How to prevent complications in breast augmentation
Introduction
Breast augmentation is the most commonly performed surgical procedure in aesthetic plastic surgery (1,2).
Evidences in aesthetic breast augmentation only derive from few randomized controlled trials comparing different types of implants and different techniques (3,4).
No high level of evidence conclusions about the best technique or the best implant to use for obtaining the best outcomes in aesthetic breast augmentation, with low complications and re-interventions rates exist from available literature.
The actual best evidence about silicone gel-filled breast implants derives from the US Food and Drug Administration (FDA) core-studies. We actually have the 10-year follow-up results about Natrelle 410 anatomical form-stable silicone-filled breast implants (Allergan Inc., Irvine, California) use in aesthetic and reconstructive breast surgery (5). The Allergan core-study investigated the safety and effectiveness of Natrelle 410 breast implants reporting complications and re-interventions rates, reporting the cumulative risk of a subject experiencing an adverse event at any time during the 10 years.
Capsular contracture rates (Baker scale grades III and IV) at 10-year follow-up were 9.2% for augmentation and 14.5% for reconstruction. The confirmed rupture rate was 9.4% without any report of extracapsular silicone gel migration. Other major complications (>5%) were implant malposition (4.7% for augmentation) and asymmetry (6.9%). The seroma rate was 1.6% for augmentation subjects, 0.6% occurring more than 1 year after implantation (late seroma). A single case of breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) was reported.
The 410 Allergan core-study concludes the most commonly reported complication in breast implant surgery is capsular contracture, the risk of this complication increasing over time, even though capsular contracture rates being lower than those observed in the Natrelle round gel (fourth generation) core study, mostly including smooth implants (56.2%) (6).
Similarly the 6-year data about the form-stable Mentor Contour Profile Gel (CPG) implants (Mentor Worldwide LLC, Santa Barbara, California) showed lower contracture rates for the CPG implants when compared with predominantly smooth-surface round gel breast implants (7,8).
The 10-year data also show a very low rate of implant rippling or wrinkling (0.9% for augmentation, 6.2% for reconstruction).
In this paper we would like to present the actual evidences about the etiopathogenesis of main complications in aesthetic breast augmentation, trying to identify some basic rules to follow in order to reduce complication rates in our daily activity, minimizing re-interventions, obtaining long lasting results and high women’s satisfaction levels with their surgery.
Complications in breast augmentation
Potential surgical complications in breast implant surgery could be classified in pre- and intra-operative complications and early and late post-operative complications.
Pre-operative and intra-operative complications derive from poor planning (wrong choice of the surgical access, incorrect measurement) or poor surgical technique (over-dissection of the implant pocket, implant malpositioning, excessive bleeding).
Early post-operative complications are haematoma, seroma, infection, implant malposition and pain. Late post-operative complications are infection, seroma, capsular contracture, poor muscular animations (excessive, unusual, painful) or distortions, implant visibility, implant malposition (descent, double bubble, waterfall deformity, etc.), implant rippling, wrinkling and palpability, implant rupture, symmastia, poor scar healing or scar hypertrophy.
The role of bacterial biofilm in implant-associated infection, capsular contracture, late seromas and BIA-ALCL
Breast implants are placed in a potentially contaminated pocket, bacteria being present in breast ducts and glandular parenchyma (9,10).
Several in vitro studies demonstrated how bacteria could bind to breast implants’ surface despite the type of surface (11).
These bacteria could form a biofilm, that is a combination of glycoprotein and latent bacteria binding to the breast implant silicone envelope. When forming a biofilm, bacteria are resistant to antibiotics (12).
When overcoming the local host defenses, the biofilm will continue proliferating leading to local inflammation and fibrosis, causing capsular contracture (13).
An experimental model in pigs was presented by Hu and colleagues in 2015 (14), showing that capsular contracture Baker grade is directly linked to the number of bacteria for increasing and a threshold of bacterial biofilm exists above which host responses lead to capsular contracture, due to an inflammatory response leading to fibrosis.
A great T-cell response to the presence of bacteria has been described by Hu and colleagues, particularly in textured implants when compared with smooth implants, texturization representing a more ideal surface for biofilm formation. However the infectious hypothesis does not mean that textured implant will be necessarily associated with higher contracture rates, remaining determinant the threshold of infection above which local inflammation is initiated.
Chronic biofilm infection of breast implants and the predominant T-cell lymphocytic infiltrate could acquire a particular importance in the etiopathogenesis of late seromas and breast-implant associated Anaplastic Large Cell Lymphoma (BIA-ALCL) as well.
Chronic bacterial infection has been shown to be associated with the development of lymphomas (15) and similarly chronically infected breast implants could be extremely rarely linked with inflammatory processes leading to T-cell lymphoma development. Obviously this will be a multistep process with fundamental impact of patient genotypes and immunomediated factors contributing to BIA-ALCL development.
Double capsules
Many authors reported about double capsule formation around textured breast implants (16).
Double capsule could be defined as two distinct capsular layers around a breast implant with an intercapsular space: the inner layer adheres to the implant envelope and the outer one to the breast tissue. Between the two capsular layer could has been described the presence of seroma-like fluid. Double capsules could be partial or complete. When complete, double capsules could be linked to rotation of the implant due to the interface between the inner and the outer layers. In these cases the tissue in-growth into the textured surface could not prevent rotation, textured implants acting as smooth ones, due to the intercapsular space, where synovial metaplasia has been described.
The etiopathogenesis of double capsules is controversial with four main hypothesis. The first theory is based on movement of the implant within an oversized pocket, where adhesion of the implant with the surrounding tissues is precluded (17).
The second hypothesis propose a mechanical etiology: the detachment of the implant from the capsule would be determined by shear stresses applied to the implant-capsule complex, leading to the creation of a new inner layer of capsule over the implant, from seeding of cells coming from the seroma-like fluid accumulating between the implant and the original capsule (16).
The third hypothesis is based on seroma formation around the implant (from an infectious, allergic or hemorrhagic origin), subsequently leading to the development of a new inner capsule (18).
The fourth hypothesis also propose a mechanical etiology with shear forces causing detachment of the implant-capsule complex from the surrounding breast tissue, with a new capsular layer developing outside the original capsule (19,20).
A recent study by Giot and colleagues (21) observed that bacterial load and biofilm presence within the intercapsular space was lower or absent while bacteria could always be seen in the prosthesis interface, so the two spaces do not share the same initial fluid, as necessarily would be in the case of the first three hypotheses.
Moreover the histological findings reported in the same studies confirmed a layered appearance of the inner capsule and delamination at the more solicited locations of the capsule (outer breast quadrants), supporting the fourth hypothesis.
Late seromas
The term “seroma” is generic and describes collections of clear serous fluid developing in dissected spaces following surgery. The fluid could be defined as a serous effusion if on examination it appears acellular with small quantities of proteins (<2.0 g/dL), as an exudate if it contains cells and proteins (> 2.9 g/dL) and as an hematoma, if it predominantly contains red blood cells. Moreover it as an inflammatory effusion if the cells are predominantly white blood cells and a malignant effusion if it contains cancer cells.
Late seroma is defined as a periprosthetic fluid collection occurring more than 1 year following breast augmentation.
Few data exists about the epidemiology and etiology of this clinical entity in patients with breast implants.
Bengston and colleagues presented a literature review and a consensus panel recommendation about late periprosthetic seromas in patients with breast implants (22).
The exact epidemiology of late periprosthetic fluid collections in patients with breast implants is not defined with reported incidences ranging from 0.88 to 1.84% (23,24).
The cause and pathophysiology of late periprosthetic fluid collections could be linked with the infectious theory or deriving from mechanical shear forces or sliding surfaces generated by micromovements between the implant and the surrounding tissue, as for double capsule formation (25).
Breast Implant Associated Anaplastic Large Cell Lymphoma
Late periprosthetic fluid collections in patients with breast implants have also been reported in association with Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
This is why a correct diagnostic pathway should always be followed when dealing with late seromas. Late seroma does not represent a direct precursor of BIA-ALCL, but all late seromas should be thoroughly investigated with cytological examination through fine needle aspiration, flow cytometry and CD30 IHC of effusion.
Two-thirds of BIA-ALCL patients present as a malignant effusion associated with the fibrous capsule surrounding an implant occurring on average 8 to 10 years after implantation.
Therefore any seroma occurring greater than 1 year after implantation not readily explainable by infection or trauma should be considered suspicious for disease. One third of patients present with a mass which may indicate a more aggressive clinical course (26).
Any aspiration of peri-prosthetic fluid should be sent to pathology for cytologic evaluation and include a clinical history with the aim to “rule out BIA-ALCL”. Diagnosis by hematoxylin and eosin staining alone is nearly impossible: BIA-ALCL will demonstrate strong and uniform membranous expression of CD30 immunohistochemistry (27).
Ultrasound examination may help defining the extent of an effusion and identifying associated capsule masses. Clinical examination should include evaluation of regional lymph nodes. BIA-ALCL effusions are typically more viscous than a benign seroma due to the higher protein content and cellularity. The surrounding capsule may be thickened and fibrous or may be completely normal in appearance.
If a mass is present, it can protrude into the implant creating a mass effect distortion on imaging or the mass may protrude outward into the soft tissue (28).
Patients with biopsy-proven BI-ALCL must be referred to a lymphoma oncologist ideally prior to any surgical intervention to allow for proper oncologic evaluation. Surgical treatment of BI-ALCL includes removal of the implant, complete removal of any disease mass with negative margins and total capsulectomy. Because an implant capsule may drain to multiple regional lymph node basins, there does not appear to be a role for sentinel lymph node biopsy in the treatment of BI-ALCL. Excisional biopsies of any suspicious lymph nodes should be performed (29).
BIA-ALCL is distinct from primary breast lymphoma, that is a disease of the breast parenchyma and is predominantly a B-cell lymphoma (65–90%) (30,31). BIA-ALCL is a T-cell lymphoma arising either in an effusion surrounding the implant or in the scar capsule surrounding a breast implant, is ALK negative and express the CD30 cell surface protein (32).
Most cases are diagnosed during implant revision surgery performed for a late onset (> 1 year), persistent seroma and may be associated with symptoms of pain, breast lumps, swelling or breast asymmetry.
BIA-ALCL most commonly follows an indolent course when adequate surgical removal of the implant and surrounding capsule is performed, without any systemic therapy, but aggressive cases experiencing disease progression and death have been reported.
Implant rupture
Rupture is a long-recognized complication of all breast implants. Breast implants are not lifetime devices. MRI screenings are recommended 3 years after initial implant surgery and then every two years after to detect silent rupture. Among primary causes of implant rupture in the first 5 years after implantation, instrument damage by surgeon seems to be the is the principal cause (33).
A variety of methods have been used both to detect rupture and to estimate its incidence. Most ruptures are silent and detectable only by imaging techniques such as Magnetic Resonance Imaging (MRI), the current gold standard, or high-resolution ultrasound (34). Clinical examination detects only about 30% of the rupture found at MRI (35). As a result, any report of rupture incidence that is not based on screening of all patients with an imaging modality such as MRI or ultrasound will significantly underestimate the actual rupture rate. For this reason, rupture incidence should not be derived from combined populations of MRI-screened and non-MRI-screened patients. Although product complaint reporting has also been used for assessing the incidence of rupture, it is widely recognized that for nearly all device complications, such reports represent only a fraction of the actual complications occurring in patients. Incidence rates of rupture increase over time following implantation, so the follow-up is particularly important to be considered in any reported estimates of rupture rates. Rupture rates are very low in the first few years after implantation. A MRI-based study from Denmark reported a rupture-free survival of 98% at 5 years and 83% to 85% at 10 years (36).
When estimating rupture rates from prospective Core Clinical Study data, the most appropriate and rigorous method is to use follow-up data only through the patients’ last MRI exam, rather than through their last office visit, as most ruptures are detected via MRI as discussed earlier. This method of rupture calculation, however, is not yet standard, and until such methods are standardized, direct comparisons of rupture rates among studies are not reliable or meaningful.
Breast implant durability is a highly debated issue between surgeons, patients and regulators as FDA. To determine the useful life of a breast implant, it is necessary to determine the primary cause of failure (Figures 1-3); instrument damage seems to be an important “failure” factor (33).
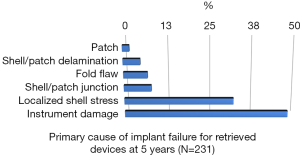
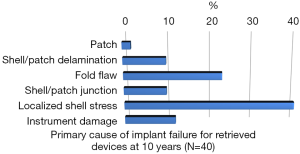
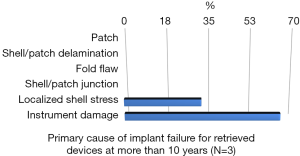
This instrument implant damage can be initiated at different times:
- During or before insertion;
- During implant removal (i.e. explantation);
- During medical maneuvers (punctions, biopsies, revisions);
- In situ while in use by the patient.
How to prevent complications in breast augmentation
Basic rules to minimize contamination in aesthetic breast augmentation
All the reported evidences about the role of bacterial biofilm in implant-associated infection, capsular contracture, late seromas and BIA-ALCL stress the importance of applying accurate pre-operative and intra-operative strategies to reduce the risk of breast implants’ bacterial contamination and biofilm formation.
Our experience leads to several recommendations in order to minimize contamination and reducing bacterial access during breast augmentation surgery, in accordance with the strategies for prevention of device-associated infection in breast prostheses proposed by Deva and colleagues (38).
Minimizing implant contamination starts pre-operatively advising patients to perform an accurate skin cleaning, taking a shower with an antibacterial foam gel before undergoing surgery.
Some surgical advices start with the antibiotic prophylaxis at anesthetic induction. Peri-areolar incisions should be generally not preferred in order to avoid breast implant contamination through the contact with bacteria present within breast ducts and tissues. We also suggest to avoid parenchymal dissections preferring subfascial, dual-plane techniques. An accurate surgery should be pursued, reducing bleedings and tissues devascularization, through careful atraumatic dissections with proper surgical tools. We advise to perform implant pocket irrigation with triple antibiotic solution as suggested by Adams and colleagues (39-41) or 500 cc of saline solution with one vial of amikacine (for each breast). We also advice to fit the implant pocket with saline solution wet gauzes for five minutes to remove any residual dust inside the pocket, then washing the skin with antibacterial solution and remove the gauzes. Other useful tricks when placing a breast implant could be the use of nipple shields (sterydrap) to prevent contamination through bacteria coming from the ducts, the possible use of introduction sleeves and changing all surgical instruments, drapes and gloves before opening the implant. Handling of the implant should be minimized as well as repositioning of the implant within the pocket. We suggest to seat the patient and gently move the implant inside the pocket with an oblique insertion, avoiding the use of sizers.
We also suggest to close the skin incision immediately after the implant positioning with three to five stitches between the muscle (inferiorly) and the Superficial Fascia System (SFS) superiorly, knotting them at the end in order to avoid any implant damage. Then we suggest to protect the sutured skin with a tape, left in place as long as possible and to use sterile ice around the breast to avoid even minimal bleeding and seroma formation.
Moreover, we suggest not to use any suction or drainage and to avoid implant external compression: compression means inflammatory reaction and a post-operative bra is enough. The post-operative bra must be worn day and night for two months, thus avoiding sliding of implants that can reduce or prevent tissue adherence and tissue ingrowth, meaning more local inflammation.
Careful surgical technique to reduce complications in aesthetic breast augmentation
Haematoma and seroma rates could be reduced performing a proactive haemostasis, using the electrocautery for sharp dissections, leaving the connective tissue on the ribs, through a tailored pocket dissection, irrigating the pocket with topic antibiotics, using the proper tools and applying the “no touch” technique.
Tips to minimize iatrogenic rupture:
- Not allowing sharp instruments such as scalpels or needles to contact the device;
- Ensuring that excessive force is not applied to a small area of the shell when inserting the device;
- Making incision of reasonable length to accommodate the selected style, size and profile of the implant;
- Avoiding creation of wrinkles or folds in the device during implantation.
How to minimize poor animations
Poor animation is surprisingly common after breast augmentation. If the distal/medial origin of the pectoralis muscle is not properly divided when creating the implant pocket, unnatural movements of the implant may occur during muscle activity. This complication could be minimized thanks to selective fiber release, with a pectoralis major division 2–3 cm above the inframammary fold line and 2–3 cm along the sternum.
Conclusions
Every choice in breast implant-based surgery presents trade-offs: risks can be reduced by making the best surgical and implant choices, based on good team decisions. Good team decisions start with well-informed patients and technically-prepared surgeons.
We firmly believe that the best outcomes in breast augmentation could only be achieved through a careful and standardized pre-operative planning of the surgery, a complete knowledge of the available devices, the application of an impeccable surgical technique and a scheduled follow-up.
The breast augmentation decisional process remains a complex choice: only pursuing a standardized decisional process, performing an accurate surgery aiming to reduce trade-offs and minimizing contaminations (that does not necessarily mean longer operative times), with a tight-knit and “oiled” surgical team, we could aspire to obtain the best, tailored and long-lasting results with low complication rates.
We firmly believe a scientific and rigorous approach towards breast augmentation to be mandatory in order to obtain good outcomes, long-lasting results, low complication and re-intervention rates and high women’s satisfaction levels.
Even following the best pathway in planning and performing a breast augmentation, short- and long- term post-operative complications can occur, including infection, capsular contracture, haematoma and seroma, double capsules, late seromas, rupture of the implant and implant malpositioning.
Our aim as “evidence-based surgeons” should be trying to diligently use all the available evidences in order to reduce our complication rates, trying to pursue a “flawless” surgery, achieving good outcomes and reducing short- and long-term complication rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Society of Plastic Surgery, 2015 Cosmetic Plastic Surgery Statistics. Available online at https://www.plasticsurgery.org/news/plastic-surgery-statistics. Accessed March 2017.
- International Society of Aesthetic Plastic Surgery. 2015 Cosmetic Plastic Surgery Italian Statistics. Available online: http://www.isaps.org/Media/Default/global-statistics/2016%20ISAPS%20Results.pdf. Accessed March 2017.
- Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006;118:1224-36. [Crossref] [PubMed]
- Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg 2006;117:2182-90. [Crossref] [PubMed]
- Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J 2015;35:145-55. [Crossref] [PubMed]
- Spear SL, Murphy DK, Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133:1354-61. [Crossref] [PubMed]
- Hammond DC, Migliori MM, Caplin DA, et al. Mentor Contour Profile Gel implants: clinical outcomes at 6 years. Plast Reconstr Surg 2012;129:1381-91. [Crossref] [PubMed]
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [Crossref] [PubMed]
- Bartsich S, Ascherman JA, Whittier S, et al. The breast: A clean-contaminated surgical site. Aesthet Surg J 2011;31:802-6. [Crossref] [PubMed]
- Thornton JW, Argenta LC, McClatchey KD, et al. Studies on the endogenous flora of the human breast. Ann Plast Surg 1988;20:39-42. [Crossref] [PubMed]
- Jennings DA, Morykwas MJ, Burns WW, et al. In vitro adhesion of endogenous skin microorganisms to breast prostheses. Ann Plast Surg 1991;27:216-20. [Crossref] [PubMed]
- Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J 2000;46:S47-S52. [Crossref] [PubMed]
- Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device¬associated infection. Plast Reconstr Surg 2013;132:1319-28. [Crossref] [PubMed]
- Hu H, Jacombs A, Vickery K, et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg 2015;135:319-29. [Crossref] [PubMed]
- Szczepanik M. Interplay between Helicobacter pylori and the immune system: Clinical implications. J Physiol Pharmacol 2006;57:15-27. [PubMed]
- Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg 2011;127:56-66. [Crossref] [PubMed]
- Goes JC, Landecker A. Optimizing outcomes in breast augmentation: seven years of experience with the subfascial plane. Aesthetic Plast Surg 2003;27:178-84. [Crossref] [PubMed]
- Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg 2011;35:303-14. [Crossref] [PubMed]
- Pandya AN, Dickson MG. Capsule within a capsule: an unusual entity. Br J Plast Surg 2002;55:455-6. [Crossref] [PubMed]
- Robinson HN. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg 2011;128:818; author reply 818-9. [Crossref] [PubMed]
- Giot JP, Paek LS, Nizard N, et al. The double capsules in macro-textured breast impants. Biomaterials 2015;67:65-72. [Crossref] [PubMed]
- Bengtson B, Brody GS, Brown MH, et al. Managing Late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg 2011;128:1-7. [Crossref] [PubMed]
- Mazzocchi M, Dessy LA, Carlesimo B, et al. Late seroma formation after breast surgery with textured silicone implants: A problem worth bearing in mind. Plast Reconstr Surg 2010;125:176e-177e. [Crossref] [PubMed]
- Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg 2011;35:303-14. [Crossref] [PubMed]
- Hasham S, Akhtar S, Fourie LR. Persistent seroma following breast prosthesis explantation: A case report and review. Eur J Plast Surg 2006;28:490-3. [Crossref]
- Clemens MW, Miranda RN. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma After 18 Years of Investigation. Clin Plast Surg 2015;42:605-13. [Crossref] [PubMed]
- Hart AM, Lechowicz MJ, Peters KK, et al. Breast Implant-Associated Anaplastic Large Cell Lymphoma: Report of 2 Cases and Review of the Literature. Aesthet Surg J 2014;34:884-94. [Crossref] [PubMed]
- Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015;135:695-705. [Crossref] [PubMed]
- Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8. Erratum in: J Clin Oncol. 2016 Mar 10;34(8):888. DiNapoli, Arianna [corrected to Di Napoli, Arianna]. [Crossref] [PubMed]
- Cao YB, Wang SS, Huang HQ, et al. Primary breast lymphoma--a report of 27 cases with literature review. Ai Zheng 2007;26:84-9. [PubMed]
- Gholam D, Bibeau F, El Weshi A. Primary breast lymphoma. Leuk Lymphoma 2003;44:1173-8. [Crossref] [PubMed]
- Kim B, Roth C, Chung KC, et al. Anaplastic large cell lymphoma and breast implants: a systematic review. Plast Reconstr Surg 2011;127:2141-50. [Crossref] [PubMed]
- Brandon HJ, Young VL, Jerina KL, et al. Scanning electron microscopy characterization of surgical instrument damage to breast implants. Plast Reconstr Surg 2001;108:52-61. [Crossref] [PubMed]
- Bengtson BP, Eaves FF 3rd. High-resolution ultrasound in the detection of silicone gel breast implant shell failure: background, in vitro studies, and early clinical results. Aesthet Surg J 2012;32:157-74. [Crossref] [PubMed]
- Hölmich LR, Fryzek JP, Kjoller K, et al. The diagnosis of silicone breast-implant rupture. Clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg 2005;54:583-9. [PubMed]
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003;138:801-6. [Crossref] [PubMed]
- Data on file with Mentor Worldwide LLC. 2011. MemoryGel® Breast Implant 9 -Year Core Clinical Study Report. Available online: http://www.mentorwwllc.com/global-us/
- Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg 2013;132:1319-28. [Crossref] [PubMed]
- Adams WP Jr, Conner WC, Barton FE Jr, et al. Optimizing breast pocket irrigation: An in vitro study and clinical implications. Plast Reconstr Surg 2000;105:334-8; discussion 339-43. [Crossref] [PubMed]
- Adams WP Jr, Conner WC, Barton FE Jr, et al. Optimizing breast-pocket irrigation: The post-betadine era. Plast Reconstr Surg 2001;107:1596-601. [Crossref] [PubMed]
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: Six-year prospective clinical study. Plast Reconstr Surg 2006;118:46S-52S. [Crossref] [PubMed]


