Surgical prophylaxis with gentamicin and acute kidney injury: a systematic review and meta-analysis
Introduction
Surgical site infections accounted for 17% of healthcare associated infections and were found in 2–5% of patients undergoing inpatient surgery. These infections are estimated to cost up to $10 billion annually (1). Prophylactic antibiotics have been found to reduce the incidence of postoperative wound infections (2). According to clinical practice guidelines for antimicrobial prophylaxis in surgery, antimicrobials from the cephalosporin class are recommended as the first-line agents for the majority of procedures. However, studies have found that cephalosporins are among the common antimicrobials associated with Clostridium difficile-associated diarrhea (CDAD). Other agents associated with CDAD include clindamycin, fluoroquinolones, and extended-spectrum penicillins (3). Given this concern over the increasing incidence of CDAD, alternative regimens with decreased risk of CDAD, including ones containing gentamicin, have been increasingly used in current practice (2,4).
Gentamicin has been in use for more than 40 years and remains popular not only because its effectiveness but also because of its low rate of antimicrobial resistance, low cost, and low risk of CDAD (5). It belongs to the aminoglycoside class of drugs; its main bactericidal mechanism is inhibition of protein synthesis in susceptible organisms. Gentamicin has activity against a wide range of aerobic gram-negative bacteria, including Escherichia coli, Proteus species, Pseudomonas aeruginosa, species of the Klebsiella-Enterobacter-Serratia group, and Citrobacter species. It is also active against methicillin-susceptible Staphylococcus aureus (MSSA) but not methicillin-resistant Staphylococcus aureus (MRSA) or Streptococci (6).
The major disadvantages of gentamicin are its requirement for drug level monitoring and the risks of ototoxicity and nephrotoxicity. The pathogenesis of gentamicin-induced nephrotoxicity is related to tubular necrosis and glomerular injury through the induction of mesangial cell contraction, proliferation, and apoptosis.
Studies have shown that the rate of nephrotoxicity secondary to therapeutic use of gentamicin is between 8% and 26%. Patients at risk include patients with elevated gentamicin trough level, elevated area under the plasma drug concentration-time curve, prolonged duration of treatment, concomitant use of vancomycin, flucloxacillin or furosemide, elevated baseline serum creatinine, or volume depletion (5). Most data on gentamicin-induced nephrotoxicity have been obtained in patients who had received therapeutic doses of this drug. The data on kidney injury associated with prophylactic use of gentamicin have been reported in small studies. We, therefore, wanted to systematically review the association between the risk of acute kidney injury (AKI) and prophylactic use of gentamicin in the setting of different types of surgery in adult patients.
Methods
Identification of studies
Two main authors performed electronic searches in January 2016 on PubMed and Embase. First, MeSH terms (PubMed) and Emtree terms (Embase) were used to search for available articles. Subsequently, to broaden the search results, the authors repeated the search without using the MeSH/EMTREE functions. The search strategy included terms for AKI, gentamicin, and surgical prophylaxis. The authors then reviewed all titles and abstracts for subsequent selection of articles.
Inclusion and exclusion criteria
Studies were included if: the incidence of AKI was reported; a control group was included; they were published in English.
Studies were excluded if: the study did not report all incidence of AKI; the study was performed in pediatric patients; the study was published in languages other than English; a full text was not available. There were no limitations in the publication dates.
Data extraction
All titles and abstracts were reviewed by two reviewers independently. In abstracts which information was insufficient, the full text was also reviewed. Any discrepancies were resolved through consensus.
Quality assessment
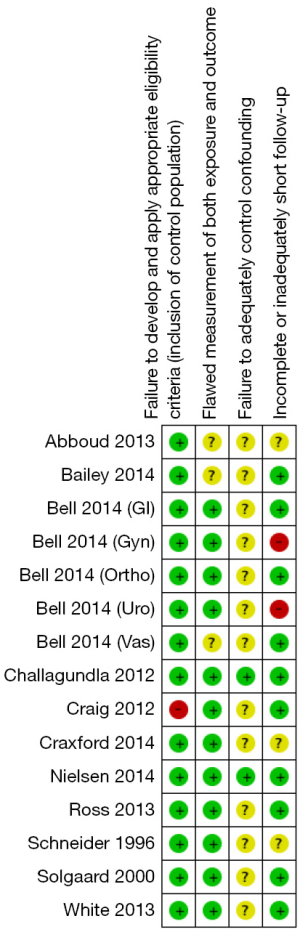
All included studies are either prospective or retrospective cohort studies. No randomized controlled trials were found during the electronic search. We used the risk of bias assessment tool for observational studies recommended in GRADE working group to assess the quality of each study. The assessment tool addressed the following four aspects: failure to develop and apply appropriate eligibility criteria, flawed measurement of both exposure and outcome, failure to adequately control confounding and incomplete or inadequately short follow-up (7). The risk of bias for each domain was categorized into three levels: low, high and unclear.
Statistical analysis
The authors used the Review Manager 5.3 software (The Cochrane Collaboration, Oxford, UK) for data analysis as well as quality of evidence assessment. The risk ratios (RR), risk differences (RD) and 95% confidence intervals were calculated using a random-effect model. Pooled RR was then calculated and Forest plots were drawn. I2 was calculated to measure heterogeneity across studies.
Funnel plot was utilized to allow for visual assessment of publication bias.
Since the included studies reported the incidence of AKI related to gentamicin-containing surgical prophylactic regimen in several types of surgery, subgroup analysis was performed according to the types of surgery (orthopedic, cardiac, and urologic surgery).
Results
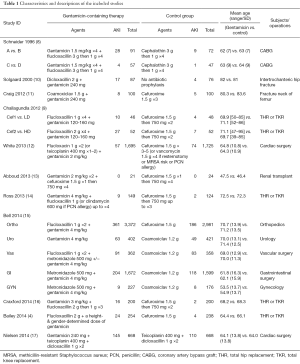
Three hundred and fourteen articles were identified using our search strategy. Two hundred and ninety studies were excluded after title and abstract review as they were not relevant to the study question. The authors then reviewed the remaining 24 studies in full length to evaluate their study designs, patient cohorts, and outcomes. After full-length review, 13 studies were excluded as they met exclusion criteria. Finally, 11 studies (15 cohorts) with 18,354 patients were included in the statistical analysis. Figure 1 demonstrates the flow diagram of the study selection process. Table 1 contains the characteristics of the included studies. The study conducted by Bell et al. consisted of 5 cohorts, with each cohort representing one surgical subspecialty (15). There were 4 study groups in each of the studies done by Challagundla et al. (9) and Schneider et al. (8), so we coupled each 2 study groups together in order to simplify the comparisons.
Full table
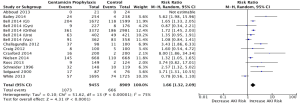
Figure 2 demonstrates the analysis with all studies included, regardless of surgery type. The pooled RR of all studies regardless of surgery type was found to be 1.66 (1.32, 2.09), whereas the pooled RD of this analysis was found to be 0.05 (0.03, 0.07). The calculated heterogeneity value (I2) was 75%.
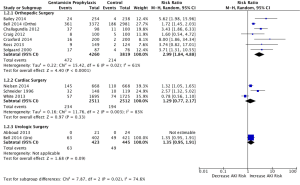
Figure 3 demonstrates the subgroup analysis according to surgery type. The pooled RR of orthopedic surgery only was found to be 2.99 (1.84, 4.88), whereas the pooled RD of this analysis was found to be 0.07 (0.04, 0.11) with I2 of 61%. The pooled RR of cardiac surgery only was found to be 1.29 (0.77, 2.17), whereas the pooled RD of this analysis was found to be 0.05 (−0.03, 0.13) with I2 of 83%. The pooled RR of urologic surgery only was not applicable as one study (13) had zero AKI events thus RR was not estimable.
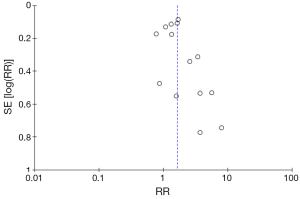
Figure 4 illustrates the funnel plot of all studies included in this meta-analysis which is used to visually assess the publication bias. Figure 5 and Figure 6 show the risk of bias assessment. These trials had methodological limitations mainly in failure to control confounding factors since all of them were observational studies and most did not take possible confounding factors into account i.e., pre- or perioperative medications, perioperative hypotension or blood loss. One study had high risk of bias in terms of appropriateness of eligibility criteria due to lack of exclusion criteria (11). In another study, only 47% and 52% of patients undergoing gynecologic and urologic operations respectively, were able to document postoperative renal function which raised a major concern over incomplete follow-up (15).
Discussion
The included studies reported the incidence of AKI secondary to gentamicin prophylaxis in several types of surgery. Studies in orthopedic surgery were the most frequent (7 cohorts). The characteristics of the gentamicin-containing arm and the control arm were the most homogeneous in the orthopedic studies, with penicillin antibiotic (mostly flucloxacillin) and gentamicin in the exposure arm and cefuroxime in the control arm, except for one study (10) in which the control group used no antibiotic prophylaxis.
The second most frequent type of surgery in the included studies was cardiac surgery. With three cohorts, the characteristics of the gentamicin-containing arm and the control arm were less homogeneous compared to the orthopedic surgery studies. Gentamicin and flucloxacillin were the main regimen in the exposure arm, whereas the control arm mainly used beta-lactam antibiotics (with or without teicoplanin).
Two urologic studies were included here; the exposure arm and the control arm were not as homogeneous as in the orthopedic studies.
It is noteworthy that dosing, frequency, and timing of antibiotic administrations were different across studies. Most studies defined AKI as a rise in serum creatinine of greater than 50% of the baseline (4,9,11,14,16), while two studies used the aforementioned criteria or an increase in serum creatinine of >0.3 mg/dl (or >26.5 µmol/L) (17), and >26.4 µmol/L (15). One study not only defined AKI as an increase of >50% in the serum creatinine but the serum creatinine value must also be >120 µmol/L on the first postoperative day (8). Solgaard et al. defined a reversible nephrotoxicity as an increase of serum creatinine above the upper standard 95% limit, and irreversible kidney damage as uremia leading to death (10). White et al. defined renal impairment as serum creatinine >200 µmol/L (or 2.26 mg/dL) or requirement for renal replacement therapy (12). Abboud et al. did not define AKI in their study, however, the investigated patients did not have worsening renal function in that study—most likely because the study was done in renal transplantation (13).
Most studies reported the mean preoperative creatinine values which ranged between 71–98 µmol/L in the control groups and 65–98 µmol/L in the gentamicin groups. Bailey et al. excluded patients with baseline glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2 (4). Craxford et al. also excluded patients with abnormal baseline renal function although the cutoff GFR was not mentioned (16). Five studies did not mention the use or requirement of renal replacement therapy. The requirement rates of renal replacement therapy ranged between 0–2.2% in the control groups and 0–2.88% in the gentamicin groups.
Our meta-analysis showed that there was a significant increase in risk of AKI related to gentamicin therapy used as a surgical prophylaxis. The analysis of orthopedic studies had the largest effect size and the greatest degree of homogeneity. Gentamicin and penicillin-antibiotic therapy as a surgical prophylaxis carries more risk for the development of AKI for patients undergoing orthopedic surgery as compared to cefuroxime therapy. In a subtype of orthopedic surgery, Craxford et al. showed that total knee replacement (TKR) had a significantly higher rate of kidney injury than total hip replacement (THR) (16). They suggested that using pneumatic tourniquet in TKR produced ischemic metabolites and further increased risk of AKI when combined with gentamicin use.
Although, gentamicin-containing regimen was associated with higher risk of developing AKI, the degree and severity of AKI in these studies were mild and transient. Bell et al. showed that the majority of affected patients had transient stage1 AKI by Kidney Disease Improving Global Outcomes (KDIGO) classification and the median length of stay in patients with AKI was 8 days compared to 7 days in patients without AKI (15).
Our meta-analysis showed that gentamicin-containing therapy slightly increased risk of AKI compared to the control group treated with beta-lactam antibiotics (with or without teicoplanin) in cardiac surgery patients, but this did not reach statistical significance. In one cardiac surgery study, gentamicin had a protective effect compared to cefuroxime. The author suggested that gentamicin might protect against perioperative infection, but this did not reach statistical significance (12). Gentamicin-containing therapy as a surgical prophylaxis in urologic surgery also had an increased risk of AKI compared to the beta-lactam prophylaxis but this did not reach statistical significance.
The combination of gentamicin and flucloxacillin was used in most studies, and these drugs had a significant increase in risk of AKI. However, it is difficult to determine whether gentamicin, flucloxacillin, or both increase the risk of AKI development. Gentamicin is a common cause of tubular injury, whereas flucloxacillin is an uncommon cause of interstitial nephritis. Challagundla et al. reported a significant decrease in the incidence of AKI after reducing the dose of flucloxacillin from 8 to 4 g (9).
AKI in postsurgical patients is probably multifactorial (hypotension, medication, infection, etc.). It may be hard to blame one factor, but gentamicin has definite nephrotoxicity and could have a role in patients who are at risk for AKI and prolong the renal function recovery period. Despite the increased risk of AKI in gentamicin-containing regimens compared to beta-lactam regimens, the benefits of gentamicin over beta-lactam antibiotics need to be considered. The rate of Clostridium difficile infection with the use of beta-lactam antibiotics is an important concern. Further analysis of this outcome should be conducted in the future.
Limitations exist for our study. First, all studies included were retrospective/prospective cohort studies which could have confounding factors affecting the results. A randomized controlled trial could eliminate these biases. Second, the heterogeneity of regimen across studies (types of regimen and dosage) might lead to difficulty in interpretation. Additionally, the calculated heterogeneity values (I2) in all analysis were considered high. Third, the definition of AKI, the change in creatinine, and length of creatinine monitoring varied among studies. Fourth, the sample sizes of cardiac and urologic studies were not large enough to reflect any statistical significance. Finally, the asymmetrical funnel plot may suggest some degree of publication bias.
Conclusions
Our meta-analysis demonstrates that antibiotic prophylaxis with gentamicin containing regimen has significant risk for developing postoperative AKI in orthopedic surgery, and therefore should be avoided if possible especially in patients with pre-existing risks of AKI. The results were inconclusive in other types of surgery. Physicians should consider risks and benefits of using this regimen in individual patients. To confirm the outcome, prospective randomized control trial needs to be conducted in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35 Suppl 2:S66-88. [Crossref] [PubMed]
- Luo S, Lai Y, Liu C, et al. Prophylactic use of gentamicin/flucloxacillin versus cefuroxime in surgery: a meta analysis of clinical studies. International journal of clinical and experimental medicine 2015;8:17856-67. [PubMed]
- Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:238-42. [Crossref] [PubMed]
- Bailey O, Torkington MS, Anthony I, et al. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014;96B:395-8. [Crossref] [PubMed]
- Selby NM, Shaw S, Woodier N, et al. Gentamicin-associated acute kidney injury. QJM 2009;102:873-80. [Crossref] [PubMed]
- Product Monograph: Gentamicin(e). BAXTER CORPORATION.: prepared 1994 Mar 08; updated 2012 Aug 28.
- Schünemann H BJ, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available online https://gdt.gradepro.org/app/handbook/handbook.html#h.m9385o5z3li7
- Schneider M, Valentine S, Clarke GM, et al. Acute renal failure in cardiac surgical patients, potentiated by gentamicin and calcium. Anaesth Intensive Care 1996;24:647-50. [PubMed]
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013;28:612-9. [Crossref] [PubMed]
- Solgaard L, Tuxoe JI, Mafi M, et al. Nephrotoxicity by dicloxacillin and gentamicin in 163 patients with intertrochanteric hip fractures. Int Orthop 2000;24:155-7. [Crossref] [PubMed]
- Craig P, Starks I, Bancroft G, et al. Is prophylactic Gentamicin associated with acute kidney injury in patients undergoing surgery for fractured neck of femur? Injury 2012;43:2152-5. [Crossref] [PubMed]
- White RW, West R, Howard P, et al. Antimicrobial regime for cardiac surgery: the safety and effectiveness of short-course flucloxacillin (or teicoplanin) and gentamicin-based prophylaxis. J Card Surg 2013;28:512-6. [Crossref] [PubMed]
- Abboud CS, Bergamasco MD, Sousa EE, et al. Successful use of gentamycin as an antibiotic prophylaxis regimen to reduce the rate of healthcare-associated infections after renal transplantation. Braz J Infect Dis 2013;17:254-5. [Crossref] [PubMed]
- Ross AD, Boscainos PJ, Malhas A, et al. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013;58:209-12. [Crossref] [PubMed]
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. Journal of the American Society of Nephrology: JASN 2014;25:2625-32. [Crossref] [PubMed]
- Craxford S, Bayley E, Needoff M. Antibiotic-associated complications following lower limb arthroplasty: a comparison of two prophylactic regimes. Eur J Orthop Surg Traumatol 2014;24:539-43. [Crossref] [PubMed]
- Nielsen DV, Fedosova M, Hjortdal V, et al. Is single-dose prophylactic gentamicin associated with acute kidney injury in patients undergoing cardiac surgery? A matched-pair analysis. J Thorac Cardiovasc Surg 2014;148:1634-9. [Crossref] [PubMed]