Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation
Introduction
α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II) have been used as important tumor markers in the diagnosis of hepatocellular carcinoma (HCC) (1,2). Several groups have recently reported correlations between AFP, PIVKA-II level and histopathological finding such as tumor size and microvascular invasion (MVI) (3,4). These findings may indicate that pretreatment serum AFP and PIVKA-II levels have an important role on patient outcomes, because MVI increases the risk of intrahepatic metastasis or distant recurrence (5,6). Indeed, previous studies demonstrated that a high AFP and PIVKA-II levels at the time of HCC diagnosis are associated with poor prognosis (7-9). Moreover, these high tumor marker levels have been considered as a predictor of recurrence in patients treated with conventional treatments such as surgical resection, transarterial chemoembolization (TACE) and percutaneous radio-ablation therapy (RFA) (6,10,11). Recently, living donor liver transplantation (LDLT) is the one of the effective treatments of HCC (12-14). Many centers have recently proposed new extended criteria beyond Milan criteria (MC) for HCC based on tumor number and size (15-17). However, only a few studies have been made to clarify the usefulness of preoperative AFP and PIVKA-II level as predictors of HCC recurrence after LDLT (6,18). We thought that AFP and PIVKA-II levels before liver transplantation can provide additional important information related to the recurrence of HCC and patient outcomes after LDLT. Hence, we performed this study analyzing the utility of AFP and PIVKA-II as predictors of recurrence of HCC after LDLT.
Methods
Patients
Between May 2007 and December 2013, 1,570 patients underwent adult LDLT at the Asan Medical Center (AMC). Of these, 461 patients with HCC who received LDLT using hemi-liver graft were analyzed in this study, excluding 16 patients with incidental HCC. Of the 461 patients, 275 patients (59.7%) had a history of previous treatment for HCC using various non-operative methods including TACE, RFA, percutaneous ethanol injection therapy (PEIT), radiotherapy (RTx) and chemotherapy (CTx). These treatments were not intended for a bridge or down-staging before LDLT, but performed for curative managements. Since 2008, we have adopted Asan criteria as an inclusion criterion of LDLT for HCC that maximal tumor size and number is 5 cm and 6 nodules, respectively. Patients with extrahepatic metastases or major vascular invasion of HCC on preoperative imaging work-up were contraindicated for LDLT. The standard immunosuppression protocol which is consisted of tacrolimus, mycophenolate mofetil (MMF) and corticosteroids, the latter of which were usually tapered off 3 months after LT. There was no difference in immunosuppression protocol between HCC and non-HCC recipients after LDLT at our institution. All patients were followed up regularly in the same team of surgeons. No patients were lost to follow-up. The last census date for this study was April 2016. The median duration of follow-up was 55 months (range, 1–108 months). The study was approved by institutional review board of University of Ulsan College of Medicine (S2014-0898-0010).
Staging classification
Staging of HCC was based on pre-transplantation imaging work-up. We performed evaluation of tumor extent basically by using dynamic liver transplantation computed tomography (LTCT) was held within 1 month before LDLT. Tumor staging was determined by counting and measurement of only viable and enhancing nodules on LTCT.
Preoperative measurement of serum AFP and PIVKA-II levels
AFP and PIVKA-II were measured within 7 days before liver transplantation. The upper normal ranges of AFP and PIVKA-II in our institution are 7.5 nag/mol and 40 maul/mol, respectively.
Receiver operating characteristic (ROC) analysis
ROC analysis was used to evaluate the ability of tumor variables to predict postoperative recurrence and to choose the optimal cut-off value for subsequent analysis. For indication of LDLT, high specificity is necessary to avoid excluding a large number of patients who would not develop recurrence. We therefore defined the optimal cut-off value as the point showing the highest C-index among values with specificity ≥0.85 (17).
Statistical analysis
Cumulative overall survival (OS) and recurrence free survival (RFS) rates were calculated using Kaplan-Meier methods, and differences between curves were evaluated using log-rank testing. The χ2 test or Student’s t-test was used to compare differences between subgroups. The Cox proportional hazards model was used to identify the independent risk factors for recurrence. The values of P<0.05 were considered significant. All statistical analyses were performed using the SPSS (version 20; IBM, New York, NY, USA).
Results
Preoperative patient profile
Patient profiles and preoperative clinical characteristics are shown in Table 1. The median age for the 461 patients (395 men, 66 women) was 54 (range, 31–69) years. Hepatitis B (HBV) was the most common original liver disease, followed by hepatitis C (HCV). The median serum PIVKA-II was 27.0 (5.0–19,400.0) maul/mol, and the median serum AFP was 10.9 (0.36–42,200.0) nag/mL. Thirty four percent of patients with HCC had a normal PIVKA-II level (<40 maul/mol) and 40.1% had a normal AFP level (<7.5 nag/mol). The median size and the number of HCCs detected on preoperative CT scan were 2.6 cm (range, 0.5–11.0 cm) and 2 nodules (range, 1–31 nodules), respectively. TACE was the most common treatment (54.4%) among pretransplant managements of HCC.
Full table
Postoperative HCC recurrence and ROC curve analysis
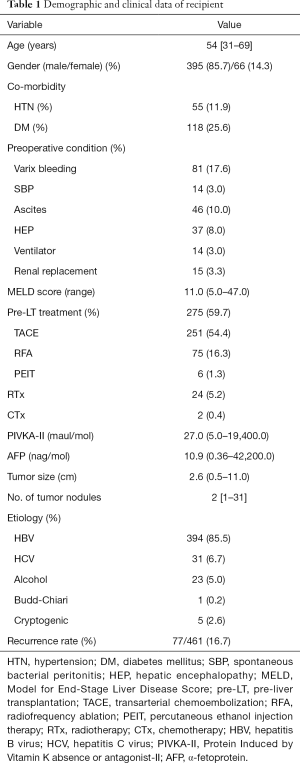
Postoperative recurrence of HCC was identified in 77 (16.7%) patients. The ability of preoperative tumor variables to predict HCC recurrence was analyzed by ROC curves. Areas under the curve (AUCs) for PIVKA-II, AFP, tumor size and tumor number were 0.62, 0.59, 0.72 and 0.70, respectively (Figure 1). AUC was largest for tumor size followed by that of number of tumor nodules, although no significant differences were seen among the four variables. Among the cut-off values with sufficient specificity, the cut-off point with the highest C-index was chosen as the optimal cut-off value for subsequent analysis. The selected cut-off values were 100 maul/mol for PIVKA-II [(C-index, sensitivity, specificity) = (0.71, 0.55, 0.93)], 150 nag/mol for AFP (0.68, 0.46, 0.90), 5 cm for tumor size (0.62,0.29, 0.95) and 3 for tumor number (0.60, 0.23, 0.97).
Patients outcome after adult LDLT
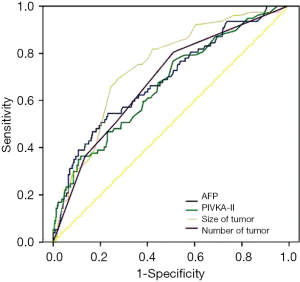
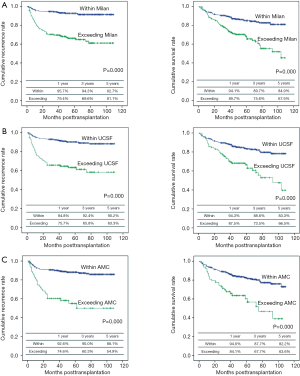
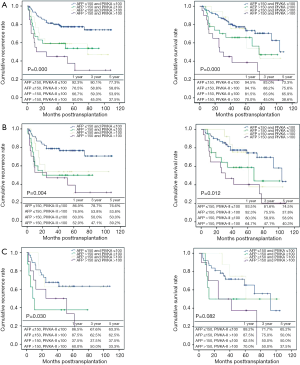
Overall patient survival and RFS rates at 1-, 3-, and 5-year were 92.6%, 85.05%, 79.2% and 90.2%, 85.9%, 83.7%, respectively. Five-year survival rates were 84.6% for patients within MC and 70.2% for those beyond MC (P=0.000). Five-year survival rates were 83.3% for patients within University of California and San Francisco (UCSF) and 66.5% for those beyond UCSF (P=0.000). Five-year survival rates were 82.2% for patients within AMC and 63.6% for those beyond AMC (P=0.000) (Figure 2). By using cut-off level of AFP and PIVKA-II, OS and RFS of four subgroups at 1-, 3-, 5-year are shown in Figure 3. The 1-, 3-, 5-year OS and RFS of AFP >150 ng/mL and, PIVKA-II>100 mAU/mL group were the lowest, 73.9%, 47.8%, 42.5% and 52.2%, 47.8%, 41.8%, respectively. The OS and RFS of AFP<150 ng/mL and PIVKA-II <100 mAU/mL group were 94.7%, 89.1%, 83.0% and 96.1%, 92.2%, 91.9%, respectively.
Preoperative risk factors for recurrence
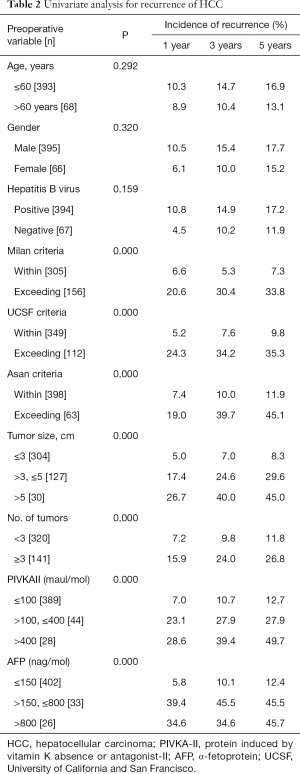
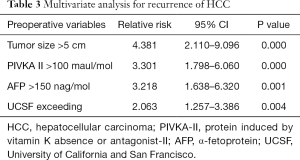
For the total of 461 patients, overall cumulative recurrence rate was 16.7% at 5 years. Univariate analysis was performed to identify risk factors for recurrence among preoperative variables. In univariate analysis, MC (P<0.05), UCSF criteria (P<0.05), AMC criteria (P<0.05), tumor size >5 cm (P<0.05), number of tumors ≥3 (P<0.05), PIVKA-II >100 mAU/mL (P<0.05) and AFP >150 ng/mL (P<0.05) were identified as significant risk factors for recurrence (Table 2). The multivariate Cox regression analyses revealed four variables including tumor size >5 cm, PIVKA-II >100 mAU/mL, AFP >150 ng/mL, and UCSF criteria were independently significant risk factors for the recurrences. Tumor size >5 cm was the strongest predictor of recurrence (relative risk =4.381), followed by PIVKA-II >100 mAU/mL, AFP >150 ng/mL, and UCSF criteria (Table 3).
Full table
Full table
Discussion
Several liver transplant centers have recently proposed new expansion criteria of LDLT for HCC patient mainly based on tumor number and size because MC is too strict and exclude many HCC patients who might have long term survival after liver transplantation (19-21). Biologic tumor markers, such as AFP and PIVKA-II might be additional useful variables to optimize the criteria that can predict the risk of recurrence, more precisely. Several groups have identified MVI and tumor size as independent predictors of recurrence after liver transplantation (18,22). However, histological information such as MVI is difficult to know prior to LDLT. The preoperative needle biopsy is not recommended because of the risk of tumor seeding along the biopsy tract or tumor rupture (23). If there is a way to obtain histological information prior to LDLT without invasive methods, patient prognosis could be predicted more accurately. We routinely measured preoperative serum AFP and PIVKA-II levels, which might be correlated with histological results of the explanted liver and also with recurrence of HCC after LDLT. ROC analysis revealed that the abilities of preoperative tumor size to predict HCC recurrence after LDLT tended to be superior to PIVKA-II, AFP and number, but it was not shown difference significantly. According to C-index analysis based on ROC, optimal cut-off values to predict recurrence were set at 100 maul/mol for PIVKA-II, 150 nag/mol for AFP, 5 cm for tumor size and 3 for tumor number. Among significant variables for the recurrence after LDLT in univariate analysis, tumor size, number, AFP and PIVKA-II were significant in multivariate analysis. These findings indicate that the HCC patients with AFP >150 nag/mol and/or PIVKA-II >100 maul/mol might have aggressive tumor characteristics having higher chance of vascular invasion. PIVKA-II is known to be related with the development of portal invasion and early intrahepatic recurrence after HCC treatment such as TACE or RFA (17,24-26). In addition, the biologic tumor markers significantly correlate with tumor size and status of the MVI on χ2 test (P<0.001). In this study, tumor size and MVI were risk factors for recurrence in univariate analysis, but MVI was not analyzed in multivariate analysis because preoperative factors alone should be used for preoperative patient selection. These correlations between preoperative tumor markers and histological findings indicated that elevation of tumor markers can be important predictors of HCC recurrence after LDLT among preoperative variables. By using preoperative AFP and PIVKA-II levels, we might select patients having higher chance of recurrence among HCC patients belonging to the indication criteria of LDLT for HCC, and also select patients having lower chance of recurrence among HCC patients beyond the indication criteria. Based on our study, HCC patients with PIVKA-II ≤100 maul/mol and AFP ≤150 nag/mol preoperatively might have least chance of recurrence after for LDLT regardless of tumor morphology on LDCT.
Posttransplant OS and RFS rate above indication criteria according to four tumor marker groups was shown in Figure 4. Although patients with HCC did not satisfy the previously mentioned criteria, the 1-, 3-, 5-year OS and RFS was 94.5%, 83.0%, 73.3% and 92.3%, 80.1%, 77.3% in beyond Milan criteria (MC), 93.5%, 81.6%, 74.5% and 86.9%, 78.7%, 76.6% in beyond UCSF criteria, 89.2%, 71.7%, 65.2% and 86.5%, 67.6%, 63.3% in beyond AMC criteria when preoperative AFP and PIVKA-II levels meet both AFP ≤150 ng/mL and PIVKA-II ≤100 mAU/mL. The number of patients satisfying both AFP ≤150 ng/mL and PIVKA-II ≤100 mAU/mL despite beyond indication criteria were 92/156 (59.8%) in of MC, 62/112 (55.4%) in UCSF, 37/63 (59.7%) in AMC (Table 4). These result indicated that more than half of the patient with HCC of beyond criteria could be the candidate for LDLT when the preoperative level of tumor marker was within PIVKA-II ≤100 mAU/mL and AFP ≤150 ng/mL.

Full table
In conclusion, not only tumor morphology related to tumor size and MVI obtained by explant pathology but also the preoperative AFP and PIVKA-II level can give us important information about the post-transplant prognosis after LDLT. Highly selected HCC patients satisfying both PIVKA-II <100 AU/mol and AFP<150 ng/mol can be indicated for LDLT regardless of morphologic appearance of tumor on LDCT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of University of Ulsan College of Medicine (S2014-0898-0010).
References
- Peng SY, Chen WJ, Lai PL, et al. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer 2004;112:44-50. [Crossref] [PubMed]
- Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology 2003;37:1114-21. [Crossref] [PubMed]
- Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2007;95:235-40. [Crossref] [PubMed]
- Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int 2013;2013:310427.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330-9. [Crossref] [PubMed]
- Hakamada K, Kimura N, Miura T, et al. Des-gamma-carboxy prothrombin as an important prognostic indicator in patients with small hepatocellular carcinoma. World J Gastroenterol 2008;14:1370-7. [Crossref] [PubMed]
- Hamamura K, Shiratori Y, Shiina S, et al. Unique clinical characteristics of patients with hepatocellular carcinoma who present with high plasma des-gamma-carboxy prothrombin and low serum alpha-fetoprotein. Cancer 2000;88:1557-64. [Crossref] [PubMed]
- Kim HS, Park JW, Jang JS, et al. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: a prospective study. J Clin Gastroenterol 2009;43:482-8. [Crossref] [PubMed]
- Suehiro T, Sugimachi K, Matsumata T, et al. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer 1994;73:2464-71. [Crossref] [PubMed]
- Nanashima A, Morino S, Yamaguchi H, et al. Modified CLIP using PIVKA-II for evaluating prognosis after hepatectomy for hepatocellular carcinoma. Eur J Surg Oncol 2003;29:735-42. [Crossref] [PubMed]
- Kim DY, Paik YH, Ahn SH, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology 2007;72 Suppl 1:52-7. [Crossref] [PubMed]
- Mazzaferro V, Chun YS, Poon RT, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001-7. [Crossref] [PubMed]
- Hwang S, Lee SG, Joh JW, et al. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl 2005;11:1265-72. [Crossref] [PubMed]
- de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist 2007;12:1321-31. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765-74. [Crossref] [PubMed]
- Fujiki M, Takada Y, Ogura Y, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant 2009;9:2362-71. [Crossref] [PubMed]
- Parfitt JR, Marotta P, Alghamdi M, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl 2007;13:543-51. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832-8. [Crossref] [PubMed]
- Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer 2000;88:538-43. [Crossref] [PubMed]
- Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol 2005;100:2708-16. [Crossref] [PubMed]
- Takamori R, Wong LL, Dang C, et al. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl 2000;6:67-72. [PubMed]
- Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol 2000;32:269-78. [Crossref] [PubMed]
- Koike Y, Shiratori Y, Sato S, et al. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer 2001;91:561-9. [Crossref] [PubMed]
- Kaibori M, Ishizaki M, Matsui K, et al. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol 2010;102:462-8. [Crossref] [PubMed]


