Novel treatments for anaplastic thyroid carcinoma
Introduction
Among the deadliest human cancers, anaplastic thyroid cancer (ATC) is less than 2% of thyroid carcinoma (TC). It has a very rapid course of disease progression and it shows poor treatment outcomes, accounting for 15–40% of TC deaths (1-3).
According to the American Joint Committee on Cancer, ATC is classified as Stage IV TC, independently from tumor size or presence of lymphnode or distant metastasis (4), and it is commonly aggressive or metastatic at the initial presentation (5,6).
The standard treatment of ATC includes surgical debulking, accelerated hyperfractionated “external beam radiation therapy” (EBRT), and chemotherapy, achieving 10 months of median survival (7). Doxorubicin, docetaxel/paclitaxel and platins are endorsed by ATA guidelines in ATC, even though with no improvement of the survival in advanced ATC (8).
A systematic review was conducted on the published papers from 1995 to 2017 about “anaplastic thyroid” and “treatment” (9). Forty studies were returned from the search and, among these, 25 met the inclusion criteria, that established to consider papers comparing patients who received any type of therapy for ATC and measuring survival, as primary outcome, or the percentage of patient surviving more than 1 year, as secondary outcome. The best chance of disease control was an early multidisciplinary approach with extensive radical surgery, combined with adjuvant chemoradiation (with cisplatin or docetaxel/paclitaxel). Targeted multi-tyrosine kinases inhibitors (TKIs) were associated with limited disease progression. Moreover, the presence of foci of differentiated thyroid cancer (DTC) within the ATC was associated with a higher long-term survival (9).
Since ATC is a rare and aggressive tumor, it is still challenging to predict the patient clinical therapy responsiveness. Several genetic mutations have been described in ATC, that are involved in different molecular pathways linked to tumor progression (8,10), and novel therapies acting on these molecular pathways have been investigated (8), in order to improve the quality of life in these patients (11,12). Here we review the new targeted therapy of ATC.
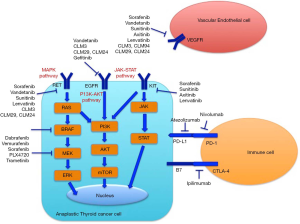
Molecular targets of ATC (Figure 1)
The ATC mutational status can be used to understand the pathogenesis of this tumor and to guide the selection of the right systemic therapy (8,13). Mutation of the tumor suppressor p53 gene is commonly detected among ATC (70–88%), whereas it is less frequently found in follicular thyroid cancer (FTC) and papillary thyroid cancer (PTC) (14).
All tumors should be evaluated for the presence of the most studied mutations, including BRAF (15-17). BRAF V600E occurs in approximately 45% of PTC, but also it is found in 25% of ATCs (17,18). The mutated BRAF V600E is a permanently activated kinase and phosphorylates its downstream targets, such as MEK and ERK, and it is associated with more aggressive features including larger tumor mass, lymph node or extrathyroidal metastasis, which in turn cause a poorer prognosis (19-21).
RET/PTC rearrangements have been reported in 3 cases of ATC tissues (22), perhaps owing to the coexistence of ATC and PTC in the same tissue.
The vascular endothelial growth factor (VGF)-A plays a physiological role in the survival and proliferation of endothelial cells (23). Cancer cells expressing VEGF show a more aggressive behavior, growing more rapidly and metastasizing to distant organs. In fact, VEGF is commonly found on highly malignant ATC cells (24). Also, DTC express elevated burden of VEGF-receptor (VEGFR), in particular VEGFR-2, and VEGF-A, compared to control thyroidal tissue (25). Furthermore, an augmented VEGF expression was associated with the incidence of local and distant metastasis in PTC (26). Moreover, the expression of VEGF and microvessel density in TCs and the effect of VEGF expression in TC cells on the dendritic cells were evaluated in 65 patients with different types of TCs: PTC, oncocytic (OTC), FTC and ATC (27). PTC expressed VEGF more significantly than ATC (92.3% versus 60.0%, P=0.025). The microvessel density (identified by antibodies against CD31) in the tumor border of PTC was significantly more elevated with respect to FTC (P=0.039), but not to ATC and OTC (P=0.337 and 0.134). This paper confirmed the hypothesis that the VEGF expression on TC cells can induce neovascularization (27).
Regarding RAS mutations [KRAS (in codon 13/12), HRAS, and NRAS (in 61 codon)], point mutations are present in about 40% of FTCs, 15% PTCs, and 50% of ATCs, activating PI3K/AKT and MAPK pathways, and they are linked to aggressiveness and a poor prognosis (28,29). As several studies suggest, the evaluation of the mutational status of TC could be of help to detect those genetic mutations linked to a more aggressive clinical course of the tumor, such as the telomerase reverse transcriptase promoter (TERTp) especially when associated with BRAF V600E or RAS mutations (12,30-32).
As what concerns epidermal growth factor receptor (EGFR), its misregulation, amplifications, or mutations are involved in approximately 30% of epithelial carcinoma (33). EGFR was linked to tumor invasiveness and progression of TC (34,35), and it is overexpressed in ATC.
In DTC, a copy number gain is present in the genes of receptor tyrosine kinase (i.e., VEGFR1, PIK3Ca, VEGFR2, EGFR, PDGFRα, KIT, PDGFRβ, PIK3Cb, PDK1, and MET) (22). However, copy number gains were more prevalent in ATC, with respect to DTC (36). Most of these genes are determinant in ATC carcinogenesis, for this reason it has been hypothesized that gene copy number variations are implicated in the aggressiveness and progression of this neoplasm (37).
A common feature of neoplastic cells is the dysregulation of the activity of histone deacetylase (HDAC) or histone acetyltransferase: a more favourable chromatin configuration is obtained for gene transcription (38,39). The anticancer effect of CUDC-101, the first-in-class dual inhibitor of EGFR, HER2 and HDACs, was evaluated in ATC (40), showing its association with an elevated expression of p21 and E-cadherin, and a diminished expression of survivin, XIAP, β-catenin, Vimentin, and N-cadherin. In an in vivo mouse model of metastatic ATC, CUDC-101 inhibited tumor growth and metastases, and prolonged significantly the survival, leading to evaluate the potential of CUDC-101 therapy in ATC (40).
In ATC, the augmented synthesis of miR-20a seems to oppose progression, with significant therapeutic consequences (41). The inhibitory receptor programmed death (PD)-1 is expressed on the membrane of activated T cells. In tumor microenvironment, the binding of PD-1 to its ligands (PD-L1 and PD-L2), inhibits T cell function, through a mechanism called “adaptive resistance” (42). PD-1 and PD-L1 have a crucial role in the capability of tumor cells to escape the host’s immune system. For this reason, the block of their binding in vitro had shown to enhance the immune response and the antitumor activity in preclinical models (43). PD-L1 expression is considered a potential biomarker for response of anti-PD-1 or anti-PD-L1 agents in different tumors (44). About 22.2% ATC, 7.6% FTC and 6.1% PTC expressed PD-L1, among the 407 evaluated primary TCs. The authors concluded that advanced TC markedly expressed PD-L1 (44).
More recently, a massively-parallel DNA sequencing was conducted in 27 ATC and 86 advanced DTC, and RNA sequencing was done on transcriptomes of 13 ATC and 12 advanced DTC (45). AKT1, TERT, EIF1AX, and PIK3Ca were often co-mutated with driver genes (i.e., BRAF V600E and RAS) in advanced DTC and ATC, while tumor suppressor genes (i.e., TP53 and CDKN2A) were particularly altered in ATC. In patients with ATC or advanced DTC, CDKN2A loss was significantly associated with poor disease survival and up-regulation of PD-L1 and PD-L2 (45).
Targeting angiogenesis
Vascular disrupting mechanism
Combretastatin
Combretastatin A4 phosphate (CA4P or fosbretabulin) is a microtubule depolymerizing drug acting against tumor vascular microenvironments, preventing the tumoral blood supply, causing necrosis (46). A complete response (CR) was evidenced in one patient administered with CA4P, still living 30 months after the therapy (47).
In ATC patients, the FACT trial (a randomised, controlled phase II/III study) investigated the effect of carboplatin/paclitaxel, combined with CA4P (experimental group), or not (control group) (48). Eighty patients were enrolled (55% of them had been treated by surgery and, among them, 70% by near total or total thyroidectomy). Patients belonging to the CA4P arm had 8.2 months of median survival with respect to 4.0 months in the control group. In the CA4P arm 1-year survival was 33.3%, while in the control arm 7.7%. These results suggested that thyroidectomy followed by carboplatin/paclitaxel, combined with CA4P, showed a not significant trend toward improvement in survival in ATC patients (48).
Moreover, an open-label trial was conducted in 80 patients with ATC, administered with carboplatin/paclitaxel, combined or not with fosbretabulin, reporting no differences between the 2 arms in progression-free survival (PFS) (49).
VEGF pathway
Sorafenib
Sorafenib is an orally active multikinase inhibitor (mKI) that targets BRAF, c-Kit, RET, and VEGFR-1 and -2, and exerts anti-neoplastic actions in patients with TC, acting on the BRAF pathway, RET, and angiogenesis (50-53).
A phase III trial showed that sorafenib is an effective therapy in progressive radioactive iodine-refractory DTC (53).
A phase II trial has tested sorafenib in 20 patients with ATC (not submitted to previous therapies). It was administered 400 mg twice daily. Ten percent of patients had a partial response (PR), and 25% a stable disease (SD). The median PFS was 1.9 months and the median survival 3.9 months, and 1-year survival was 20%. It was concluded sorafenib was not effective in ATC patients (54).
Moreover, a combination of sorafenib and metformin showed in vitro to inhibit the growth of ATC cells and stem cells (55). An open-label, single-arm, multicenter, uncontrolled phase II study investigated the effect of sorafenib. In ATC median overall survival (OS) was 5.0 months (95% CI, 0.7–5.7) and median PFS 2.8 months (95% CI, 0.7–5.6), [overall response rate (ORR) 0%; disease control rate (DCR) 40%]. The authors concluded that sorafenib could be not useful to treat ATC (56).
Another phase II study was performed in 36 patients with metastatic, radioactive iodine-refractory TC of follicular origin (including PTC, FTC, Hürthle cell, or ATC), who were treated with oral sorafenib (200 mg twice daily) combined with intravenous temsirolimus (25 mg weekly) (57). PR was 22%, SD 58%, and progressive disease (PD) 3%. Patients who had received any prior systemic therapy had a response rate of 10% versus 38% of those who had not received it. One/two patients with ATC had an objective response (57).
Vandetanib
Vandetanib is a multiple TKI, that targets EGFR, RET kinases, and VEGFR-2 and -3, and it has anti-angiogenetic action. It has been approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) in aggressive medullary thyroid cancer (MTC) (58,59). Vandetanib was evaluated in 1 phase III trial and 2 phase II trials in patients with advanced MTC, showing a clinically important antineoplastic activity (58,60,61).
In ATC xenografts it has been reported vandetanib reduces the tumor mass (up to 60%), and the vascularization of the neoplasm, in association with a reduced EGF-R/VEGF-R2 activity (62).
A randomised, double-blinded, phase II trial was done in locally advanced or metastatic DTC patients (63) (ClinicalTrials.gov, number NCT00537095). Eligible patients received vandetanib (300 mg per day; vandetanib group comprised 72 patients) or matched placebo (73 subjects). Patients belonging to the vandetanib group had longer PFS than subjects administered with placebo; median PFS was 11.1 months (95% CI, 7.7–14.0) for vandetanib, versus 5.9 months (4.0–8.9) for placebo (63).
Moreover, vandetanib showed antitumor and antiangiogenic activity in primary ATC cells, decreasing significantly cell growth, inducing apoptosis in a dose dependent manner, and inhibiting migration and invasion. It downregulated cyclin D1 and inhibited ERK1/2, AKT and EGFR phosphorylation (64). In continuous cell lines [the human 8305C and an ATC-cell line (AF)], the proliferation was significantly reduced by vandetanib, that induced apoptosis. Furthermore, after the subcutaneous injection of the 8305C cells in CD nu/nu mice, xenografts were treated with vandetanib (25 mg/kg/day), that inhibited significantly tumor growth, VEGF-A expression and microvessel density (64).
Sunitinib
Sunitinib is a multitarget inhibitor against VEGFR-2, RET, c-Kit, PDGFR, FLT-3, and CSF-1R (65). Sunitinib is active in vitro and in vivo in ATC cells (66). In a phase II trial performed in 28 DTC and 7 MTC patients, PR was in 28%, SD in 46%, and a CR was observed in one patient (67).
Sunitinib was proposed as salvage therapy in a patient affected by ATC (68). A CR in the neck mass was observed in this patient 12 weeks from the start of sunitinib (near the 2nd cycle). However, the disappearance of the neck mass was not associated with a response in lung metastases that remained stable during the treatment. Five months after the beginning of the therapy with sunitinib, a fatal episode of massive upper gastrointestinal bleeding occurred and the patient died (68).
A phase II trial enrolled 71 patients (45 with differentiated or anaplastic tumor: 4 ATC, 13 FTC, 21 PTC, 7 others; 26 MTC) in 1st line anti-angiogenic therapy with sunitinib at 50 mg/d, 4/6 w. In MTC patients median PFS and OS were 16.5 and 29.4 months, while in patients with aggressive DTC were 13.1 and 26.4 months (69).
Lenvatinib
The oral mKI lenvatinib is directed against fibroblast growth factor receptors-1, -2, -3, -4, VEGFR-1, -2, -3, PDGFRb, RET and c-KIT, and it has been demonstrated effective in aggressive DTC (70), for this reason FDA and EMA approved it to treat advanced radioiodine–refractory DTC.
In vivo lenvatinib has shown antitumor activity against human TC in xenografts (in nude mice) of different subtypes of TC (5 DTC, 5 ATC, 1 MTC). In these models lenvatinib showed a strong antiangiogenic action both in DTC and ATC xenografts (71).
The safety and efficacy of lenvatinib has been assessed in a phase II study (clinical trial NCT01728623) in 51 patients with radioiodine-refractory DTC (RR-DTC), MTC or ATC, who received lenvatinib (24 mg) once daily (72). All patients experienced one or more adverse event (AE); 1 patient had to discontinue the therapy. Median PFS was: 25.8 months for RR-DTC; 9.2 months for MTC; 7.4 months for ATC. Lenvatinib reported a manageable safety profile, antineoplastic activity in RR-DTC and an encouraging effectiveness in MTC and ATC (72).
In another study, surgical treatment was done in 10/33 ATC patients, and lenvatinib was used postoperatively (73). The patients had a lenvatinib response rate of 17.4% and DCR of 43.5%. Hypertension was the most common AE (91.3%). Dose interruptions or reductions were needed owing to the onset of tumor fistulas or other AEs, and 39.1% patients discontinued the therapy due to grade 3 or higher AEs. The median OS time was 166 days (73).
Moreover, lenvatinib showed an antineoplastic action in primary ATC cells, significantly reducing cell growth and increasing apoptosis (74). It inhibited migration and invasion, and downregulated cyclin D1 and inhibited EGFR, AKT and ERK1/2 activation. Moreover, lenvatinib significantly reduced 8305C and AF cell (an ATC cell line) proliferation, increasing apoptosis. After the subcutaneous injection of AF cells into CD nu/nu mice, xenografts were treated with lenvatinib (25 mg/kg/day) and it significantly reduced tumor growth, VEGF-A expression and microvessel density (74).
CLM94 and pyrazolo[3,4-d]pyrimidine derivatives
The antitumoral action of the cyclic amide CLM94, with VEGFR-2 and antiangiogenic action, was demonstrated in vitro in ATC cells and in vivo in xenografts in nude mice (75).
Furthermore, the antitumoral action of a pyrazolo [3,4-d]pyrimidine compound (CLM3), with antiangiogenic activity, and able to inhibit EGFR, VEGFR-1, and the RET TK, was reported in primary ATC cells and in human ATC continuous cells. CLM3 (76,77) can inhibit the proliferation of primary ATC cells, it induces apoptosis, and inhibits the activation of ERK1/2, EGFR, AKT, and cyclin D1, and decreases the microvessel density. The results demonstrated that CLM3 is effective in ATC, opening the doors to next clinical evaluations (77).
Moreover, the antitumor activity of 2 “pyrazolo[3,4-d]pyrimidine” compounds (CLM29 and CLM24) that inhibit the RET TK, EGFR, VEGFR, and have antiangiogenic effect, was studied in primary ATC cells and in 8305C. The BRAF V600E mutation was detected in 3 ATC samples; CLM29 and CLM24 inhibited the cell growth both in ATC tumors with and without the BRAF V600E mutation in a similar manner. In primary ATC cells, migration and invasion were inhibited by CLM29 (P<0.01) (78).
EGFR pathway inhibition
Gefitinib, a low molecular weight compound, inhibits EGFR TK reducing the TC cell growth and it enhances the ionizing radiation antiproliferative effect on DTC and ATC cells (79,80).
A paper by Nobuhara et al. (81) investigated the expression of EGFR in ATC cell lines. All the considered ATC continuous cell lines expressed EGFR. In the ACT-1 cell line, specific EGFR stimulation showed significant phosphorylation of ERK1/2 and Akt, stimulating the proliferation of these cells, that highly expressed EGFR. Gefitinib inhibited such proliferation. Moreover, gefitinib inhibited dose-dependently the growth of xenografts in mice. Inhibition of EGFR-transmitted growth stimulation by gefitinib was clearly observed in ATC cell lines.
A phase II trial was carried on in metastatic patients with aggressive TC (among whom 18 DTC) with (250 mg/daily) gefitinib. The results showed reduction of the tumor volume in 32% of patients (with no PR), SD at 3 months in 48% of patients, the OS was 17.5 months, and median PFS was 3.7 months. The authors suggested that gefitinib has no significant effect in monotherapy (82).
The continuous ATC cell line 8505c was treated with baicalein or docetaxel alone or combined (83). Proliferation was significantly inhibited and apoptosis was induced in comparison to the monotherapy. The combined therapies significantly inhibited the expression of E-cadherin, Bax, caspase-3, TGF-β1, VEGF, mTOR and N-cadherin, but decreased the expression of Bcl-2 and the phosphorylation of ERK and Akt. The Authors concluded that in ATC baicalein enhances the anticancer effect of docetaxel (83).
Targeting BRAF
Dabrafenib and its combination with trametinib
Nowadays a novel treatment for cancers harboring BRAF is represented by dabrafenib, a BRAF inhibitor. Unfortunately, many clinical trials showed that BRAF-positive tumors develop resistance to this new drug within 6 to 7 months, and to overcome this issue, the BRAF inhibitor dabrafenib has been administrated together with the MEK inhibitor trametinib (84).
The effectiveness of inhibiting the activated RAS/RAF/MEK pathway in ATC cells was investigated in 4 human ATC cell lines (ACT-1, OCUT-2, OCUT-4 and OCUT-6) (85). Dabrafenib downregulated MEK/ERK phosphorylation and inhibited the viability in BRAF mutated cells by G0/G1-arrest. Upon treatment with dabrafenib, upregulated phosphorylation of MEK was shown in RAS mutated cells leading to VEGF upregulation. Trametinib, via the downregulation of ERK phosphorylation, inhibited cell viability. In the 4 ATC cell lines, dual blockade by both inhibitors showed cytostatic effects (85).
In 2014, the combined therapy with dabrafenib and trametinib has been approved by FDA for BRAF V600E/K-mutant metastatic melanoma (86). Two cases of BRAF V600E-positive ATC receiving the BRAF inhibitor dabrafenib were reported by Lim et al. (87). The first patient had a T4bN1bM0 ATC and was a woman of 49 years with metastatic disease 8 weeks after chemoradiation therapy, administered with dabrafenib. One month later, FDG-PET scan reported a CR. Nevertheless, the therapy was withdrawn owing to disease progression, and death occurred after 11 months from the diagnosis. The other subject was a man of 67 years with a T4aN1bM0 ATC, who received dabrafenib, halving the tumor size after 10 days of therapy. Owing to disease progression, death occurred after 11 months from the diagnosis. It was shown that BRAF inhibitor monotherapy can achieve short clinical benefits in ATC. In mice, models of BRAF V600E-positive ATC have shown a significant improved survival when treated with combined dabrafenib and trametinib comparing to a BRAF inhibitor alone (87).
Another study reported the case of a woman of 81 years with a growing neck mass (88). At the beginning, a diagnosis of MTC was made and she underwent to total thyroidectomy. ATC was then diagnosed. Her tumor harbored a BRAF V600E mutation and she received EBRT. Progression in the neck and lung metastases were reported after 4 months from the initial diagnosis. Then she received other 24 Gy of EBRT to the neck, followed by pazopanib. The disease progressed rapidly in the neck and lung masses, and owing to the urgency to start treatment, dabrafenib and trametinib were used in liquid formulation. She started dabrafenib (150 mg twice/day) and trametinib (2 mg/day), and the pressure in her neck diminished 2 weeks after. One month after starting dabrafenib and trametinib, a strong treatment response in the neck and lungs was shown, and such response was sustained after 4 months of follow-up. Hypothyroidism occurred while on treatment, together with fatigue, weakness and edema, and the therapy with dabrafenib and trametinib was reduced, but without significant clinical benefit. After 6 months, owing to disease progression, the treatment was stopped and the patient died quickly (88).
Another study reported the effect of dabrafenib and trametinib in ATC patients with BRAF V600E mutation, with an ORR of 69% (95% CI, 41% to 89%), and 7 ongoing responses. Dabrafenib + trametinib had a strong clinical activity in these patients and a good tolerance (89).
Moreover, a subject with unresectable, end-stage, locally advanced ATC received combined dabrafenib and trametinib (90). At progression, immunotherapy with pembrolizumab was conducted and a PR was reached, allowing a complete surgical resection, and then postoperative chemoradiation (90).
The paper by Subbiah et al. (89) led to the approval from FDA (on May 2018) of this combination of drugs for patients with BRAF V600E mutated ATC with locally advanced, unresectable, or metastatic ATC with no locoregional treatment options.
A recent paper was conducted in 16 patients with advanced ATC (91). Ten/sixteen received lenvatinib, while 6 BRAF mutated patients were administered with dabrafenib/trametinib. This last group had improved survival versus those who received lenvatinib. For all patients, median OS was 6.3 months (95% CI, 1.8–7.6), for the lenvatinib and dabrafenib/trametinib subgroups, 3.9 and 9.3 months, respectively. For all patients, median PFS was 3.7 months (95% CI, 1.8–7.6), for the lenvatinib and dabrafenib/trametinib subgroups, 2.7 and 5.2 months, respectively (91).
Vemurafenib
The FDA approved also vemurafenib for the treatment of patients with metastatic melanomas harbouring BRAF V600E mutation. In fact, this small compound can inhibit the enzymatic activity of BRAF, blocking the MAPK pathway.
In a phase I study, three DTC patients were enrolled and treated with vemurafenib. One had a PR while the other two obtained a SD (92). In a mouse model, vemurafenib suppressed growth of BRAF-mutated human ATC (93). A marked response to vemurafenib in a 51-year-old man with BRAF-mutated ATC has been described showing a nearly total eradication of metastatic disease (94). A further study demonstrated a significant effect of vemurafenib in an ATC patient with the BRAF V600E mutation (95).
Another paper described a 51-year-old man with BRAF-mutated ATC, in whom the physical examination revealed thyroid masses and jugulodigastric lymphadenopathy, and direct laryngoscopy reported unilateral vocal cord paralysis and diffuse supraglottic edema (96). The diagnosis was of ATC associated with PTC. On days 4 and 10, the patient received low, radiosensitizing paclitaxel (45 mg per square meter of body-surface area) and carboplatin (at an area under the curve of 2). The patient experienced progressive dyspnea, and CT reported worsening pulmonary infiltrates and nodules. On day 10, the patient started the therapy with vemurafenib (960 mg orally twice daily). His condition improved rapidly, and on day 14 radiation therapy to the neck and upper mediastinum were done. On day 16 he was discharged from the hospital, in therapy with vemurafenib. The BRAF V600E mutation (T1799A) was detected by real-time polymerase-chain-reaction assay. On day 38 18F-FDG–PET and CT of the chest reported a nearly total clearing of metastatic disease (96). However, a recent paper in an ATC patient showed only a transient initial response (97).
One hundred twenty-two patients with BRAF V600 mutation tumors, including 7 with ATC, were evaluated by another paper. Anecdotal responses were reported among patients with ATC (96).
Targeting PPARγ
PPARγ are nuclear hormone receptors (98) and their activation induces antineoplastic (99) effects in different cancer cells. PPARγ activatory ligands have been shown: (I) to have antiproliferative action on PTC cells, inducing apoptosis (98); (II) to prevent in nude mice distant metastasis of BHP18–21 tumors (98); (III) to induce redifferentiation of dedifferentiated TC cells (100-102). Human ATC cells overexpressed PPARγ, and once activated it inhibits invasion and proliferation, and it induces apoptosis (103-105). The PPARγ agonist rosiglitazone showed to induce redifferentiation in ATC cells (104). Furthermore, rosiglitazone or pioglitazone inhibited the proliferation in primary ATC cells (106,107).
The effect of efatutazone (a PPARγ ligand; 0.15, 0.3, 0.5 mg twice daily) and paclitaxel (every 3 weeks) was investigated in 15 ATC patients. The median progression time and median survival were 48 and 98 days, respectively, in patients receiving efatutazone (0.15 mg), and 68 and 138 days, respectively, in those administered with efatutazone at the dose of 0.3 mg. The authors suggested that paclitaxel combined with efatutazone were tolerated and effective (108).
Targeting PD-1 and PD-L1
BRAF, KRAS, EGFR mutations and protein overexpression of C-KIT and PD-L1 were assessed in ATC. Among the 13 ATC patients, 23% had BRAF V600E mutation, and 1 (8%) patient had C-KIT overexpression. PD-L1 expression was reported in 3 (23%) patients. KRAS codon 12/13 and EGFR exon 18, 19, 20 and 21 were all wild type (WT). The authors concluded that protein kinase inhibitors and immunotherapy could be adjuvant therapies for ATC (109).
A study investigated PD-L1 effect, and of the anti-PD-L1 antibody immunotherapy, alone or combined with a BRAF inhibitor, on TC regression and intratumor immune response (110). TC cell lines and tumor samples from patients with BRAF V600E-positive tumors have higher levels of PD-L1 than either BRAF WT tumors or matched controls. The immunocompetent mice B6129SF1/J were randomized to PLX4720 (a BRAF inhibitor), control, anti PD-L1 antibody or both the combined treatments, after the implantation of syngeneic 3747 BRAF V600E/WT P53-/- murine cancer cells. Combining PD-L1 antibody and PLX4720 improved markedly the tumor shrinkage and it increased tumor infiltrating lymphocytes. Clinical trials of this therapeutic combination could be useful in ATC patients. Proper clinical trials are needed to explore this potential new therapeutic strategy (110).
Furthermore, it was evaluated, in 12 ATC patients at progression, the combination of pembrolizumab and kinase inhibitors. PR was of 42% of patients, SD 33% and PD 25%. From the start of kinase inhibitors, median OS was 10.43 months (range, 5.4–40 months). From the start of pembrolizumab, median OS and PFS were 6.93 months (range, 3–15.9 months) and 2.96 months (range, 0.57–13.14 months), respectively (111).
Targeting TERT mutations
Targeting the telomerase complex has been shown effective in preclinical studies, but a lower performance and high side effects were reported in clinical trials (112,113). The use of Bromodomains and Extraterminal inhibitors (BETi) drugs to indirectly target telomerase has been proposed (114). BETi can directly reduce TERT expression through the inhibition of BRD4 transcriptional activity, but it can also indirectly induce its transcription by repressing other BRD4-dependent transcription factors (i.e., c-Myc). Moreover, the involvement of BRD4, and HDAC (that also controls histone acetylation at the telomere level), in telomere regulation suggests the use of epigenetic drugs [i.e., BETi and HDAC inhibitors (HDACi)] to ameliorate the outcomes of telomerase-inhibiting compounds. When these therapies are used alone, they do not have substantial effects in clinical trials (115), while combined with other compounds they showed encouraging results (116). The potential effects, on TERT expression and telomeres regulation, of BETi and HDACi combined with telomerase inhibitors could improve their effectiveness.
Cancer stem cell (CSC)-targeted therapies
Many studies have suggested that among tumors with a slow cycling rate, there is a little biologically distinct subpopulation of tumoral cells (namely CSCs), able to regulate self-renewal and multilineage potential, explaining recurrence, metastasis, and resistance to therapy.
This “stem cell niche” grows in vitro as spheres, and sometimes show radio/chemo-resistance, harbouring a molecular profile similar to embryonic and/or adult CSCs. New therapies able to target CSC cell membrane markers, or signaling pathways, which modulate CSC initiation and growth, have been developed (117).
Different intracellular signal transduction pathways are determinant mediators of thyroid CSC biology: (I) insulin-like growth factor (IGF)-I/II and IGF-IR is expressed by PTC spheres, and the number and size of spheres increase when this signaling pathway is stimulated (118); (II) certain ATC cell lines (BCPAP, KAT-18, and SW1736) have an activated sonic hedgehog (Shh) pathway, and its inhibitors inhibit ALDH activity and the formation of thyrosphere (119); (III) in ATC-CD133+ cells, targeting STAT3 pathway by cucurbitacin I (a JAK–STAT inhibitor) diminishes self-renewing and radiochemoresistance (120).
As with the other subtypes of TC, the ATC pathogenesis is still unclear. Traditional treatments act against mature cancer cells, but they do not eradicate thyroid CSCs. After a cytotoxic injury, CSCs repair DNA damage, restoring the previous tumoral burden. This is the reason why it is crucial to synthetize new compounds against thyroidal CSCs (121).
Possible strategies to destroy thyroid CSCs and bypass radio/chemo-resistance may involve the following: promoting the increase of sensitization of CSCs directly by agents able to kill exactly CSCs or to promote their differentiation; blocking specific CSCs signaling pathway components [i.e., ABC subfamily G member (ABCG)2 and ABCB1, SOX2, STAT3, c-Met, CD44, RET]; and destroying CSC niches. More researches are needed to understand the biology and molecular mechanism of thyroid CSC survival, in order to define adequate therapeutic targets to obtain a complete TC eradication (122).
CD47 regulates distinct signalling systems linked to cancer biology, in particular CSC self-renewal, and tumor progression. It regulates tumor growth together with thrombospondin-1 (TSP-1) (123). CD47 is a “don’t eat me” signal, preventing tumor cells from phagocytosis through the binding to signal regulatory protein alpha on macrophages (124). A paper assessed 19 primary human ATC for macrophage markers, CD47 expression, and immune checkpoints by immunohistochemistry. Human ATC samples were markedly infiltrated by tumor-associated macrophages, and expressed CD47. ATC tissues expressed PD-1 and PD-L1. The block of CD47 promoted the phagocytosis of ATC cell lines by macrophages in vitro. The Authors concluded that targeting CD47, or CD47 in combination with PD-1, could ameliorate the outcomes in ATC (124).
Personalization of targeted therapy
Since the introduction of new genetic patient-specific tests, personalized therapies have become more available. Moreover, an in vitro screening technique involving primary tumor cells collected from each patient (125) can predict clinical activity to specific targeted drugs in vivo, with a 60% positive predictive value, and a 90% of negative predictive value (126). Therefore, thanks to these new tools, it is possible not to administer ineffective or even dangerous therapies in patients (127).
Several preclinical models have been considered as encouraging platforms for the development of precision medicine applications. Primary cultures obtained from solid tumors have gained significant importance in personalized cancer therapy (128).
The use of primary TC cells from patients has been complex until now because of their establishment from surgical biopsies. To date, ATC primary cells have been obtained directly from fine-needle aspiration cytology (FNAC), avoiding the more challenging collection from surgical biopsies, in order to test in vitro the sensitivity in every subject to different treatments (106,107,129,130).
Recently, the antineoplastic effect of lenvatinib and vandetanib has been shown in primary cells in 6 ATC patients, established from biopsy or FNAC (131). Lenvatinib and vandetanib reduced significantly the proliferation in primary cells from FNAC, and from biopsy, compared to control, and the percentage of apoptosis increased dose-dependently in both the primary cells. Primary cells from FNAC, or biopsy, had a similar sensitivity to lenvatinib and vandetanib, that are effective in reducing cell growth, increasing apoptosis in ATC. The possibility to test the sensitivity to different TKIs in each patient could open the way to personalized treatments (131).
Moreover, patient-derived xenografts are considered the strongest and most evaluated experimental platform for the development of precision medicine applications. A paper evaluated the expression of sodium iodide symporter (NIS) and radioiodine up-take (RAI-U), and the efficacy of sorafenib, selumetinib and the HDACi panobinostat in patient-derived tumor tissue (PDTT) of ATC/poorly differentiated thyroid cancer (PDTC) (132). Panobinostat had the highest cytotoxic effect (10 nM) in PDTTs and human foreskin fibroblasts (controls) and it led to a significant overexpression of NIS transcript. TKIs up-regulated NIS transcript in patient 5 and in controls. After 24 h of treatment with TKIs and panobinostat, RAI-U was up-regulated in all PDTT and controls, except in patient 5. Selumetinib suppressed significantly High Mobility Group AT-Hook 2 (HMGA2), a well-known non-transcription factor with oncogenic properties, in PDTT 1, 2, 4, 5 and controls, while sorafenib did not change the HMGA2 expression. Panobinostat induced a significant suppression of HMGA2 in PDTT 2, 4 and controls. The expression of miRNAs hsa-miR-146b-3p, hsa-let-7f-5p, hsa-miR-146b-5p and has-let-7b-5p was modulated in a heterogeneous manner. After 24 h of treatment with selumetinib, sorafenib and especially panobinostat, NIS protein level was overexpressed in 3 PDTTs (patients 1, 3 and 4), while controls had a stable NIS protein level. Panobinostat had the highest cytotoxicity in all treated PDTTs at the lowest dosage. For these reasons, the establishment of PDTT could be useful to evaluate the efficacy of compounds and to develop novel and personalized multimodal treatment options for PDTC and ATC (132).
Conclusions
To date the mainstay treatment for ATC is based on debulking surgery, accelerated hyperfractionated EBRT, and chemotherapy, in particular with cisplatin or doxorubicin.
Novel chemotherapies able to target the molecular pathways at the basis of ATC aggressiveness and progression are under evaluation. Survival ameliorated in patients with aggressive TC receiving lenvatinib, showing 10.6 (3.8–19.8) months of median OS (72).
Furthermore, the recent introduction of innovative and not expensive diagnostic tools, such as individual mutational testing and in vitro evaluation of the patient ATC cells, makes more feasible a real tailored therapy, in order to increase the therapeutic success and to avoid the use of ineffective and harmful treatments.
Furthermore, recently a great attention has been given to the epigenetic alterations underlying thyroid carcinogenesis, including those that drive PDTC and ATC (133). Dysregulated epigenetic candidates are KMT2D, multiple non-coding RNAs, the SWI/SNF chromatin-remodeling complex, PTEN, RASSF1A, and the Aurora group. Better knowledge of the signaling pathways affected by epigenetic dysregulation could ameliorate prognostic testing and support the advancement of thyroid specific epigenetic therapy.
The results of clinical trials with dabrafenib and trametinib led to the approval from FDA of this combination for patients with BRAF V600E mutated ATC with locally advanced, unresectable, or metastatic ATC. The anti-PD-L1 antibody immunotherapy, alone or combined with a BRAF inhibitor, has been shown also promising in the treatment of ATC. More researches are needed to find effective therapies for this fatal cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 Cancer 1998;83:2638-48. [see commetns]. [Crossref] [PubMed]
- Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab 1999;84:4043-9. [Crossref] [PubMed]
- Antonelli A, Miccoli P, Derzhitski VE, et al. Epidemiologic and clinical evaluation of thyroid cancer in children from the Gomel region (Belarus). World J Surg 1996;20:867-71. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th edition. Chicago: Springer, 2002.
- Miccoli P, Materazzi G, Antonelli A, et al. New trends in the treatment of undifferentiated carcinomas of the thyroid. Langenbecks Arch Surg 2007;392:397-404. [Crossref] [PubMed]
- Kebebew E. Anaplastic thyroid cancer: rare, fatal, and neglected. Surgery 2012;152:1088-9. [Crossref] [PubMed]
- De Crevoisier R, Baudin E, Bachelot A, et al. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys 2004;60:1137-43. [Crossref] [PubMed]
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [Crossref] [PubMed]
- Salehian B, Liem SY, Mojazi Amiri H, et al. Clinical Trials in Management of Anaplastic Thyroid Carcinoma; Progressions and Set Backs: A Systematic Review. Int J Endocrinol Metab 2019;17:e67759. [PubMed]
- Antonelli A, Fallahi P, Ferrari SM, et al. New targeted therapies for thyroid cancer. Curr Genomics 2011;12:626-31. [Crossref] [PubMed]
- Ljubas J, Ovesen T, Rusan M. A Systematic Review of Phase II Targeted Therapy Clinical Trials in Anaplastic Thyroid Cancer. Cancers (Basel) 2019;11:E943. [Crossref] [PubMed]
- Antonelli A, Ferrari SM, Elia G, et al. Metastases free thyroid cancer patients harbouring TERT mutations may benefit from a more intensive treatment and follow-up. Gland Surg 2019;8:298-300. [Crossref] [PubMed]
- Antonelli A, Fallahi P, Ulisse S, et al. Tyrosine kinase inhibitors for the therapy of anaplastic thyroid cancer. Int J Endocr Oncol 2015;2:135-42. [Crossref]
- Quiros RM, Ding HG, Gattuso P, et al. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer 2005;103:2261-8. [Crossref] [PubMed]
- Smith N, Nucera C. Personalized therapy in patients with anaplastic thyroid cancer: targeting genetic and epigenetic alterations. J Clin Endocrinol Metab 2015;100:35-42. [Crossref] [PubMed]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184-99. [Crossref] [PubMed]
- Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer 2009;16:17-44. [Crossref] [PubMed]
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12:245-62. [Crossref] [PubMed]
- Perri F, Pezzullo L, Chiofalo MG, et al. Targeted therapy: a new hope for thyroid carcinomas. Crit Rev Oncol Hematol 2015;94:55-63. [Crossref] [PubMed]
- Zoghlami A, Roussel F, Sabourin JC, et al. BRAF mutation in papillary thyroid carcinoma: predictive value for long-term prognosis and radioiodine sensitivity. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:7-13. [Crossref] [PubMed]
- Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943-9. [Crossref] [PubMed]
- Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 2008;93:3106-16. [Crossref] [PubMed]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581-611. [Crossref] [PubMed]
- Sato K. Vascular endothelial growth factors and thyroid disorders. Endocr J 2001;48:635-46. [Crossref] [PubMed]
- Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 1999;155:1967-76. [Crossref] [PubMed]
- Lennard CM, Patel A, Wilson J, et al. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery 2001;129:552-8. [Crossref] [PubMed]
- Gulubova M, Ivanova K, Ananiev J, et al. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip 2014;28:508-17. [Crossref] [PubMed]
- Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn 2008;8:83-95. [Crossref] [PubMed]
- Ruggeri RM, Campennì A, Baldari S, et al. What is New on Thyroid Cancer Biomarkers. Biomark Insights 2008;3:237-52. [Crossref] [PubMed]
- Penna GC, Vaisman F, Vaisman M, et al. Molecular Markers Involved in Tumorigenesis of Thyroid Carcinoma: Focus on Aggressive Histotypes. Cytogenet Genome Res 2016;150:194-207. [Crossref] [PubMed]
- Su X, Jiang X, Wang W, et al. Association of telomerase reverse transcriptase promoter mutations with clinicopathological features and prognosis of thyroid cancer: a meta-analysis. Onco Targets Ther 2016;9:6965-76. [Crossref] [PubMed]
- Donati B, Ciarrocchi A. Telomerase and Telomeres Biology in Thyroid Cancer. Int J Mol Sci 2019;20:E2887. [Crossref] [PubMed]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004;59 Suppl:21-6. [Crossref] [PubMed]
- Knauf JA. Does the epidermal growth factor receptor play a role in the progression of thyroid cancer? Thyroid 2011;21:1171-4. [Crossref] [PubMed]
- Yeh MW, Rougier JP, Park JW, et al. Differentiated thyroid cancer cell invasion is regulated through epidermal growth factor receptor-dependent activation of matrix metalloproteinase (MMP)-2/gelatinase A. Endocr Relat Cancer 2006;13:1173-83. [Crossref] [PubMed]
- Bond JA, Wyllie FS, Rowson J, et al. In vitro reconstruction of tumour initiation in a human epithelium. Oncogene 1994;9:281-90. [PubMed]
- Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 2007;13:1161-70. [Crossref] [PubMed]
- Mahlknecht U, Hoelzer D. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol Med 2000;6:623-44. [Crossref] [PubMed]
- Marks P, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001;1:194-202. [Crossref] [PubMed]
- Zhang L, Zhang Y, Mehta A, et al. Dual inhibition of HDAC and EGFR signaling with CUDC-101 induces potent suppression of tumor growth and metastasis in anaplastic thyroid cancer. Oncotarget 2015;6:9073-85. [PubMed]
- Xiong Y, Zhang L, Kebebew E. MiR-20a is upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS One 2014;9:e96103. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-65. [Crossref] [PubMed]
- Ahn S, Kim TH, Kim SW, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer 2017;24:97-106. [Crossref] [PubMed]
- Yoo SK, Song YS, Lee EK, et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun 2019;10:2764. [Crossref] [PubMed]
- Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. Int J Exp Pathol 2009;90:284-94. [Crossref] [PubMed]
- Dowlati A, Robertson K, Cooney M, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 2002;62:3408-16. [PubMed]
- Sosa JA, Balkissoon J, Lu SP, et al. Thyroidectomy followed by fosbretabulin (CA4P) combination regimen appears to suggest improvement in patient survival in anaplastic thyroid cancer. Surgery 2012;152:1078-87. [Crossref] [PubMed]
- Sosa JA, Elisei R, Jarzab B, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 2014;24:232-40. [Crossref] [PubMed]
- Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675-84. [Crossref] [PubMed]
- Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714-9. [Crossref] [PubMed]
- Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 2009;161:923-31. [Crossref] [PubMed]
- Worden F, Fassnacht M, Shi Y, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer 2015;22:877-87. [Crossref] [PubMed]
- Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013;23:600-4. [Crossref] [PubMed]
- Chen G, Nicula D, Renko K, et al. Synergistic anti-proliferative effect of metformin and sorafenib on growth of anaplastic thyroid cancer cells and their stem cells. Oncol Rep 2015;33:1994-2000. [Crossref] [PubMed]
- Ito Y, Onoda N, Ito KI, et al. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid 2017;27:1142-8. [Crossref] [PubMed]
- Sherman EJ, Dunn LA, Ho AL, et al. Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer. Cancer 2017;123:4114-21. [Crossref] [PubMed]
- Wells SA Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 2010;28:767-72. [Crossref] [PubMed]
- Thornton K, Kim G, Maher VE, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res 2012;18:3722-30. [Crossref] [PubMed]
- Robinson BG, Paz-Ares L, Krebs A, et al. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2664-71. [Crossref] [PubMed]
- Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Wunderlich A, Khoruzhyk M, Roth S, et al. Pretherapeutic drug evaluation by tumor xenografting in anaplastic thyroid cancer. J Surg Res 2013;185:676-83. [Crossref] [PubMed]
- Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 2012;13:897-905. [Crossref] [PubMed]
- Ferrari SM, Bocci G, Di Desidero T, et al. Vandetanib has antineoplastic activity in anaplastic thyroid cancer, in vitro and in vivo. Oncol Rep 2018;39:2306-14. [PubMed]
- Kim DW, Jo YS, Jung HS, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab 2006;91:4070-6. [Crossref] [PubMed]
- Ferrari SM, Centanni M, Virili C, et al. Sunitinib in the Treatment of Thyroid Cancer. Curr Med Chem 2019;26:963-72. [Crossref] [PubMed]
- Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 2010;16:5260-8. [Crossref] [PubMed]
- Grande E, Capdevila J, Díez JJ, et al. A significant response to sunitinib in a patient with anaplastic thyroid carcinoma. J Res Med Sci 2013;18:623-5. [PubMed]
- Ravaud A, de la Fouchardière C, Caron P, et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer 2017;76:110-7. [Crossref] [PubMed]
- Sherman SI, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC). J Clin Oncol 2011;29:5503. [Crossref]
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014;2014:638747. [Crossref] [PubMed]
- Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019;15:717-26. [Crossref] [PubMed]
- Iwasaki H, Yamazaki H, Takasaki H, et al. Lenvatinib as a novel treatment for anaplastic thyroid cancer: A retrospective study. Oncol Lett 2018;16:7271-7. [PubMed]
- Ferrari SM, Bocci G, Di Desidero T, et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol Rep 2018;39:2225-34. [PubMed]
- Antonelli A, Bocci G, La Motta C, et al. CLM94, a novel cyclic amide with anti-VEGFR-2 and antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab 2012;97:E528-36. [Crossref] [PubMed]
- Ferrari SM, Fallahi P, La Motta C, et al. Antineoplastic activity of the multitarget tyrosine kinase inhibitors CLM3 and CLM94 in medullary thyroid cancer in vitro. Surgery 2014;156:1167-76. [Crossref] [PubMed]
- Antonelli A, Bocci G, Fallahi P, et al. CLM3, a multitarget tyrosine kinase inhibitor with antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab 2014;99:E572-81. [Crossref] [PubMed]
- Fallahi P, Ferrari SM, La Motta C, et al. CLM29 and CLM24, pyrazolopyrimidine derivatives, have antitumoral activity in vitro in anaplastic thyroid cancer, with or without BRAF mutation. Endocrine 2016;53:136-44. [Crossref] [PubMed]
- Croyle M, Akeno N, Knauf JA, et al. RET/PTC-induced cell growth is mediated in part by epidermal growth factor receptor (EGFR) activation: evidence for molecular and functional interactions between RET and EGFR. Cancer Res 2008;68:4183-91. [Crossref] [PubMed]
- Lopez JP, Wang-Rodriguez J, Chang CY, et al. Gefitinib (Iressa) potentiates the effect of ionizing radiation in thyroid cancer cell lines. Laryngoscope 2008;118:1372-6. [Crossref] [PubMed]
- Nobuhara Y, Onoda N, Yamashita Y, et al. Efficacy of epidermal growth factor receptor-targeted molecular therapy in anaplastic thyroid cancer cell lines. Br J Cancer 2005;92:1110-6. [Crossref] [PubMed]
- Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid 2008;18:317-23. [Crossref] [PubMed]
- Park CH, Han SE, Nam-Goong IS, et al. Combined Effects of Baicalein and Docetaxel on Apoptosis in 8505c Anaplastic Thyroid Cancer Cells via Downregulation of the ERK and Akt/mTOR Pathways. Endocrinol Metab (Seoul) 2018;33:121-32. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Kurata K, Onoda N, Noda S, et al. Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells. Int J Oncol 2016;49:2303-8. [Crossref] [PubMed]
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma - a review of current and future treatment options. Acta Derm Venereol 2015;95:516-24. [Crossref] [PubMed]
- Lim AM, Taylor GR, Fellowes A, et al. BRAF Inhibition in BRAFV600E-Positive Anaplastic Thyroid Carcinoma. J Natl Compr Canc Netw 2016;14:249-54. [Crossref] [PubMed]
- Cabanillas ME, Busaidy NL, Khan SA, et al. Molecular diagnostics and anaplastic thyroid carcinoma: the time has come to harvest the high hanging fruit. Int J Endocr Oncol 2016;3:221-33. [Crossref]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36:7-13. [Crossref] [PubMed]
- Cabanillas ME, Ferrarotto R, Garden AS, et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid 2018;28:945-51. [Crossref] [PubMed]
- Iyer PC, Dadu R, Ferrarotto R, et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018;28:79-87. [Crossref] [PubMed]
- Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid 2013;23:1277-83. [Crossref] [PubMed]
- Nehs MA, Nucera C, Nagarkatti SS, et al. Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology 2012;153:985-94. [Crossref] [PubMed]
- Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med 2013;368:684-5. [Crossref] [PubMed]
- Prager GW, Koperek O, Mayerhoefer ME, et al. Sustained Response to Vemurafenib in a BRAFV600E-Mutated Anaplastic Thyroid Carcinoma Patient. Thyroid 2016;26:1515-6. [Crossref] [PubMed]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Marten KA, Gudena VK. Use of vemurafenib in anaplastic thyroid carcinoma: a case report. Cancer Biol Ther 2015;16:1430-3. [Crossref] [PubMed]
- Ohta K, Endo T, Haraguchi K, et al. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J Clin Endocrinol Metab 2001;86:2170-7. [PubMed]
- Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol 2004;5:419-29. [Crossref] [PubMed]
- Klopper JP, Hays WR, Sharma V, et al. Retinoid X receptor-gamma and peroxisome proliferator-activated receptor-gamma expression predicts thyroid carcinoma cell response to retinoid and thiazolidinedione treatment. Mol Cancer Ther 2004;3:1011-20. [PubMed]
- Park JW, Zarnegar R, Kanauchi H, et al. Troglitazone, the peroxisome proliferator-activated receptor-gamma agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid 2005;15:222-31. [Crossref] [PubMed]
- Fröhlich E, Machicao F, Wahl R. Action of thiazolidinediones on differentiation, proliferation and apoptosis of normal and transformed thyrocytes in culture. Endocr Relat Cancer 2005;12:291-303. [Crossref] [PubMed]
- Hayashi N, Nakamori S, Hiraoka N, et al. Antitumor effects of peroxisome proliferator activate receptor gamma ligands on anaplastic thyroid carcinoma. Int J Oncol 2004;24:89-95. [PubMed]
- Aiello A, Pandini G, Frasca F, et al. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology 2006;147:4463-75. [Crossref] [PubMed]
- Marlow LA, Reynolds LA, Cleland AS, et al. Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res 2009;69:1536-44. [Crossref] [PubMed]
- Antonelli A, Ferrari SM, Fallahi P, et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf) 2009;70:946-53. [Crossref] [PubMed]
- Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomed Pharmacother 2008;62:559-63. [Crossref] [PubMed]
- Smallridge RC, Copland JA, Brose MS, et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab 2013;98:2392-400. [Crossref] [PubMed]
- Wu H, Sun Y, Ye H, et al. Anaplastic thyroid cancer: outcome and the mutation/expression profiles of potential targets. Pathol Oncol Res 2015;21:695-701. [Crossref] [PubMed]
- Brauner E, Gunda V, Vanden Borre P, et al. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016;7:17194-211. [Crossref] [PubMed]
- Iyer PC, Dadu R, Gule-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer 2018;6:68. [Crossref] [PubMed]
- Chiappori AA, Kolevska T, Spigel DR, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol 2015;26:354-62. [Crossref] [PubMed]
- Xu Y, Goldkorn A. Telomere and Telomerase Therapeutics in Cancer. Genes (Basel) 2016;7:E22. [Crossref] [PubMed]
- Donati B, Lorenzini E, Ciarrocchi A. BRD4 and Cancer: going beyond transcriptional regulation. Mol Cancer 2018;17:164. [Crossref] [PubMed]
- Catalano MG, Pugliese M, Gallo M, et al. Valproic Acid, a Histone Deacetylase Inhibitor, in Combination with Paclitaxel for Anaplastic Thyroid Cancer: Results of a Multicenter Randomized Controlled Phase II/III Trial. Int J Endocrinol 2016;2016:2930414. [Crossref] [PubMed]
- Yang L, Zhang Y, Shan W, et al. Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci Transl Med 2017;9:eaal1645.
- Nagayama Y, Shimamura M, Mitsutake N. Cancer Stem Cells in the Thyroid. Front Endocrinol (Lausanne) 2016;7:20. [Crossref] [PubMed]
- Malaguarnera R, Frasca F, Garozzo A, et al. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J Clin Endocrinol Metab 2011;96:766-74. [Crossref] [PubMed]
- Heiden KB, Williamson AJ, Doscas ME, et al. The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression. J Clin Endocrinol Metab 2014;99:E2178-87. [Crossref] [PubMed]
- Tseng LM, Huang PI, Chen YR, et al. Targeting signal transducer and activator of transcription 3 pathway by cucurbitacin I diminishes self-renewing and radiochemoresistant abilities in thyroid cancer-derived CD133+ cells. J Pharmacol Exp Ther 2012;341:410-23. [Crossref] [PubMed]
- Hombach-Klonisch S, Natarajan S, Thanasupawat T, et al. Mechanisms of therapeutic resistance in cancer (stem) cells with emphasis on thyroid cancer cells. Front Endocrinol (Lausanne) 2014;5:37. [Crossref] [PubMed]
- Vicari L, Colarossi C, Giuffrida D, et al. Cancer stem cells as a potential therapeutic target in thyroid carcinoma. Oncol Lett 2016;12:2254-60. [Crossref] [PubMed]
- Sherbet GV. Notable Approaches to Cancer Immunotherapy in Molecular Approach to Cancer Management. 1st edition. Newcastle: Elsiever, 2017.
- Schürch CM, Roelli MA, Forster S, et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019;29:979-92. [Crossref] [PubMed]
- Newell DR. Flasks, fibres and flanks--pre-clinical tumour models for predicting clinical antitumour activity. Br J Cancer 2001;84:1289-90. [Crossref] [PubMed]
- Schroyens W, Tueni E, Dodion P, et al. Validation of clinical predictive value of in vitro colorimetric chemosensitivity assay in head and neck cancer. Eur J Cancer 1990;26:834-8. [Crossref] [PubMed]
- Blumenthal RD, Goldenberg DM. Methods and goals for the use of in vitro and in vivo chemosensitivity testing. Mol Biotechnol 2007;35:185-97. [Crossref] [PubMed]
- Ferrari SM, Fallahi P, La Motta C, et al. Recent advances in precision medicine for the treatment of anaplastic thyroid cancer. Expert Rev Precis Med Drug Dev 2019;4:37-49. [Crossref]
- Antonelli A, Ferrari SM, Fallahi P, et al. Evaluation of the sensitivity to chemotherapeutics or thiazolidinediones of primary anaplastic thyroid cancer cells obtained by fine-needle aspiration. Eur J Endocrinol 2008;159:283-91. [Crossref] [PubMed]
- Antonelli A, Ferrari SM, Fallahi P, et al. Primary cell cultures from anaplastic thyroid cancer obtained by fine-needle aspiration used for chemosensitivity tests. Clin Endocrinol (Oxf) 2008;69:148-52. [Crossref] [PubMed]
- Ferrari SM, La Motta C, Elia G, et al. Antineoplastic Effect of Lenvatinib and Vandetanib in Primary Anaplastic Thyroid Cancer Cells Obtained From Biopsy or Fine Needle Aspiration. Front Endocrinol (Lausanne) 2018;9:764. [Crossref] [PubMed]
- Wächter S, Wunderlich A, Roth S, et al. Individualised Multimodal Treatment Strategies for Anaplastic and Poorly Differentiated Thyroid Cancer. J Clin Med 2018;7:E115. [Crossref] [PubMed]
- Sasanakietkul T, Murtha TD, Javid M, et al. Epigenetic modifications in poorly differentiated and anaplastic thyroid cancer. Mol Cell Endocrinol 2018;469:23-37. [Crossref] [PubMed]