harding R, Higginson IJ, et al. Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliat Med 2014;28:158-75. [Crossref] [PubMed]
- Bausewein C, Daveson B, Benalia H, et al. Outcome measurement in Palliative Care - The Essentials. London: PRISMA, 2009.
- Bausewein C, Le Grice C, Simon S, et al. The use of two common palliative outcome measures in clinical care and research: a systematic review of POS and STAS. Palliat Med 2011;25:304-13. [Crossref] [PubMed]
- Bausewein C, Simon ST, Benalia H, et al. Implementing patient reported outcome measures (PROMs) in palliative care--users' cry for help. Health Qual Life Outcomes 2011;9:27. [Crossref] [PubMed]
- . Available online: https://www.hospiz-palliativ-register.de/wp-content/uploads/2017/04/Datensatz_und_Glossar.pdfNationales Hospiz- und Palliativregister.
- Stiel S, Matthes ME, Bertram L, et al. Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative care: the German version of the edmonton symptom assessment scale (ESAS). Schmerz 2010;24:596-604. [Crossref] [PubMed]
- Schildmann EK, Groeneveld EI, Denzel J, et al. Discovering the hidden benefits of cognitive interviewing in two languages: The first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat Med 2016;30:599-610. [Crossref] [PubMed]
- Eagar K, Watters P, Currow DC, et al. The Australian Palliative Care Outcomes Collaboration (PCOC)--measuring the quality and outcomes of palliative care on a routine basis. Aust Health Rev 2010;34:186-92. [Crossref] [PubMed]
- Masso M, Allingham SF, Banfield M, et al. Palliative Care Phase: inter-rater reliability and acceptability in a national study. Palliat Med 2015;29:22-30. [Crossref] [PubMed]
- Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice BMC Palliat Care 2005;4:7. [ISRCTN81117481]. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie. S3 Leitlinie Palliativmedizin für Patienten mit einer nicht heilbaren Krebserkrankung. c: https://www.awmf.org/uploads/tx_szleitlinien/128-001OLl_S3_Palliativmedizin_2015-07.pdf
- Tavares AP, Paparelli C, Kishimoto CS, et al. Implementing a patient-centred outcome measure in daily routine in a specialist palliative care inpatient hospital unit: An observational study. Palliat Med 2017;31:275-82. [Crossref] [PubMed]
- Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 2009;18:115-23. [Crossref] [PubMed]
- Grol RP, Bosch MC, Hulscher ME, et al. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Q 2007;85:93-138. [Crossref] [PubMed]
- Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care 1998;7:149-58. [Crossref] [PubMed]
- Currow DC, Allingham S, Yates P, et al. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 2015;23:307-15. [Crossref] [PubMed]