Therapeutic options for intrahepatic cholangiocarcinoma
Introduction
Biliary tract cancer (BTC) is composed of a group of neoplasms, which include intrahepatic cholangiocarcinoma (ICCA), extrahepatic cholangiocarcinoma (ECCA), gallbladder cancer and ampullary carcinoma. Although anatomically BTC arise from a contiguous anatomical region, with improved understanding of molecular biology and genetics, it has become evident that this is a very heterogeneous disease. Collectively however, BTC is associated with universally high morbidity and mortality given the diagnosis at a later stage for most (1). In the United States, it is estimated that in 2016 there will be 39,230 new cases and five year survival less than 20% (2).
ICCA is a rare tumor that arises in the peripheral branches of endothelial cells in the biliary tree and accounts for up to 30% of all primary liver malignancies (3,4). It is the second most common primary liver cancer with an increasing incidence over the past few decades, with approximately two-thirds of patients presenting with advanced or metastatic disease (5). Surgical resection is the mainstay for potentially curative therapy in patients with resectable disease. Even with optimal surgery, the 5-year overall survival (OS) and recurrence rates are 15–40% and 50–60% respectively, with a median disease free interval of 26 months necessitating the need for chemotherapy and radiation (6,7). Factors that are associated with survival and tumor recurrence are the presence of multiple primary tumors, vascular invasion and lymph node metastasis. Presence of lymph node positive disease is seen in about 22–37% and is associated with worse outcomes (7,8). Cytotoxic chemotherapy is a standard part of therapy for recurrent or metastatic disease.
Treatment options
Staging and survival of ICCA
ICCA is often diagnosed with locally advanced or metastatic disease. In patients who are diagnosed with early stage disease, the only potential curative treatment is partial hepatectomy (9). Long-term survival at 5-year is reported to be 20–40% following aggressive resection (10). Even with adequate resection, more than 50% of patients experience recurrence of tumor in the first or second year post resection (11). Factors associated with an increased survival after resection include R0 resection, mass-forming tumor types, no lymph node involvement, low tumor burden, CA 19-9 lower than 100 IU/mL, absence of satellite nodules, absence of lymphovascular involvement, and absence of perineural invasion (11).
The Role of adjuvant therapy in resectable or locally advanced ICCA
Given the high incidence of recurrence following adequate resection, adjuvant treatment strategies to diminish the risk of recurrence include chemotherapy and/or radiation. There is a noticeable absence of phase III trials supporting the use of adjuvant therapy in patients following surgical resection. Results of a large multi-center randomized phase III (BILCAP) evaluating the role of adjuvant capecitabine in bile duct or gallbladder cancer are expected in 2017. Currently, treatment strategies are mostly based on trials consisting of BTC from any origin. A recent meta-analysis evaluated the role of adjuvant chemotherapy, chemoradiation, or radiation in BTC (12). The study suggested that patients who received either chemotherapy or chemoradiation had a better outcome than those who received radiation alone [odds ratio (OR) 0.39, 0.61, and 0.98, respectively; P=0.02]. A sub-group analysis showed that patients who had lymph node positive disease (OR 0.49; P=0.004) and R1 resection (OR 0.36; P=0.002) had the greatest benefit with chemotherapy or chemoradiation as adjuvant therapy. However, ICCA was underrepresented with only 11 of 6,700 cases were of primary intrahepatic disease.
Consistent with the findings above, another study suggested that adjuvant therapy might benefit patients with adverse prognostic features (lymph node metastasis or blood vessel invasion). In this study, patients who received adjuvant therapy with gemcitabine, 5-fluorouracil (5-FU) and cisplatin (GFP) had a longer progression-free survival (PFS) at 1 year compared to those who did not (71.6% vs. 45%, P<0.02) (13). Similarly, another retrospective review of the role of adjuvant therapy in patients who had an R0 resection for intrahepatic and perihilar cholangiocarcinoma was recently conducted (14). In this study, 80 patients (58.4%) had ICCA and 35 patients (25.5%) had lymph node involvement. A total of 73 (53.3%) of patients received adjuvant therapy with chemotherapy, chemoradiation or radiation alone. The authors found that lymph node involvement (HR: 3.60; P<0.001), tumor differentiation (HR: 2.35; P=0.048) and an elevated baseline CA 19-9 (HR: 1.97; P=0.013) were all predictors of worse OS. Patients who received adjuvant chemoradiation had a longer recurrence free survival (RFS) (HR: 0.44; P=0.036) but no benefit in OS (HR: 0.56; P=0.245) in lymph node negative disease. However, there was a numerical trend for improvement in OS with adjuvant chemoradiation in patients with lymph node positive disease (HR: 0.24; P=0.097). In contrast chemotherapy alone did not appear to improve RFS or OS, and radiation therapy was associated with a shorter RFS and OS. Review of this data, suggests that the addition of radiation to chemotherapy may provide a benefit in patients with an R0 resection and lymph node positive disease, however prospective studies will need to determine the validity of this observation.
To better understand the role of radiotherapy following surgical resection, another analysis on patients from the National Cancer Database [1998–2011] was performed (15). In this retrospective analysis, a total of 405 patients were stratified based on resection margin status. Survival for R0 vs. R1/R2 was 32 vs. 16.5 months respectively (P<0.001) and radiotherapy appeared to have a trend in improved survival for R1/R2 thought not significant (P=0.191). Furthermore, in a multivariable model accounting for age, sex, comorbidities, disease stage and resection margins, radiotherapy did not appear to be a predictor for improved survival. The authors from this study concluded that radiotherapy alone does not significantly impact survival in patients with positive margins.
Liver transplantation
The role of liver transplantation in ICCA remains highly controversial with conflicting published data. ICCA has been consistently shown to have a 20–40% OS at 3–5 years after liver transplant in numerous studies. Though it is not currently an established indication for liver transplantation, it represents a potential curative option for those who do not meet strict criteria for resection (16-18). The Mayo protocol consisting of the combination of neoadjuvant chemoradiation with 5-FU followed by brachytherapy with 5-FU has been shown to have significant improvements, where 92% of patients are disease free at 37 months (19). Hong et al. described improved outcomes with the use of neoadjuvant/adjuvant chemotherapy combined with liver transplantation for ICCA (20). The authors divided patients in to three categories (low, intermediate, high) based on a prognostic scoring system composed of seven clinico-pathological risk factors (multifocal tumor, perineural invasion, infiltrative subtype, lack of neoadjuvant and/or adjuvant therapy, history of primary sclerosing cholangitis, hilar cholangiocarcinoma and lymphovascular invasion). The authors reported a 5-year RFS rate of 78% in low risk group, compared with 19% in intermediate, and 14% in high risk groups, suggesting that liver transplant is appropriate in select cases of locally advanced ICCA. More recently, a retrospective multicenter study demonstrated that liver transplantation for select cirrhotic patients with small solitary lesions (up to 2 cm) achieved a 5-year survival rate of 73% (21). Furthermore, at a median follow-up of 36 months, none of the patients had a tumor recurrence. While the data is inconclusive about the role of orthotopic liver transplantation (OLT) in patients with ICCA, but given its curative intent, liver transplantation seems as a reasonable option in ideal patients who are fit with good performance status, minimal co-morbidities and of young age. Prospective, randomized clinical trials are needed to confirm the benefits of OLT in ICCA. At least for now, we know that patients with vascular and/or lymphatic invasion and/or tumor size larger than 2 cm are likely to have a higher recurrence rate and may not benefit from OLT (22).
Therapy options for unresectable disease
In patients who present with advanced or metastatic disease, systemic chemotherapy is a standard approach in the treatment of ICCA. The Advanced Biliary Cancer (ABC-02) Trial, the largest randomized phase III study conducted in BTC, evaluated the combination of gemcitabine with or without cisplatin. Patients had a diagnosis of unresectable, recurrent, or metastatic BTC that included intrahepatic or exhepatic cholangiocarcinoma, gallbladder cancer and ampullary carcinoma. The study demonstrated that the addition of cisplatin significantly improved both PFS (8 months compared to 5 months; HR: 0.63; 95% CI: 6.6–8.6; P=0.001) and OS (11.7 months compared to 8.1 months; HR 0.64; 95% CI: 0.52–0.80; P=0.001) (23). The survival benefit was significant in all sub-groups including patients with ICCA. Similar results were observed in a Japanese randomized phase II trial (24). Based on this data, the combination of gemcitabine + cisplatin is now the current standard of care for first line therapy.
Further number of phase II studies suggested that oxaliplatin added to gemcitabine (GEMOX) may have comparable historical outcomes than cisplatin with a range of reported OS of 8.3 to 11 months (25-29). A recent randomized phase II trial suggested that GEMOX may be superior to 5-FU or best supportive care (BSC) alone (OS 9.5 vs. 4.5 vs. 4.6 months respectively, P=0.032) (30). Moreover, a pooled analysis done by Eckel et al. (31) suggested that chemotherapy with gemcitabine combined with cisplatin or oxaliplatin increases the response rate and tumor control rate in cholangiocarcinoma. A number of smaller studies evaluated the role of numerous chemotherapies in combination with gemcitabine and were shown signs of interesting activity with response rates approximately ranging between 20% and 40% (32-35) (Table 1).
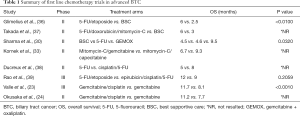
Full table
Second-line therapy
In patients who progress on gemcitabine and platinum based therapy, there is no standard option. Several trials evaluating regimens for ICCA in the second-line setting show promising activity (Table 2). Though all of the trials had few patients, mOS had ranged from 4–9 months suggesting that treatment in the refractory setting is feasible and safe, and may provide a survival advantage over BSC.
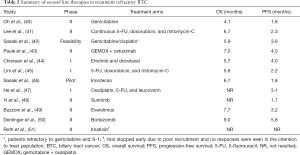
Full table
Emerging molecularly targeted therapy
Cytotoxic chemotherapy is effective and provides a benefit in the majority of patients, however its efficacy is short-lived and all patients will eventually develop resistance. With better understanding of tumor biology and molecular pathways involved in ICCA, the use of novel, targeted agents is at the forefront and provides additional treatment options. Targeted therapies are more likely to be selective for malignant cells, thus result in better efficacy and perhaps better tolerability. With the use of next generation sequencing, there is a better understanding of the landscape of ICCA.
Epidermal growth factor receptor (EGFR) inhibitors
EGFR is an extracellular receptor tyrosine kinase that when bound to its ligand causes a downstream autophosphorylation leading to activation of intracellular signaling cascades. It is overexpressed in 10–32% of patients ICCA. Overexpression of this pathway promotes intracellular activity, resulting in cell proliferation, angiogenesis and evasion of apoptosis (52). In patients with ICCA, increased activation of EGFR is associated with impaired OS and associated with presence of lymph node metastasis (53).
Erlotinib is an oral, reversible tyrosine kinase inhibitor that inhibits activation of EGFR. An initial study suggested that erlotinib monotherapy has interesting activity in BTC (54). However, when combined with other agents, the results have been relatively disappointing. The addition of erlotinib to GEMOX or bevacizumab was mostly disappointing and not historically much different than GEMOX or bevacizumab alone (55,56).
Cetuximab and panitumumab are EGFR directed monoclonal antibodies. An initial single arm study suggested a potential survival benefit with panitumumab, where the combination of gemcitabine, irinotecan and panitumumab in patients with cholangiocarcinoma resulted in a median PFS of 9.7 months and OS of 12.9 months (57). However, a large, randomized phase II trial, BINGO, failed to confirm the efficacy of anti-EGFR monoclonal antibodies, where the combination of cetuximab with gemcitabine and oxaliplatin failed to improve patient outcomes (58). This was a small, non-randomized study and future randomized studies are needed to better understand the role of anti-EGFR therapy. Similar disappointing findings were observed when panitumumab was combined with GEMOX followed by capecitabine in patients with ICCA or ECCA who were KRAS WT (59).
Human growth factor receptor 2 (HER-2/neu) as a target
HER-2/neu promotes cell growth, survival and motility. When amplified or overexpressed, HER-2/neu has been shown to have a pivotal role in the development and progression of solid tumor malignancies in general. Inhibition of HER-2/neu has been shown to be effective in gastric and breast cancers (60,61); however this has not been demonstrated in BTC. There have been two phase II trials evaluating lapatinib as monotherapy in biliary malignancies suggesting no meaningful activity observed (62,63). Contrarily, results from My Pathway study, from ASCO, evaluated six patients with HER-2/neu amplification or overexpression and found a clinical benefit in all patients (50% CR/PR, 50% with SD >120 days). This is a small study and prospective studies need to be done to validate these findings.
Vascular endothelial growth factor (VEGF) inhibitors
VEGF pathway is a potent stimulator of angiogenesis, highly expressed in BTCs and associated with a more aggressive phenotype (64). Bevacizumab is a humanized monoclonal antibody which inhibits VEGF and has been evaluated in BTC. A single arm phase II study of bevacizumab with GEMOX in patients with advanced BTC showed a median PFS of 7 months and OS of 12.7 months (65). These findings suggested that targeting VEGF might be a potential relevant therapeutic strategy in BTC. Based upon these results, ABC-03, a randomized phase II trial was conducted investigating the combination of chemotherapy with or without cediranib, an oral VEGF receptor inhibitor in patients with advanced biliary cancer, with its primary endpoint being PFS. One hundred and twenty four patients were enrolled in this study, of which 29 patients had ICCA (14 in cediranib and 15 in placebo). The results, unfortunately, were disappointing with no significant difference observed between the two arms (8 months in cediranib vs. 7.4 months in placebo; P=0.72) (66). Currently, VEGF inhibition does not appear to be a relevant therapeutic target in BTC. Furthermore, a recent randomized phase II study evaluated VEGF vs. EGFR inhibition with combination chemotherapy in patients with KRAS WT non-resectable BTC (67). In this study, patients were randomized to either bevacizumab or panitumumab in a crossover design. The study showed no significant difference in the blockade of either target.
MET inhibitors
The binding of hepatocyte growth factor (HGF) to its receptor (c-MET) activates signaling through pathways such as RAS/MAPK and PI3K/AKT that are important in proliferation and survival of tumor cells. Gene expression profiling revealed that c-MET overexpression is found in 20–60% of patients with ICCA and is associated with poor prognosis (68). Further, there is some suggestion that overexpression of c-MET can lead to EGFR resistance; thus leading to the use of c-MET inhibitors in cholangiocarcinoma (69). A recent study evaluated the combination of tivantinib (ARQ 197) with gemcitabine in patients with solid tumors (70). In this study, 20% of the patients achieved a partial response including one patient with cholangiocarcinoma. Another trial evaluated the use of cabozantinib in 19 cholangiocarcinoma patients where c-MET expression was unknown and found no objective responses (71). Overall, the role of inhibitors of c-MET is unknown in this ICCA.
Isocitrate dehydrogenase (IDH) inhibitors
A mutation in isocitrate dehydrogenase 1/2 (IDH1/2) causes formation of onco-metabolites that reduce alpha-ketoglutarate to 2-hydroxyglutarate leading to altered cell maturation, differentiation and survival. IDH1 and 2 mutations exist in numerous tumors and were recently identified in BTC. Further, IDH1/2 were evaluated in a large cohort of ICCA patients and was found to be associated with better OS (72). In contrast, patients with cholangiocarcinoma and IDH1/2 mutations had shorter OS compared to those who are wild type (3-year survival of 33% in IDH mutants vs. 81% in IDH wild-type) (73). The frequency of these mutations ranges from 22–36% in ICCA and is associated with clear cell or poorly differentiated histology (74,75). Pre-clinical studies suggest that IDH mutations inhibit hepatocyte differentiation, induce proliferation of hepatic progenitors and ultimately leading to the development of premalignant lesions (76). IDH inhibitors are currently being evaluated in clinical trials (Table 3).
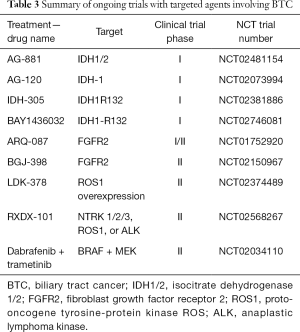
Full table
Fibroblast growth factor receptor (FGFR) inhibitors
Fibroblast growth factor receptor 2 (FGFR2) is a member of the FGFR and is associated with cell differentiation, proliferation and apoptosis (77). Recently with the use of whole exome sequencing, FGFR2 alterations were identified in 6–50% of ICCA (78). Alterations in FGFR2 pathway occur in 13% of patients with ICCA and are associated with improved survival (79). Pre-clinical data and isolated case reports suggest that there is anti-tumor activity with FGFR inhibition; however, the therapeutic benefit it currently unknown. Recently, it was reported that patients with FGFR mutated disease had similar response with first line chemotherapy as those without FGFR mutation (80). The authors also reported that when patients were treated with FGFR directed inhibitors after failure of first-line chemotherapy; those with FGFR mutation had a superior OS (P=0.010). A recent study reported results of BGJ398 (a oral small molecule pan-FGFR inhibitor) in patients who failed or were intolerant to platinum-based therapy (81). Of the 22 patients who were evaluable at the time of analysis, 3 achieved a partial response, 15 patients had stable disease and overall disease control rate was 82%. This suggests that ICCA patients with FGFR mutations may have a benefit from treatment with an FGFR inhibitor.
ROS1 inhibitors
ROS1 is a proto-oncogene belonging to the subfamily of tyrosine kinase receptors and increased expression can be seen in up to 9% of patients with ICCA (82). Preclinical data shows that inhibition of ROS1 can lead to potent anti-tumor effects (83). The benefit of ROS1 inhibition has been confirmed in non-small lung cancer (84). Similar studies are needed in patients with ICCA to confirm the benefit of targeted therapy in patients ROS fusions.
BRAF inhibitors
BRAF is a proto-oncogene, which is involved in directing cell growth. The incidence of BRAF mutations in ICCA has been reported to be about 5% (85). Its presence is associated with a more aggressive phenotype, higher tumor stage and likelihood of lymph node involvement at diagnosis (86). At this time, the benefit of BRAF inhibition with small molecules is unknown but could represent an interesting treatment option, especially in combination with other targeted therapies.
Conclusions
Patients with early stage ICCA and adverse prognostic features may benefit from adjuvant chemotherapy with a possible added benefit from radiation therapy. For locally advanced or metastatic ICCA standard treatment consists of the combination of gemcitabine with cisplatin. With the use of next generation sequencing, actionable alterations have been identified and can be matched with molecularly targeted therapies that hold promise to improve outcomes. Future trials will continue to focus on dissecting the molecular landscape of ICCA to identify novel potential therapeutic targets. As newer therapies emerge, it will be important to identify potential biomarkers that will help select patients who are most likely to benefit from combined therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008;13:415-23. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology and End Results Program. U.S. Population Data - 1969-2014. Available online: http://www.seer.cancer.gov/popdata
- Bartella I, Dufour JF. Clinical Diagnosis and Staging of Intrahepatic Cholangiocarcinoma. J Gastrointestin Liver Dis 2015;24:481-9. [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 2016. [Epub ahead of print]. [PubMed]
- Yoh T, Hatano E, Nishio T, et al. Significant Improvement in Outcomes of Patients with Intrahepatic Cholangiocarcinoma after Surgery. World J Surg 2016;40:2229-36. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 2007;11:1488-96; discussion 1496-7. [Crossref] [PubMed]
- Simo KA, Halpin LE, McBrier NM, et al. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J Surg Oncol 2016;113:62-83. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Morine Y, Shimada M, Imura S, et al. Treatment strategy for intrahepatic cholangiocarcinoma: From optimal surgical management to adjuvant therapy. J Clin Oncol 2016;34:abstr 392.
- Kim YS, Park I, Oh SY, et al. The role of adjuvant therapy after R0 resection for patients with intrahepatic and perihilar cholangiocarcinoma. J Clin Oncol 2016;34:abstr 302.
- Hammad AY, Younan G, Rajeev R, et al. Radiotherapy for intrahepatic cholangiocarcinoma: An analysis of the National Cancer Database. J Clin Oncol 2016;34:abstr 379.
- Becker NS, Rodriguez JA, Barshes NR, et al. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg 2008;12:117-22. [Crossref] [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [Crossref] [PubMed]
- Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl 2001;7:1023-33. [Crossref] [PubMed]
- De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl 2000;6:309-16. [Crossref] [PubMed]
- Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9. [Crossref] [PubMed]
- Sapisochin G, Rodríguez de Lope C, Gastaca M, et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7. [Crossref] [PubMed]
- Sapisochín G, Fernández de Sevilla E, Echeverri J, et al. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol 2015;7:2396-403. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [Crossref] [PubMed]
- André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862-7. [Crossref] [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 2006;95:848-52. [Crossref] [PubMed]
- Jang JS, Lim HY, Hwang IG, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol 2010;65:641-7. [Crossref] [PubMed]
- Wagner AD, Buechner-Steudel P, Moehler M, et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase-II trials. Br J Cancer 2009;101:1846-52. [Crossref] [PubMed]
- Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581-6. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 2006;5:110-4. [PubMed]
- Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol 2004;15:478-83. [Crossref] [PubMed]
- Ridwelski K, Fahlke J, Kuhn R, et al. Multicenter phase-I/II study using a combination of gemcitabine and docetaxel in metastasized and unresectable, locally advanced pancreatic carcinoma. Eur J Surg Oncol 2006;32:297-302. [Crossref] [PubMed]
- Williams KJ, Picus J, Trinkhaus K, et al. Gemcitabine with carboplatin for advanced biliary tract cancers: a phase II single institution study. HPB (Oxford) 2010;12:418-26. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Takada T, Nimura Y, Katoh H, et al. Prospective randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C for non-resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology 1998;45:2020-6. [PubMed]
- Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer 2005;41:398-403. [Crossref] [PubMed]
- Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer 2005;92:1650-4. [Crossref] [PubMed]
- Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2011;29:1066-72. [Crossref] [PubMed]
- Lee S, Oh SY, Kim BG, et al. Second-line treatment with a combination of continuous 5-fluorouracil, doxorubicin, and mitomycin-C (conti-FAM) in gemcitabine-pretreated pancreatic and biliary tract cancer. Am J Clin Oncol 2009;32:348-52. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs 2011;29:1488-93. [Crossref] [PubMed]
- Paule B, Herelle MO, Rage E, et al. Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 2007;72:105-10. [Crossref] [PubMed]
- Chiorean EG, Ramasubbaiah R, Yu M, et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101. Oncologist 2012;17:13. [Crossref] [PubMed]
- Lim KH, Han SW, Oh DY, et al. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology 2012;83:57-66. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. A pilot study of salvage irinotecan monotherapy for advanced biliary tract cancer. Anticancer Res 2013;33:2619-22. [PubMed]
- He S, Shen J, Sun X, et al. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother 2014;26:243-7. [Crossref] [PubMed]
- Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012;48:196-201. [Crossref] [PubMed]
- Buzzoni R, Pusceddu S, Bajetta E, et al. Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: a phase II ITMO study. Ann Oncol 2014;25:1597-603. [Crossref] [PubMed]
- Denlinger CS, Meropol NJ, Li T, et al. A phase II trial of the proteasome inhibitor bortezomib in patients with advanced biliary tract cancers. Clin Colorectal Cancer 2014;13:81-6. [Crossref] [PubMed]
- Roth A, Schleyer E, Schoppmeyer K, et al. Imatinib mesylate for palliative second-line treatment of advanced biliary tract cancer: a bicentric phase II study. Onkologie 2011;34:469-70. [Crossref] [PubMed]
- Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 2002;7 Suppl 4:2-8. [Crossref] [PubMed]
- Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98:418-25. [Crossref] [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069-74. [Crossref] [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [Crossref] [PubMed]
- Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 2013;24:3061-5. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Jensen LH, Lindebjerg J, Ploen J, et al. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann Oncol 2012;23:2341-6. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Peck J, Wei L, Zalupski M, et al. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology 2012;82:175-9. [Crossref] [PubMed]
- Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009;64:777-83. [Crossref] [PubMed]
- Quan ZW, Wu K, Wang J, et al. Association of p53, p16, and vascular endothelial growth factor protein expressions with the prognosis and metastasis of gallbladder cancer. J Am Coll Surg 2001;193:380-3. [Crossref] [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [Crossref] [PubMed]
- Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015;16:967-78. [Crossref] [PubMed]
- Jensen LK, Fernebro E, Ploen J, et al. Randomized phase II crossover trial exploring the clinical benefit from targeting EGFR or VEGF with combination chemotherapy in patients with non-resectable biliary tract cancer. J Clin Oncol 2015;33:abstr 4071.
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [Crossref] [PubMed]
- Marquardt JU, Andersen JB. Next-generation sequencing: application in liver cancer-past, present and future? Biology (Basel) 2012;1:383-94. [Crossref] [PubMed]
- Pant S, Saleh M, Bendell J, et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol 2014;25:1416-21. [Crossref] [PubMed]
- Goyal L, Yurgelun MB, Abrams TA, et al. A phase II trial of cabozantinib (XL-184) in patients with advanced cholangiocarcinoma. J Clin Oncol 2015;33:abstr 800.
- Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene 2013;32:3091-100. [Crossref] [PubMed]
- Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470-3. [Crossref] [PubMed]
- Grassian AR, Pagliarini R, Chiang DY. Mutations of isocitrate dehydrogenase 1 and 2 in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2014;30:295-302. [Crossref] [PubMed]
- Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol 2012;43:1552-8. [Crossref] [PubMed]
- Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 2014;513:110-4. [Crossref] [PubMed]
- Tiong KH, Mah LY, Leong CO. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis 2013;18:1447-68. [Crossref] [PubMed]
- Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 2014;45:1630-8. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- Jain A, Shroff RT, Kelley RK, et al. FGFR pathway genetic aberrations in cholangiocarcinoma: Demographics and experience with targeted therapy. J Clin Oncol 2016;34:abstr 109.
- Javle MM, Shroff RT, Zhu A, et al. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. J Clin Oncol 2016;34:abstr 335.
- Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One 2011;6:e15640. [Crossref] [PubMed]
- Saborowski A, Saborowski M, Davare MA, et al. Mouse model of intrahepatic cholangiocarcinoma validates FIG-ROS as a potent fusion oncogene and therapeutic target. Proc Natl Acad Sci U S A 2013;110:19513-8. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Santoro A, Gebbia V, Pressiani T, et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol 2015;26:542-7. [Crossref] [PubMed]
- Robertson S, Hyder O, Dodson R, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol 2013;44:2768-73. [Crossref] [PubMed]


