Smoking and mortality in women diagnosed with breast cancer—a systematic review with meta-analysis based on 400,944 breast cancer cases
Introduction
Although improved prognosis, breast cancer is responsible for 540,000 deaths worldwide each year (1). Essential in reducing and fighting these deaths is the identification of the modifiable factors influencing incidence and mortality of breast cancer. Identifying these, could permit intervention, and help reduce breast cancer-related deaths. One possible modifiable factor is smoking.
The mortality among current smokers is 2 to 3 times as high as that among persons that have never smoked. In the US alone, smoking is responsible for more than 480,000 deaths each year (2,3). As 18% of American adults are estimated to be current smokers it is safe to assume that there is a substantial portion of women diagnosed with breast cancer that are current smokers or that have previously smoked (2).
When advising our patient with newly diagnosed breast cancer on their smoking habits we advise against it on the basis of the increase in surgery related complications and mortality seen in the general population. Recent studies of breast cancer survivors find that a surprisingly low number of breast cancer patients quit or reduce smoking after diagnosis. Between zero to thirty percent change their smoking habits after diagnosis (4-6). This is low compared to other diseases. Lung cancer is highly associated with smoking and smoking cessation after diagnosis has been reported in around 40–87% of patients (7,8).
Little is known about the specific biological effect of smoking on breast cancer. Tobacco contains over 4,000 potentially harmful substances, with the most studied being nicotine. An increasing amount of published research is now indicating that tobacco smoke can facilitate tumor growth, angiogenesis, chemoresistance, and survival by regulating diverse signaling pathways (9,10). Biopsies made on lung cancer patients have shown that the smoke-specific chemical compounds, promotes progression of lung cancer via DNA alterations and modification of various protein expressions (11). A meta-analysis published in the British Medical Journal found a tendency of smoking after lung cancer diagnosis decreasing prognosis of death from all causes (12). Regarding breast cancer, there are some studies indicating that smoking among breast cancer patients is associated with several negative prognostic factors such as increased number of, and larger lymph node metastasis at diagnosis, and increased risk of metastases to lungs after diagnosis (13,14)
Vital, in order to motivate smoking cessation, is the investigation on the possible correlation between smoking and its effect on the nature and mortality of the disease. If present, this would be a powerful additional argument for smoking cessation.
This systematic review and meta-analysis aggregates previously published data from cohort studies in order to identify if there exists an excess mortality from breast cancer due to persistent smoking among breast cancer patients.
Methods
This systematic review and meta-analysis was conducted using the well-recognized methodology and reported in accordance with the PRISMA statement (15).
Eligibility criteria
Only cohort studies reporting on mortality among breast cancer patients with information on smoking as an exposure were included in the analysis.
Study selection and collection process
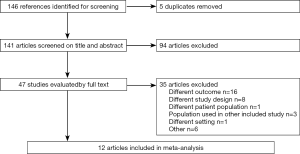
A computerized search was performed using MEDLINE, EMBASE, Cochrane Library and Cinahl. The last search was performed on the 27.07.2016. Reference lists of identified studies and previously published reviews were also explored. The search was limited to publication during the last 10 years and publications in the English language. The author MS searched the databases assisted by a research librarian. The search was performed using the terms; smoking, breast cancer/breast neoplasm, mortality/survival and cohorts studies. Variation of these terms was used depending on what database was being searched. All searches included MESH terms as well as a free words search. Search strategies for each individual database can be found in Supplementary file, appendix. Both authors reviewed the 146 identified articles by title and summary and the appropriate 49 were read in full to determine inclusion. Any disagreements between the reviewers were resolved by consensus and 12 articles were found to fulfill the inclusion criteria.
Risk of bias in individual studies
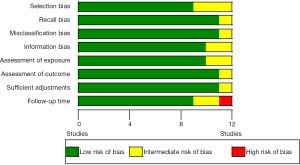
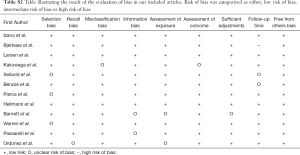
To determine the risk of bias in the individual studies both authors evaluated the included articles on the risk of selection bias, recall bias, information bias, misclassification bias, as well their assessment of exposure, outcome, follow-up time and the adjustments made. Each outcome was deemed “low risk of bias”, “intermediate risk of bias” or “high risk of bias”. “Low risk of bias” was given to those articles who were thoroughly discussing their article in relation to the specific bias. “Intermediate risk of bias” was given to those who were not specifically stating how the bias affected their results, but where we as readers had to evaluate this. “High risk of bias” was given to the articles where we did not feel an evaluation of the bias could be made sufficiently (Figure S1).
Synthesis of results
We categorized deaths into “all-cause death” and “breast cancer-related death”. Patients were subcategorized depending on their current smoking status; never smoker, former smoker or current smoker. In all articles, never smokers were used as the baseline and the unit of outcome was hazard ratio (HR). Not all included articles had information on both all-cause and breast cancer-related death. Therefore, analysis on the different subcategories were based on between eight and eleven studies, even though the systematic review included twelve articles (Table S1). Regarding the article by Pierce et al., former smoking was stratified into three groups depending on the number of pack years (py) smoked. No data on former smoking was therefore included in the meta-analysis.
HRs and confidence intervals (CIs) for all studies were collected and used to calculate the log(HR) and standard error (SE). The meta-analysis applied the random-effects model as we assumed that there had been some clinical heterogeneity. This resulted in studies being treated as random samples, with the assumption that the true effect varied between studies. Statistical heterogeneity was examined as between-study variation and quantified with the I2 value calculated by RevMan. The I2 measures the proportion of variation in the included studies. A value of 0% indicates no inconsistency between the results and a value of 100% indicates maximal inconsistency. A Tau2 value was also calculated. The Tau2 estimates the between-study variance and in general, a value >1 suggests a substantial statistical heterogeneity. The estimated Chi2 is also an indicator for possible heterogeneity, where a Chi2 larger than the degrees of freedom indicates heterogeneity. The associated p-value has a point of significance of 0.1.
The systematic review was performed using the Covidence software. The meta-analysis was performed using the Review Manager program (RevMan). Both developed and supported by the Nordic Cochrane Centre (16,17)
Results
Study selection
The database search provided 146 citations. After removal of duplicates 141 were screened on title and abstract. Forty-seven articles were retrieved and read in full. Twelve articles were included in the final analysis (Figure 1).
Study characteristics
Characteristics of the included studies can be found in Table S1. Four of the included studies originated from the USA, two from Denmark and the remaining six from; Norway, Japan, Germany, Canada, United Kingdom and a European/American-cohort. Data originated from a large period of time 1974–2008 and included a total of 400,944 women diagnosed with breast cancer.
The median or mean age of the women included in the studies ranged from 44 to 67 years. Assessment of smoking status was, in 10 studies, based on questionnaires, filled out by the patients themselves. Bérubé et al. performed interviews and Seibold et al. combined these to determine correct status (18,19). Assessment of smoking status was, in six studies, performed at the time of breast cancer diagnosis and up to 24 months later (18,20-24). Four studies ascertained the information at the time of enrolment in the cohort, before breast cancer diagnosis (25-28) and the remaining two studies gathered data on smoking retrospectively, after the time of diagnosis (19,29). Smoking status was defined by information given by the patients in the questionnaires. Only Seibold et al. and Passarelli et al. had a minimum number of cigarettes that had to have been smoked in order to classify a patient as a smoker. In these articles >100 cigarettes smoked was the cut-off point of whether someone would classify as a smoker (19,24). The term former smoker was in all articles given to patients stating to have smoked in the past, but that was currently not smoking. The number of breast cancer patients included in the cohorts ranged from a total of 528 in the smaller Danish study by Hellmann et al. to 350 918 breast cancer patients in the large European/American-cohort by Ordóñez-Mena et al. (25,26). The median in follow-up ranged from 6.0 years to 14.0 years.
Risk of bias within studies
Assessment of risk of bias within studies is shown in Table S2 and Figure S1, appendix. In general, the studies did well when reporting on these issues.
Results of the individual studies
Characteristics and results of the individual studies can be seen in Table S1.
All-cause death
Ten articles reported HRs for all-cause deaths in former smokers (18-25,27,29). Four found a statistically significant increase in mortality with HRs ranging from 1.11–1.47 (18,21,23,24).
Ten articles reported HRs for all-cause deaths in current smokers (18-25,27,29). Eight found a significant increase in mortality with HRs ranging from 1.16–2.45 (18,19,21-25,27).
Breast cancer-associated death
Nine articles reported HRs for breast cancer associated deaths in former smokers (18-22,24-26,28). Ordóñez-Mena et al. was the only one finding a statistically significant increase in mortality, HR 1.15 (1.50, 1.27) (26). Pierce et al., who stratified their group of former smokers into subgroups depending on pack-years (py) smoked found a statistically significant increase among those who had smoked >35 py (22).
Ten articles reported HRs for breast cancer associated deaths in current smokers (18-26,28). Five detected a statistically significant increase in mortality with HRs ranging from 1.15–1.73 (22-24,26,28).
Synthesis of results
All-cause death
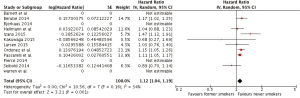
Our meta-analysis found HRs of all-cause death of 1.12 (1.04, 1.19) and 1.52 (1.32, 1.76) among former and current smokers, respectively, when compared to never smokers. (Figures 2,3)
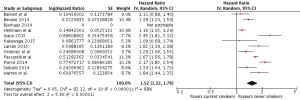
Breast cancer-associated death
The corresponding results for the meta-analysis of breast cancer associated deaths were HRs of 1.02 (0.93, 1.12) and 1.28 (1.17, 1.42), when former smokers and current smokers, respectively, were compared to never smokers (Figures 4,5).
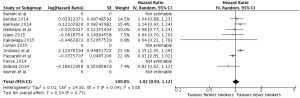
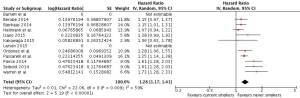
Discussion
This systematic review and meta-analysis found a significant increase in both all-cause and breast associated mortality in women diagnosed with breast cancer who were current smokers when they were compared to never smokers. Where former smokers were found to have an increase in all-cause mortality, no increase was found in breast-cancer associated death.
Adjustment for confounders
The populations in our included articles all consist of women diagnosed with invasive breast cancer. When comparing these articles, it is vital that they have made adjustments for risk factors known to modify breast cancer risk, in order to reduce possible confounders. An article in the database UpToDate summarizes these factors. Among the most important are age, race/ethnicity, weight, increased estrogen exposure/age at menarche and menopause (30). Most articles have also adjusted for tumor size, lymph node status, histological grade and other variables important for cancer staging, all influencing survival of breast cancer. All articles presented comprehensive lists of their adjustments and 11 articles adjusted for all of the above-mentioned covariates. All adjustments made can be seen in Table S1. Regarding weight/BMI, the article by Larsen et al. chose to look at BMI as an independent exposure (27). Larsen et al. had a broad focus, focusing on metabolic indicators, smoking, alcohol and socioeconomic position, rather than smoking alone. Bjerkaas et al. did not perform an adjustment on staging, but as our focus is breast cancer as a whole, this should not affect our results (28). Barnett et al. did not perform any adjustments to their statistics. They were simply investigating the impact of each individual lifestyle factor (29). Still, we regard all the articles as fulfilling our inclusion criteria. The different adjustments made in the individual articles is a source of uncertainty and this has been taken into consideration when concluding on our results. A great limitation of our study is the lack of follow-up information on our patients. There are many factors influencing breast cancer survival among these; therapies received after diagnosis, concurrent alcohol use and dietary factors to name a few. Information on these factors would without a doubt, have strengthened our results and would have allowed us to conclude beyond smoking status at time of diagnosis.
Assessment of smoking status
Underreporting of smoking
The questionnaires used in the different studies have not been published and therefore we cannot conclude on how uniform they were in content. Bjerkaas et al. included data from three different cohorts resulting in three different questionnaires (28). This is also the case in the large cohort study by Ordóñez-Mena et al. (26). When investigating smoking habits by questionnaire there is a substantial risk of underreporting. A large review by Gorber et al. investigated 54 published studies on the accuracy of self-reported smoking and found that in most cases patients underestimated their smoking. In 41 out of 54 studies underreporting was observed ranging from a few per cent to forty-six per cent (31). In most of the studies included in our analysis, the questionnaire was filled out by the patient’s themselves. The effect of possible underreporting should, however, be equally present in most of the included articles. Seibold et al. conducted interviews to fill out these forms and their patient’s answers might be more uniform, and possibly more accurate, than the self-reported ones (19).
Classification of exposure variable: time of assessment of smoking status
Six of the included studies ascertained smoking status within a period of two years after breast cancer diagnosis (18,20-24). Four studies gathered status before breast cancer diagnosis (25-28), while the remaining two studies gathered data on smoking after the time of diagnosis (19,29). A risk of misclassification is present in all studies as patients might change smoking habits after breast cancer diagnosis. None of the included studies have adjusted for this in their analysis. Seibold et al. did, however, investigate potential changes in smoking habits by making a follow-up phone call a few years after diagnosis (19). This showed that about one-third did not change their smoking habits, nearly 40% stopped and one-quarter smoked less than before breast cancer diagnosis. Only 1% smoked more. Sprague et al. observed the same tendency in their study, where women were 35% less likely to be smokers in the years after breast cancer diagnosis compared to 1 year prior to being diagnosed (32). This indicates that there is a real possibility that the group called current smokers in our studies, might be a very diverse group consisting of a large portion of patients that may have seized or at least reduced their smoking substantially. This would imply that the true HR is larger than the estimated HR for current smokers.
Classification of exposure variable: definition of smoking status
The three categories of smoking status in the included population were determined by self-reported patient data. Having the patients themselves report smoking status is an uncertainty in our analysis, as there is a possibility of patients falsely categorizing themselves as one or the other. Most of the included articles did not specify exactly what defines “a smoker”. Only two of the articles specifies this, with a cut-off point between “a smoker” and “non-smoker” of a total of >100 cigarettes smoked (19,24). In the other studies, there is therefore potentially a group of smokers having smoked a small amount of cigarettes but still defining themselves as smokers. One hundred cigarettes is, however, a relatively small amount, and the group of people having smoked below this number and categorized themselves as smokers should not significantly affect our results. Regarding the issue of self-reporting and the accuracy of these reports the main limitation today is that there is no clinical test to accurately quantify the amount of cigarettes previously smoked. To accurately determine whether a former smoker and a no smoker, is not using tobacco, the use of blood samples for serum-nicotine is an option. The pragmatic approach of patients self-reporting is arguably better as this is the situation we meet in daily clinical life.
Passive smoking as a confounder
In daily life, passive smoking is a big contributor to the total smoking exposure, especially when living with a smoking spouse. We have not been able to identify any studies on passive smoking and mortality from breast cancer. Only one of the included articles in our review, by Kakugawa et al., investigated passive smoking in their population and adjusted for this in their analysis (20). Passive smoking could have affected our results as both an underestimation or overestimation of a patient’s exposure to tobacco smoke could have taken place.
Classification of the outcome: mortality
All articles, except two, split mortality into the two groups; all-cause mortality and breast cancer-associated mortality. All of the included articles used the International Classification of Diseases (ICD-9/10) published by the World Health Organization. Uniformity across the included studies regarding this outcome classification resulted in very comparable results. However, different quality of outcome assessments was used. The majority relied solely on death certificates. Seibold et al. validated the death certificates by revising medical records to further secure correct cause of death (19). Bérubé et al. primarily used death certificates, but relied on family and relatives to fill out missing records, a possible source of error (18). Johansson et al. investigated the validity of death certificates and found that, regarding malignant neoplasms, there was generally an agreement between death certificates, hospital records, and cause of death (33). They also concluded that caution had to be taken with increasing periods of time since last discharge from the hospital. This due to the fact that deaths outside of hospital wards were often registered by a physician other than the ones that had taken part in their primary care. This could also affect the accuracy of cause of deaths in our included studies.
Duplicate cases
Two studies consisted of data from different cohorts within the same geographical area, and with an overlap in time. Duplicates could, therefore, be present (22,28). Bjerkaas et al. screened for this and stated that when a patient was included in more than one cohort, only the first entry was used in their statistics (28). Pierce et al. did not specify whether or not duplicates were actively screened for, but as they obtained written consent from all included patients prior to sending them their questionnaires, this should not have been a problem (22). Even though a few duplicate cases exist, it would probably not be associated with neither the exposure nor the outcome variable and a possible bias due to duplicates is unlikely.
Statistical heterogeneity
When looking at heterogeneity between our studies the I2 value calculated when doing the meta-analysis, ranged between 34–88%. The highest I2 of 88% might indicate substantial inconsistency, between the groups included in our study. The calculated Tau2 ranged from 0.00–0.05, not suggesting a substantial variation. The Chi2 for all meta-analysis are also indicating heterogeneity. As there is evidence of possible heterogeneity across studies, extra care should be taken when concluding on our results.
Risk of bias between studies
Looking at Table S2 and Figure S1, illustrating bias between our studies, there is a strong trend of low bias in all articles. This is, in our opinion, one of the strongest aspects of our article. Together with the, over 400,000 breast cancer cases included, this is the biggest and most comprehensive meta-analysis performed on this subject.
The importance of smoking cessation
From a clinical perspective, it is important to know what information to pass on to our patient. Previous smoking is not a factor possible to alteration, but future smoking is. Will cessation of smoking at the time of diagnosis improve chances of survival? As our article only investigates current, former and never smoking, this is not an easy question to answer. When looking at our included studies it is evident that current smoking increases mortality drastically. The trend regarding former smoking is that it increases mortality, but not to the same extent as current smoking. When looking at all-cause mortality, former smokers are still statistically significant at a higher risk of dying compared to never smokers. This increase in mortality was not observed when looking at former smoking and breast cancer associated death. There is, however, a probability that the effect seen is not limited to a patient’s own smoking, but that other sources of smoke, such as passive smoking, could affect mortality. A recommendation of total smoke-abstinence may, therefore, be the best way of eliminating the effects of smoking on mortality.
Conclusions
The present systematic review and meta-analysis found that current smoking is associated with an increase in breast cancer-related mortality of 28%. No increase was observed in those who were former smokers. Smoking was associated with an increase in all-cause mortality for all patients. This study, therefore, supports previously published evidence of smoking increasing cancer-related mortality, and that seizing to smoke could reduce the risk of dying from breast cancer. Our findings provide good arguments for clinicians when advising on smoking cessation.
Supplementary
Search 1—Search strategies
Search date 27.07.16
Search strategy, MEDLINE, 28.07.16
((((("smoking"[MeSH Terms] OR "smoking"[All Fields]) AND ("breast neoplasms"[MeSH Terms] OR ("breast"[All Fields] AND "neoplasms"[All Fields]) OR "breast neoplasms"[All Fields] OR ("breast"[All Fields] AND "cancer"[All Fields]) OR "breast cancer"[All Fields]) AND ("mortality"[Subheading] OR "mortality"[All Fields] OR "mortality"[MeSH Terms])) AND "cohort studies"[MeSH Terms])) AND "last 10 years"[PDat]) AND ("last 10 years"[PDat])
Results 104 hits
Search strategy, EMBASE, 28.07.16
1. Exp cohort analysis/
2. Limit 1 to (English language and last 10 years)
3. *Smoking habit/ or *adolescent smoking/ or *smoking/ or *smoking cessation/
4. Exp breast cancer/ or exp breast tumor/ or exp breast dysplasia/ or exp breast carcinoma/
5. 1 and 2 and 3 and 4
Results 44
Search strategy, CINAHL
(MH "Prospective Studies+") AND (MH "Breast Neoplasms+") AND "breast cancer AND smoking AND mortality AND cohort studies" AND (MH "Mortality+") AND (MH "Smoking+")
Results 0
Search strategy, COCHRANE LIBRARY
ID SearchHits
#1 MeSH descriptor: [Breast Neoplasms] explode all trees 9,702
#2 Smoking 19,027
#3 MeSH descriptor: [Cohort Studies] explode all trees 126,470
#4 MeSH descriptor: [Mortality] explode all trees 12,384
Results 0
Acknowledgements
The authors thank the librarians at the University Hospital Odense for their help with planning our search strategy. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- WHO methods and data sources for global causes of death 2014. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalCOD_method_2000_2012.pdf
- The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. In: services USDoHaH, editor. Atlanta, GA2014. Available online: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality--beyond established causes. N Engl J Med 2015;372:631-40. [Crossref] [PubMed]
- Steinhilper L, Geyer S, Sperlich S. Health behavior change among breast cancer patients. Int J Public Health 2013;58:603-13. [Crossref] [PubMed]
- Bidstrup PE, Dalton SO, Christensen J, et al. Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: a prospective study in the Danish Diet, Cancer and Health Cohort. Acta Oncol 2013;52:327-35. [Crossref] [PubMed]
- Skeie G, Hjartaker A, Braaten T, et al. Dietary change among breast and colorectal cancer survivors and cancer-free women in the Norwegian Women and Cancer cohort study. Cancer Causes Control 2009;20:1955-66. [Crossref] [PubMed]
- Hopenhayn C, Christian WJ, Christian A, et al. Factors associated with smoking abstinence after diagnosis of early stage lung cancer. Lung Cancer 2013;80:55-61. [Crossref] [PubMed]
- Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2370-7. [Crossref] [PubMed]
- Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 2001;7:833-9. [Crossref] [PubMed]
- Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol 2011;2011:456743. [PubMed]
- Yoshino I, Maehara Y. Impact of smoking status on the biological behavior of lung cancer. Surg Today 2007;37:725-34. [Crossref] [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Bmj 2010;340:b5569. [Crossref] [PubMed]
- Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest 2001;119:1635-40. [Crossref] [PubMed]
- Daniell HW, Tam E, Filice A. Larger axillary metastases in obese women and smokers with breast cancer--an influence by host factors on early tumor behavior. Breast Cancer Res Treat 1993;25:193-201. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 2009;339:b2535. [Crossref] [PubMed]
- Review Manager (RevMan). Version 5.3 ed. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration; 2014.
- Covidence systematic review software. : Veritas Health Innovation, Melbourne, Australia; 2016.www.covidence.org
- Bérubé S, Lemieux J, Moore L, et al. Smoking at time of diagnosis and breast cancer-specific survival: new findings and systematic review with meta-analysis. Breast Cancer Res 2014;16:R42. [Crossref] [PubMed]
- Seibold P, Vrieling A, Heinz J, et al. Pre-diagnostic smoking behaviour and poorer prognosis in a German breast cancer patient cohort - Differential effects by tumour subtype, NAT2 status, BMI and alcohol intake. Cancer Epidemiol 2014;38:419-26. [Crossref] [PubMed]
- Kakugawa Y, Kawai M, Nishino Y, et al. Smoking and survival after breast cancer diagnosis in Japanese women: A prospective cohort study. Cancer Sci 2015;106:1066-74. [Crossref] [PubMed]
- Izano M, Satariano WA, Hiatt RA, et al. Smoking and mortality after breast cancer diagnosis: the health and functioning in women study. Cancer Med 2015;4:315-24. [Crossref] [PubMed]
- Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst 2014;106:djt359. [Crossref] [PubMed]
- Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013;132:401-10. [Crossref] [PubMed]
- Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette Smoking Before and After Breast Cancer Diagnosis: Mortality From Breast Cancer and Smoking-Related Diseases. J Clin Oncol 2016;34:1315-22. [Crossref] [PubMed]
- Hellmann SS, Thygesen LC, Tolstrup JS, et al. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev 2010;19:366-73. [Crossref] [PubMed]
- Ordóñez-Mena JM, Schöttker B, Mons U, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med 2016;14:62. [Crossref] [PubMed]
- Larsen SB, Kroman N, Ibfelt EH, et al. Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol 2015;54:780-8. [Crossref] [PubMed]
- Bjerkaas E, Parajuli R, Engeland A, et al. The association between lifetime smoking exposure and breast cancer mortality--results from a Norwegian cohort. Cancer Med 2014;3:1448-57. [Crossref] [PubMed]
- Barnett GC, Shah M, Redman K, et al. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol 2008;26:3310-6. [Crossref] [PubMed]
- Patient information: Factors that modify breast cancer risk in women (Beyond the Basics). Wolters Kluwer 2015. Available online: http://www.uptodate.com/contents/factors-that-modify-breast-cancer-risk-in-women-beyond-the-basics
- Connor Gorber S, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12-24. [Crossref] [PubMed]
- Sprague BL, Trentham-Dietz A, Nichols HB, et al. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat 2010;124:487-95. [Crossref] [PubMed]
- Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol 2000;29:495-502. [Crossref] [PubMed]