
Extra-corporeal membrane oxygenation in paediatric acute respiratory distress syndrome: overrated or underutilized?
Introduction
Extracorporeal membrane oxygenation (ECMO) or as it is also known extracorporeal life support (ECLS) is a modified form of cardiopulmonary bypass, in which an artificial lung (membrane oxygenator) provides oxygenation and removal of carbon dioxide via an extracorporeal circuit (1).
ECMO is used to provide support for both failing heart and lungs and is recognised as a treatment option for severe paediatric acute respiratory distress syndrome (PARDS). ARDS is an acquired lung disease which is characterized by hypoxaemia and poor lung compliance (2). ARDS can lead to a failure of oxygenation and ventilation despite maximal conventional therapy including mechanical ventilation. Prolonged mechanical ventilation can lead to ventilator-induced lung injury (VILI) and the combination of pulmonary inflammation and aggressive ventilation may lead to multiorgan failure (MOF) (3).
Severe PARDS is defined as PARDS with severe hypoxaemia and characterized by an oxygen index of greater than 16 (4). Oxygenation index (OI) is a measure of both oxygenation and lung compliance and is calculated by the following formula:
OI = Fraction of inspired oxygen (FiO2) × Mean airway pressure (MAP)/Partial pressure arterial oxygen (PaO2)
Severe PARDS has a high mortality rate (20–40%) and high morbidity (3-6). ECMO may potentially provide rescue oxygenation and ventilation, preventing VILI, MOF and death. However, despite being used for respiratory failure in neonates, children and adults for many years, there has been little evidence (and no grade I evidence) to support the use of ECMO in PARDS (7).
Any potential survival benefit needs to be weighed against morbidity outcomes and hospital costs. Faraoni et al. found that median cost of ECMO in children was US $230,425 per patient. This cost was higher in survivors (US $519,450) and was more than double the cost per survivors of bone marrow, renal or liver transplantation (8).
This paper will introduce the concept of ECLS or ECMO in severe PARDS, describe its current use, review the evidence to support its use (neonatal, paediatric and adult) and describe some of the potential pitfalls and complications associated with ECMO in children. Finally current or future research in this area will be discussed (Figure 1).
Overview of ECMO in PARDS
ECMO was pioneered as a technique to support term infants with severe respiratory failure which was unresponsive to conventional management (9). The use of ECMO was then expanded to support older children and adults with cardiorespiratory failure. By pumping blood through an extracorporeal circuit containing a membrane oxygenator, ECMO can provide both oxygenation and carbon dioxide removal to replace the failing lung as well as providing mechanical cardiovascular support to replace or augment the failing circulatory system.
Particularly in severe PARDS, ECMO can allow ‘lung rest’ and prevent or reduce VILI
While the use of other supportive treatments such as inhaled nitric oxide and high frequency ventilation may have reduced the requirement for ECMO in neonates, the use of ECMO for paediatric and adult cardiorespiratory failure has increased exponentially (10).
Most of the information supporting the use of ECMO comes from the International Extracorporeal Life Support Organization (ELSO) Registry which has been collecting data on over 70,000 neonates, children and adults who have been supported on ECMO over the past 25 years.
Overall, 7,552 children (>30 days till <18 years) with respiratory failure have been supported on ECMO with an ECMO survival rate of 67% and a survival to hospital discharge of 58%. Survival to hospital discharge for children with respiratory failure is inferior to survival in neonatal respiratory failure (74%), which is predominantly due to the high survival rates in neonates with meconium aspiration syndrome (MAS). However, survival in children is similar to survival in adults with respiratory failure (58%). Survival to discharge for children with respiratory failure has been consistent over the past decade with a survival of 60% in the last published year [2015].
The number of paediatric respiratory ECMO runs has increased steadily over the past 12 years, from just under 200 reported cases to ELSO in 2003 to more than 500 cases in 2015. This increase likely reflects increased overall support amongst paediatric intensive care specialists for ECMO as a rescue therapy for children.
The most common aetiologies for respiratory failure requiring ECMO support are viral, bacterial and aspiration pneumonia and the survival to hospital discharge has been highest in those with viral (66%) and aspiration (69%) pneumonia.
While VA ECMO has been more commonly used in the total cohort, VV ECMO has been increasingly used for paediatric respiratory failure (60% of cases in 2015) and survival in VV ECMO is improved (64–69%) compared to VA ECMO (52%), although this may be related to severity of illness and aetiological factors.
Frequency of complications in paediatric ECMO remains a concern with significant neurological complications (infarction and haemorrhage) in more than 10% of cases (10).
Evidence for ECMO in ARDS
The first successful use of ECMO in newborns was published in 1976 (9). Two early studies using ‘play the winner’ and adaptive methodology (11,12), suggested ECMO may improve survival in severe PPHN before this was confirmed in the UK collaborative randomised trial of neonatal ECMO in 1996. In this landmark study, 185 neonates with respiratory failure were randomised to ECMO centre referral and consideration of ECMO versus conventional management. Referral to an ECMO centre resulted in a significant reduction in mortality (OR 0.55; 95% CI, 0.39–0.77). Further follow-up showed that neurological outcome was equivalent in survivors. Of the patients referred for ECMO, 84% were actually cannulated to ECMO. One of the major criticisms of this study was the possible benefit of medical care in an ECMO referral centre affecting outcome rather than the institution of ECMO itself (13).
The CESAR trial, published in 2009, demonstrated a survival benefit for adults with severe acute respiratory failure when randomised for consideration of ECMO versus conventional management. One hundred eighty adults with Murray lung injury score >3 or pH <7.2 were randomised. Patients referred for ECMO consideration had a significantly better survival without disability compared to conventional management (63% vs. 47%). Of those referred for ECMO, 75% were cannulated to ECMO (14).
While a randomised, controlled trial of ECMO in PARDS has not been completed successfully, there have been several attempts to demonstrate the benefits of ECMO in PARDS published in the literature.
In a multicentre, retrospective cohort analysis, Green et al. compared paediatric patients with acute respiratory failure who were supported with ECMO to those who were not. They used logistic regression analysis to identify factors associated with survival and then compared pairs of ECMO and non-ECMO patients who were matched by severity of disease and respiratory diagnosis.
The comparison of ECMO and non-ECMO matched pairs showed a reduction in mortality with ECMO (26.3%) compared to non-ECMO (49.1%) patients when matched for diagnosis and worst OI (P<0.01). The difference was most significant in the 50–75% mortality risk quartile (28.6% vs. 71.4%) (P<0.05) (15).
This however was not supported by another case-matched cohort study (16), using the RESTORE (Randomised Evaluation of Sedation Titration for Respiratory Failure) database as a source (17). Pairs were matched based on individual case matching (based on OI, age, days of ventilation and underlying aetiology) and propensity score matching using multivariate logistic regression to estimate the probability of receiving ECMO.
Of the 879 patients with severe ARDS, not supported by ECMO, 61 were matched to the 61 patients with severe ARDS who were managed with ECMO. Non-ECMO patients were excluded from this process if they had contraindications to ECMO, such as children post-BMT or with severe neurological injury.
The 90-day hospital mortality was identical between the matched pairs (25%) and similar between the propensity-matched pairs [25% vs. 30% (P=0.70)]. ECMO survivors had a longer PICU and hospital length of stay compared to non-ECMO survivors but there was no difference in Pediatric Overall Performance Category (POPC) or Pediatric Cerebral Performance Category (PCPC) outcomes between the groups.
These studies are difficult to interpret as they were both non-randomised, controlled trials and therefore only measured confounding factors (such as OI, aetiology) can be accounted for. The numbers of patients in both studies undergoing ECMO was small and patient care in an ECMO-centre, irrespective of whether the child was cannulated for ECMO could potentially be a confounding factor. While Green’s study did not include long-term follow-up, POPC and PCPC were equivalent between the groups in Barbaro’s study.
Given the supportive evidence in neonates (13) and adults (14) and the widespread use of ECMO for severe paediatric respiratory failure, it seems unlikely that a traditional randomised trial comparing the ECMO versus conventional therapy will be feasible or even considered ethical. In an era where ECMO technology and equipment is improving and ECMO complications are possibly diminishing it would appear that studying the precise role of ECMO (indications, trigger-points, contraindications) in severe respiratory failure would be the next step.
Complications
Despite improvements in equipment (pumps, oxygenators) complications from ECMO, especially central nervous system (CNS) complications, remain a significant concern.
ECMO complications or adverse events can be classified into:
- Mechanical (related to pump or oxygenator problems);
- Surgical site/cannula complications;
- Multi-organ complications.
Based on ELSO registry data, mechanical complications occur in up to 10–15% of children (oxygenator failure most common). Mechanical failure is more likely with increasing duration of ECMO but the frequency of mechanical failure appears to have reduced over the past 2 decades (18).
Surgical or cannula site haemorrhage occurs in up to 18% of children (19).
The most concerning multi-organ complications are neurological complications and infection. CNS complications are most likely to result or contribute to poor outcomes for children on ECMO and include CNS haemorrhage (6.4%), CNS infarction (4.2%). CNS complications in children on ECMO are less likely than CNS complications in neonates but more common than CNS complications in adults. It is also clear on analysis of ELSO data that the occurrence of CNS complications has a negative impact on survival and functional outcomes (10,19) (Figure 2).
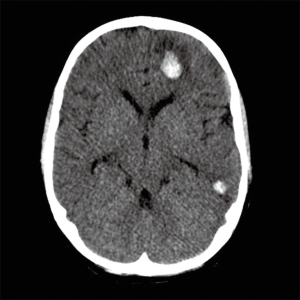
Infection is a common complication in paediatric ECMO. ELSO data analysis demonstrated an infection rate of 18% of total cases or 15.4 per 1,000 ECMO days. Infection rate was proportional to duration of ECMO and the presence of an infection significantly increased risk of mortality [OR 1.91 (95% CI, 1.75–2.08)]. Common organisms included coagulase negative Staphylococci (15.9%), Candida species (12.7%) and Pseudomonas aeruginosa (10.5%) (20).
A review of children undergoing ECMO for pneumonia showed at least one patient related complication in 82.7% of children, the most common being secondary bacteraemia (38.5%), pulmonary haemorrhage (5.8%), cerebral infarction (5.8%) and seizures (3.8%). Equipment related complications were seen in 75%, most commonly clots in the circuit (48%), oxygenator failure (11.5%), pump failure (9.6%) and cannula malposition (9.6%) (21).
Outcomes
Estimating the overall outcome of children with ARDS supported with ECMO is difficult. ELSO registry data gives an overall picture in children classified as respiratory ECMO and further analysis of the data gives a breakdown of aetiology and outcome. There are also several observational cohort studies previously referred to in this review. There are however no randomised trials in paediatric ARDS. The UK Neonatal ECMO Trial (13) demonstrated an overall survival of 68% in babies allocated to ECMO versus 41% in those managed without ECMO. The CESAR trial in adult patients (14) demonstrated that 63% of patients allocated to ECMO survived to 6 months without a disability compared to 47% in the conventional management group.
Green et al. (15) demonstrated a 73.6% survival in children with acute respiratory failure supported with ECMO. Secondary analysis of RESTORE data (17) demonstrated a survival rate of 75% in children with severe ARDS supported with ECMO. A small case series in children with refractory pneumonia supported with ECMO showed a survival to hospital discharge of 69% (21). ELSO registry data shows an overall ECMO survival of 67% but survival to hospital discharge of 58% in children with respiratory failure (10).
There are many factors which may influence ECMO survival in this group. Consideration for ECMO is dependant both on inclusion criteria as well as exclusion criteria due to comorbidities. An analysis of ELSO registry data (22) showed that the presence of comorbidities significantly reduced survival and that while overall survival for paediatric respiratory ECMO was unchanged over the period from 1993–2007, the survival rates in the subset of patients without comorbidities supported by ECMO increased from 57% to 72% over the study period. Published ELSO registry data suggests a gradual trend to increased overall survival rates over the previous 25 years (10). When broken down into aetiological categories, viral and aspiration pneumonia had slightly better outcomes than bacterial pneumonia and children cannulated for VV ECMO (64%) had better survival compared to VA ECMO (52%), although this is likely to influenced by other factors (severity of illness, multi-organ failure).
Other aetiological categories consistently show worse survival rates. Children supported with ECMO for ARDS associated with sepsis (40%), pertussis pneumonia (39%) and opportunistic infections (fungal 23%, pneumocyctis pneumonia 48%) all have poor survival rates (10,22,23).
Long-term neurological outcomes are difficult to estimate and there are no good data published on this in children. Long-term follow from the UK Neonatal ECMO trial (now up to 7 years published) shows consistent superiority in the ECMO group with equivalent frequency of neurological deficits between ECMO survivors and controls (24,25). A review of case series suggests that the rate of moderate-severe neurological deficits post-ECMO ranges between 3–13% for neonates (26-28) and 3–16% in children (29,30).
There is limited follow-up data regarding pulmonary morbidity but several case series demonstrate frequent subsequent respiratory admissions (29), pulmonary function abnormalities (31,32) and reduced exercise tolerance (33) although the clinical significance of these changes is unclear.
Practical aspects-timing, initiation, duration
Indications and contraindications for ECMO support have changed over the past decade. The original neonatal guidelines were based on severity of type 1 (OI) and type 2 (Ventilation index) respiratory failure and the estimated risk of mortality (34).
With the advent of inhaled nitric oxide, high frequency oscillation and other advances in both NICU and PICU, mortality has probably improved overall and this has led to a less rigid adherence to published guidelines. However mortality in severe PARDS with conventional support is still unacceptably high.
Multiple case series and reviews have demonstrated an association between severity of hypoxia (OI, PF ratio), poor lung compliance (OI) and mortality (3,5,6). A recent analysis of two large data sets (CHLA data and ANZICS data) by the PALICC group demonstrated that an OI >16 (severe PARDS) predicted a mortality rate of approximately 40% (35). This is equivalent to the mortality rate for paediatric respiratory ARDS requiring ECMO support (10,17), suggesting that an OI of >16 may be an appropriate cut-off to initiate ECMO support. As OI increases there appears to be a linear increase in mortality, demonstrating a mortality range of 43–56% at an OI of >20 (35). However it is recommended that the decision to support ECMO is based on overall evaluation of all clinical data with an emphasis on the trajectory of disease severity (Table 1).
Exclusion criteria, although seemingly self-explanatory, have changed over the past decade. ECMO is being offered progressively more complex patients and the number of absolute contraindications to ECMO continues to shrink (22). However, ECMO should not be offered if it is likely to be futile. Contemporary contraindications can be categorized as absolute and relative.
An analysis of ELSO data from 1993–2007 by Zabrocki et al. showed a significant increase in the number of children with co-morbidities who underwent ECMO support over a span of 10 years (47% vs. 19%). This is despite a clear relationship between the presence of comorbidities and increasing mortality. Survival with ECMO support was significantly reduced with prominent comorbidities such as renal failure (survival 33%), oncological disease (30%), immunodeficiency (34%) and stem cell transplantation (5%). The presence of chronic lung disease or congenital heart disease however did not impact significantly on survival (22). The poor outcomes in children with immunodeficiency or oncological disease described are consistent with other published data (38-40).
Physician’s opinions on exclusion criteria remain varied however. A survey looking at attitudes towards children with co-morbidities revealed some interesting outcomes. While predictably, support with ECMO was low for a child with Trisomy 18 (31%), a majority of respondents would offer ECMO to a child with cerebral palsy and severe developmental delay (76%), a child with cardiac arrest with uncertain neurological status (76%) and newly diagnosed patient with acute leukemia and septic shock (67%). Support for ECMO cannulation was also dependant on number of circuits available and geographical region (41).
These papers reiterate the need to consider the long term impact of comorbidities on survival and quality of life outcomes. Although there are few absolute contraindications to ECMO support, chronic organ disease may significantly impact on long-term outcome. Acute neurological injury also has a significant impact on both ECMO survival and long-term quality of life. The risk of secondary neurologic injury (haemorrhage and infarction) on ECMO support also needs to be considered in those with acute neurology.
Prolonged ventilation prior to ECMO support has also been a consistent contraindication to ECMO and the negative impact of prolonged ventilation (>14 days) prior to ECMO cannulation is supported by available data (7,15,22,23,42) (Table 2).
Veno-venous versus veno-arterial ECMO
ELSO data and published case series demonstrate increasing preference for veno-venous ECMO for paediatric respiratory disease. There is also some evidence from case series and ELSO data analysis that survival may be improved with VV ECMO compared to VA ECMO (10,21,22). Although without randomised trials, case-matched controls or even logistic regression analysis, confounding factors, in particular disease severity and multi-organ failure, may explain this observation. In Zabrocki’s analysis of ELSO data, survival for children commenced on VV ECMO was 66–70% compared to 51% with VA ECMO. VV ECMO was an independent predictor of survival after logistic regression analysis. In the small proportion of children that required conversion from VV to VA ECMO (6%), survival was 49% (22). Two reports describing institutional experiences with VV ECMO for neonatal (43) and paediatric (44) respiratory failure showed VV ECMO was successful despite high pre-ECMO vasopressor requirements.
There are several potential advantages to VV ECMO including a reduced risk of CNS complications (45), less complicated weaning and possible lower overall mortality (10,21). However the presence of cardiovascular compromise in paediatric ARDS may preclude the option of VV ECMO.
Duration of ECMO
Duration of ECMO is primarily dependant on lung recovery. Factors influencing lung recovery include aetiology of lung disease, duration of mechanical ventilation prior to ECMO (22), degree of lung injury secondary to ventilation (VILI) and presence of secondary pulmonary complications (pulmonary infection, pulmonary haemorrhage). Other factors influencing duration of ECMO requirement include fluid management, sedation strategies, non-pulmonary complications (brain injury, critical illness neuromyopathy) and co-morbidities (10).
While attempts should be made to wean ECMO as soon as lung recovery has occurred, prolonged ECMO can be successful. An analysis of ELSO registry data shows that survival in children on ECMO support for >7 weeks is not significantly different from those supported for less than 2 weeks (46). However evidence on this is conflicting with a large case series of children with respiratory failure showing a significantly better survival (61%) in those supported on ECMO for less than 14 days compared to those requiring more than 21 days (38%) (23,47).
Future research
A randomised controlled trial in children with ARDS comparing outcomes with ECMO versus conventional management only is unlikely to occur, despite the fact that similar trials have been conducted in neonates and adults (13,14). In fact, the positive outcomes from these trials have in all likelihood, convinced the majority of PICU practitioners that ECMO should be offered in the absence of contra-indications. Future research is likely to be directed at trigger points for initiating ECMO-at what severity of PARDS do the potential benefits of ECMO outweigh the risks. As discussed in this review, propensity scoring and comparative outcome studies have attempted to address this precise issue.
Conclusions
Despite the use of ECMO in an increasingly complex cohort of children, survival to discharge has remained consistent (57%) and while morbidity is a concern, it appears at least comparable to morbidity in similar children managed with conventional therapy.
Well-defined criteria and practice parameters for children with ARDS requiring ECMO support are not yet published. However available data support the use of ECMO as rescue therapy for children with severe ARDS and a high risk of mortality and suggest that despite an increase in use over the past decade that ECMO is still currently under-utilized in children with severe ARDS.
While mortality rates in severe ARDS are similar both with and without the use of ECMO, ECMO support potentially has other benefits including reducing VILI and multi-organ failure associated with prolonged mechanical ventilation. However mortality and morbidity of children with severe ARDS requiring ECMO support remain high and this needs to be taken into account, along with the substantial hospital costs and the burden on hospital resources, when a child is considered for ECMO support. Patient selection is crucial as there is consistent evidence that aetiology of lung disease, the presence of comorbidities, presence of multi-organ failure and duration of mechanical ventilation prior to institution of ECMO all strongly influence outcome.
Acknowledgments
Dr. Graeme MacLaren (Cardiothoracic Intensive Care Unit, National Health System, Singapore) for permission to use adapted tables. Dr. Anusha Ganeshalingham (Starship Children’s Hospital, Auckland, New Zealand) for assistance with manuscript review.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- O'Rourke PP, Crone RK. Pediatric applications of extracorporeal membrane oxygenation. J Pediatr 1990;116:393-4. [Crossref] [PubMed]
- Bernhard G, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trials coordination. Am J Respi Crit Care Med 1994;149:818-24. [Crossref]
- Erickson S. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicentre, observational study. Pediatr Crit Care Med 2007;8:317-23. [PubMed]
- Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16:S23-40. [Crossref] [PubMed]
- López-Fernández Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012;40:3238-45. [Crossref] [PubMed]
- Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171:995-1001. [Crossref] [PubMed]
- Moler FW, Palmisano J, Custer JR, et al. Extracorporeal life support for pediatric respiratory failure: Predictors of survival from 220 patients. Crit Care Med 1993;21:1604-11. [Crossref] [PubMed]
- Faraoni D, Nasr VG, DiNardo JA, et al. Hospital Costs for Neonates and ChildrenSupported with Extracorporeal Membrane Oxygenation. J Pediatr 2016;169:69-75.e1. [Crossref] [PubMed]
- Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Trans Am Soc Artif Intern Organs. 1976;22:80-93. [PubMed]
- Thiagarajan RR. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics 1985;76:479-87. [PubMed]
- O’Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics 1989;84:957-63. [PubMed]
- UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trial Group. Lancet 1996;348:75-82. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Green TP, Timmons OD, Fackler JC, et al. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Crit Care Med 1996;24:323-9. [Crossref] [PubMed]
- Barbaro RP. Does Extracorporeal Membrane Oxygenation Improve Survival in Pediatric Acute Respiratory Failure. Am J Respir Crit Care Med 2018;197:1177-86. [Crossref] [PubMed]
- Curley MA, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomised clinical trial. JAMA 2015;313:379-89. [Crossref] [PubMed]
- Fleming GM, Gurney JG, Donohue JE, et al. Mechanical component failures in 28,171 neonatal and pediatric extracorporeal membrane oxygenation courses from 1987 to 2006. Pediatr Crit Care Med 2009;10:439-44. [Crossref] [PubMed]
- Extracorporeal Life Support Organization: ECLS Registry Report. Ann Arbor, MI, Extracorporeal Life Support Organization, 2012.
- Bizzarro MJ, Conrad SA, Kaufman DA, et al. Extracorporeal Life Support Task Force on Infections, Extracorporeal Membrane Oxygenation: Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med 2011;12:277-81. [Crossref] [PubMed]
- Smalley N, MacLaren G, Best D, et al. Outcomes in children with refractory pneumonia supported with extracorporeal membrane oxygenation. Intensive Care Med 2012;38:1001-7. [Crossref] [PubMed]
- Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med 2011;39:364-70. [Crossref] [PubMed]
- Nehra D, Goldstein AM, Doody DP, et al. Extracorporeal Membrane Oxygenation for Nonneonatal Acute Respiratory Failure. The Massachusetts General Hospital Experience From 1990 to 2008. Arch Surg 2009;144:427-32. [Crossref] [PubMed]
- Bennett CC, Johnson A, Field DJ, et al. UK Collaborative ECMO Trial Group. A comparison of clinical variables that predict adverse outcome in term infants with severe respiratory failure randomised to a policy of extracorporeal membrane oxygenation or to conventional neonatal intensive care. J Perinat Med 2002;30:225-30. [Crossref] [PubMed]
- McNally H, Bennett CC, Elbourne D, et al. UK Collaborative ECMO Trial Group. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 2006;117:e845-54. [Crossref] [PubMed]
- Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: A nationwide evaluation at 5 years of age. Crit Care 2006;10:R127. [Crossref] [PubMed]
- Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: A population-based study. Crit Care 2009;13:R47. [Crossref] [PubMed]
- Khambekar K, Nichani S, Luyt DK, et al. Developmenta outcome in newborn infants treated for acute respiratory failure with extracorporeal membrane oxygenation: Present experience. Arch Dis Child Fetal Neonatal Ed 2006;91:F21-5. [Crossref] [PubMed]
- Jen HC, Shew SB. Hospital readmissions and survival after nonneonatal pediatric ECMO. Pediatrics 2010;125:1217-23. [Crossref] [PubMed]
- Taylor AK, Cousins R, Butt WW, et al. The long-term outcome of children managed with extracorporeal life support: An institutional experience. Crit Care Resusc 2007;9:172-7. [PubMed]
- Boykin AR, Quivers ES, Wagenhoffer KL, et al. Cardiopulmonary outcome of neonatal extracorporeal membrane oxygenation at ages 10-15 years. Crit Care Med 2003;31:2380-4. [Crossref] [PubMed]
- Hamutcu R, Nield TA, Garg M, et al. Long-term pulmonary sequelae in children who were treated with extracorporeal membrane oxygenation for neonatal respiratory failure. Pediatrics 2004;114:1292-6. [Crossref] [PubMed]
- van der Cammen-van Zijp MH, Gischler SJ, Hop WC, et al. Deterioration in exercise capacity after neonatal extracorporeal membrane oxygenation. Eur Respir J 2011;38:1098-104. [Crossref] [PubMed]
- Rivera RA, Butt W, Shann F. Predictors of mortality in children with respiratory failure: possible indications for ECMO. Anaesth Intensive Care 1990;18:385-9. [Crossref] [PubMed]
- Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence and Epidemiology: Proceedings from the Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16:S23-40. [Crossref] [PubMed]
- MacLaren G, Conrad S, Dalton H. Extracorporeal life support. In: Shaffner H, Nichols D. editors. Roger’s Textbook of Pediatric Intensive Care. Fifth edition. Philadelphia: Lippincott Williams & Wilkins, 2015.
- MacLaren G, Conrad S, Peel G. Indications for Pediatric Respiratory Extracorporeal Life Support. Extracorporeal Life Support Organization (ELSO) Guidelines 2015.
- Gupta M, Shanley TP, Moler FW. Extracorporeal life support for severe respiratory failure in children with immune compromised conditions. Pediatr Crit Care Med 2008;9:380-5. [Crossref] [PubMed]
- Gow KW, Heiss KF, Wulkan ML, et al. Extracorporeal life support for children with malignancy and respiratory or cardiac failure: The Extracorporeal Life Support Organization experience. Crit Care Med 2009;37:1308-16. [Crossref] [PubMed]
- Gow KW, Wulkan ML, Heiss KF, et al. Extracorporeal membrane oxygenation for support of children after hematopoietic stem cell transplantation: The Extracorporeal Life Support Organization experience. J Pediatr Surg 2006;41:662-7. [Crossref] [PubMed]
- Kuo KW, Barbaro RP, Gadepalli SK, et al. Should Extracorporeal Membrane Oxygenation Be Offered? An International Survey. J Pediatr 2017;182:107-13. [Crossref] [PubMed]
- Nance ML, Nadkarni VM, Hedrisk HL, et al. Effect of preextracorporeal membrane oxygenation ventilation days and age on extracorporeal membrane oxygenation survival in critically ill children. J Pediatr Surg 2009;44:1606-10. [Crossref] [PubMed]
- Roberts N, Westrope C, Pooboni SK, et al. Venovenous extracorporeal membrane oxygenation for respiratory in inotrope dependant neonates. ASAIO J 2003;49:568-71. [Crossref] [PubMed]
- Pettignano R, Fortenberry JD, Heard ML, et al. Primary use of the veno-venous approach for extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Crit Care Med 2003;4:291-8. [Crossref] [PubMed]
- Rollins MD, Hubbard A, Zabrocki L, et al. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg 2012;47:68-75. [Crossref] [PubMed]
- Dalton HJ, Macrae DJ. Extracorporeal support in children with Pediatric Acute Respiratory Distress Syndrome: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16:S111-7. [Crossref] [PubMed]
- Brogan TV, Zabrocki L, Thiagarajan RR, et al. Prolonged extracorporeal membrane oxygenation for children with respiratory failure. Pediatr Crit Care Med 2012;13:e249-54. [Crossref] [PubMed]




