Surgical options for intrahepatic cholangiocarcinoma
Introduction
Intrahepatic cholangiocarcinoma (ICC) is one of the hepatobiliary malignancies arising from epithelial cells of the small intrahepatic ductules or large intrahepatic ducts that are proximal to the bifurcation of the hepatic ducts, even hepatocytes (1). Distinguished by anatomic location, cholangiocarcinoma can be classified as intrahepatic, perihilar, or distal. Although the annual incidence of ICC remains low during recent decades, it has dramatically increased from 0.32 per 100,000 in 1975 to 1 per 100,000 in 2000, making it the second most common primary liver cancer (2). ICC tends to be advanced upon diagnosis due to difficulties in detection and treatment. Despite advances in modern surgery and medical technology, survival after curative surgical resection for ICC remains low because it is difficult to achieve tumor-free margins due to tumor locations and technical challenges. Either local or distant recurrence may hamper the resectability of ICC in a large number of patients. Lymph node involvement and vascular invasion are considered negative predictive factors for survival of ICC patients. In the following sections, we will review the epidemiology and staging of ICC, and highlight the selection of surgical modalities and postoperative outcomes of ICC patients.
Epidemiology
Approximately 35,660 new cases of primary liver and intrahepatic bile duct cancer are diagnosed each year in the United States (3), of which about 15% are ICC. Data from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program suggest that the overall incidence of ICC is 0.95 cases per 100,000 adults (4,5). The incidence of ICC in Europe, North America, Asia, Japan and Australia has been rising over the past two decades (5-7), with the highest incidence of 96 per 100,000 men reported in Thailand (8). Some of these changes can be attributed to the alteration in disease classification (9) as supported by the evidence of concurrent drop in the incidence of extrahepatic cholangiocarcinoma. Some also suggested that part of the increase in ICC may be due to the advantages in modern diagnostic modalities that could identify early lesions and biliary malignancies that were undiagnosed previously (10). However, a study has demonstrated that the increase in the incidence of ICC is independent of the increased proportion of early-stage ICC or smaller size and un-staged diseases (5). For instance, the increased incidence might be associated with a rise in certain newly recognized risk factors such as viral hepatitis and non-viral chronic liver diseases (11). Like other biliary tract malignancies, the incidence of ICC increases with age, peaking between 55 and 75 years old, and is slightly higher in males than that in females (5).
A number of pathologies may affect the biliary system to produce chronic biliary inflammation, bile stasis and cirrhosis, thus predisposing individuals to the development of ICC and other biliary malignancies. Intrahepatic lithiasis, primary sclerosing cholangitis (PSC), congenital abnormalities of the bile ducts, parasite infection and toxic exposure all have been associated with an increased risk of ICC (12,13). It is also worth to note that chronic liver diseases such as viral infection and cirrhosis are now recognized as risk factors for cholangiocarcinoma, especially ICC. In addition, some recent studies have revealed an increased link between the risk of ICC and metabolic abnormalities, such as type II diabetes and obesity (14), as well as thyrotoxicosis and chronic pancreatitis (15).
Diagnosis
A large proportion of patients with ICC are asymptomatic, and the lesions are often detected incidentally by radiologic examinations for other purposes (16). Patients may exhibit right upper abdominal pain, weight loss, and elevation of alkaline phosphatase (ALP) level, but rarely fever or jaundice. In addition, elevation of gamma-glutamyl transpeptidase (GGT), 5'-nucleotidase (5'NT), and tumor markers CA19-9, CEA and CA-125 can be observed in some ICC patients. Imageologically, ICC lesions usually present as malignant-appearing masses in cirrhotic or non-cirrhotic livers. Contrast-enhanced CT scan and MRI with MR cholangiopancreatography (MRCP) can be useful in differentiating ICC from HCC when intrahepatic metastatic diseases are ruled out, the lesion site is precisely located, and biliary duct involvement is confirmed. CT scan is useful in detecting intrahepatic tumors, the level of biliary obstruction, and the presence of liver atrophy or hypertrophy. Multiphasic contrast-enhanced multidetector-row CT (MDCT) can also help identify causes of intrahepatic bile duct strictures, and assess the tumor stage and resectability of ICC (17). The typical radiographic characteristics of ICC include a hypodense hepatic lesion without a capsule, and distal biliary dilatation. Capsular retraction could be seen in individual patients with fibrotic tumors. Rim enhancement can be seen in both arterial and venous phases after administration of the contrast agent (17). The enhancement pattern in some ICC cases, especially those with small sizes, can be similar to that in HCC (18). ICC often appears as a hypointense lesion on MR T1-weighted images, and a heterogeneous hyperintense lesion on T2-weighted images (19). After gadoxetic acid enhancement, the lesion may appear as a target-shaped mass with a lobulated shape and weak rim. CT and MRI/MRCP may have some value in identifying metastatic tumors in the most common sites such as lymph nodes, peritoneum, lungs and pleura. Meanwhile, positron emission tomography with fluorodeoxyglucose (FDG-PET) scan may play an important role in preoperative evaluation of potentially occult metastatic disease (20).
Tumor staging
A variety of ICC staging systems have been proposed, but all of them are based on western populations. The main difference between these staging systems is the factors included in T staging classification. The latest AJCC/UICC staging system (7th edition) assesses the predictive factors for ICC separately, which is a significant improvement as compared with the previous staging systems in which ICC and HCC are both regarded as “primary liver cancer”. In addition, the 7th edition no longer regards tumor size as a prognostic factor. Instead, it considers the number of lesions, vascular invasion, intrahepatic metastasis, and adjacent tissue invasion as the important factors affecting the T stage of ICC (21). Staging of ICC is a systematic process that starts with the evaluation of local invasion by imaging study of the abdomen, or the chest and pelvis in some cases. A PET scan can be considered if no distant metastasis, extra-regional node involvement or critical adjacent structural invasion is detected by routine examinations. Previously, the staging criteria for HCC were applied to ICC, knowing that ICC is a relatively rare malignancy and the etiological and pathological characteristics of ICC are not well documented (8). However, it has been realized that ICC represents a distinct hepatobiliary malignancy that is different from HCC in both carcinogenesis and biological behaviors. Therefore, it should be investigated by using ICC-specific staging systems and prognostic models. Currently, four models for TNM staging and prognostic assessment of ICC have been developed based on oriental populations, including the Okabayashi staging system (22), the Liver Cancer Study Group of Japan (LCSGJ) staging system (2), and the Prognostic Score of Fudan University and the Nomogram of Eastern Hepatobiliary Surgery Hospital in China (23,24). Among them, LCSGJ defines three gross pathological types of ICC: mass-forming (MF) type, periductal infiltrating (PI) type, and intraductal growth type (IG) (22). The MF type is the most common type, accounting for 86% of all ICC cases, and the other two are very uncommon. The two staging systems in Japan are specific for the MF type ICC. In addition, other than the TNM staging system, several new prognostic models have been reported both in Eastern and Western worlds, including scoring systems and related nomograms. These prognostic models include more comprehensive contributing factors affecting prognosis as compared with the TNM staging system, and therefore their predictive power for prognosis is improved significantly. For instance, nomograms can integrate and graphically display the risk factors affecting tumor prognosis, thus giving a probability value to the occurrence of clinical events, based on which decisions on individualized treatment and adjuvant therapy can be made. The result of prognostic assessment of a broad range of tumors has shown that as compared with the conventional TNM staging system, the predictive performance of nomograms is enhanced at least in terms of precision (24,25). However, some authors believe that the system needs to be improved to increase the accuracy of prognostic stratification, and that clinicians should include tumor size, cancer cell differentiation and other prognostic predictors in the current AJCC criteria (26).
Surgical management
Surgical resection is the most effective treatment for ICC at present, but its resectability and curability remain low. Unlike HCC, most ICC cases have poor blood supply and rare liver cirrhosis. Thus extended hepatectomy is often required, including bloc resection with resection of the vessel, bile duct and adjacent tissue invaded by the tumor in some cases (27). The extent of resection should be determined by the size and location of the lesion, satellite situation, and the degree of tumor infiltration. R0 resection is the only curative treatment for ICC at present. The ICC lesion is frequently multifocal due to the tendency of high invasiveness, node metastasis and vascular invasion, which are the main reasons for poor long-term survival of ICC patients after resection. It was found that nearly 30% ICC patients could no longer be treated with curative resection because of intrahepatic metastasis involving more than two lobes or peritoneal metastasis during laparotomy or laparoscopic exploration (27-31). Some studies reported that the 5-year overall survival (OS) rate was 39–41% in patients with negative margins and 0% in those with positive margins (32-35). Hence, aggressive surgical strategies aiming to achieve a R0 resection are vital for long-term survival of ICC patients including those with relapsed ICC. Prognosis after resection is affected by the size and number of lesions, lymph node metastasis, satellite, vascular invasion (hepatic vein or portal vein), margins and other factors. Lymph node metastasis is an important prognostic factor, but there are still controversies over whether routine lymph node dissection improves the survival outcome. It was reported that the long-term survival of ICC patients after liver resection is associated with clinical pathological factors including no clinical symptoms, an early tumor stage, the papillary mass forming type and postoperative chemotherapy (33,36-42).
Preoperative evaluation and staging laparoscopy
Radical surgery should ensure a complete tumor resection and negative resection margin invasion. The prognosis of ICC patients is closely related to whether radical resection can be performed. However, the radical resection rate of ICC is only 15–20%, which is far lower than 70% for distal bile duct carcinoma (34). Preoperative clinical data and radiographic data should be collected to evaluate the potential of radical tumor resection, while the physical status score, nutritional condition and morbidities should also be considered. Albumin and total bilirubin levels can be used to predict the risk of postoperative hepatic failure, and preoperative serum albumin <3 g/dL and bilirubin >10 mg/dL often indicate poor prognosis of ICC patients (35). Preoperative treatment includes preoperative jaundice-reducing and PVE, respectively for patients with obstructive jaundice caused by hilar bile duct invasion and patients with postoperative residual liver volume deficiency. ICC invading the secondary or above biliary branch is considered to be one of the contraindications to surgical resection. But for some patients with jaundice who have been carefully evaluated for the invasion of the hilar bile duct and received R0 hepatic resection treatment, preoperative biliary drainage to reduce bilirubin can reduce the occurrence of postresectional hepatic insufficiency. The criteria for evaluation of tumor resection include the extent of intra- or extrahepatic bile duct invasion, vascular invasion, liver lobe atrophy, local and distant metastasis. Preoperative biliary drainage should be considered for patients with obstructive jaundice caused by tumor invasion, and preoperative portal vein embolization (PVE) can be performed for patients with future liver remnant (FLR) volume less than 30%, knowing that it can promote the compensatory proliferation of the reserved residual liver and reduce postoperative complications and mortality of the patients (43). Portal vein invasion is known as an independent risk factor for unresectable tumors, but many signs, such as peritoneal implantation, intrahepatic metastasis, lymph node invasion and the actual tumor invasive range sometimes cannot be accurately predicted by preoperative radiological presentations until abdominal exploration is performed. Laparoscopic or mini laparotomy can effectively evaluate the patient’s tumor stage and unresectability. There is no guide to confirm whether ICC patients who receive accurate preoperative prediction of respectability can benefit from liver resection, but a recent study shows that an appropriate treatment option can be obtained by combining nomograms including preoperative factors and a regret-based decision curve analysis (44). Imaging examinations, including abdominal CT and various forms of (MR, endoscopic or transhepatic) cholangiopancreatography are helpful in the diagnosis and staging of ICC. PET scan may be done to detect possible occult metastasis.
The role of staging laparoscopy in the management of ICC has not been well documented, but it may be useful in ruling out small peritoneal implants before proceeding with a laparotomic incision. Approximately 36% ICC cases were found to be unresectable due to laparoscopic detection of peritoneal or intrahepatic metastasis (31). In addition, the presence of intrahepatic metastasis and vascular invasion may be detected with a combination of ultrasound during operation.
Lymphadenectomy
Lymphatic metastasis is a significant prognostic factor in ICC. The role of routine lymphadenectomy is still controversial. Few data regarding the benefits of lymphadenectomy for ICC have been reported. Some studies suggest that routine lymphadenectomy should be performed to provide better prognostic assessment and reduce local recurrences (45,46). The 2015 expert consensus on ICC treatment recommends that regional lymphadenectomy should be performed as a standard part of surgical therapy due to high incidence of node metastasis, its prognostic importance and the potential therapeutic benefit in decreasing locoregional recurrence (47). Despite the fact that lymph node involvement is an important prognostic factor for ICC, lymphadenectomy does not appear to provide proven therapeutic benefits, and there is a lack of consensus as to whether or not it should be routinely performed (21,33). There is evidence that the lymph node dissection group failed to achieve better prognosis as compared with the control group, mainly due to uncontrolled postoperative intrahepatic metastasis and lymph node metastasis, which indicates late stage and metastatic lesions beyond the scope of lymphadenectomy. As is reported that lymph node metastasis has negative impact on prognosis and the incidence of node metastasis is as high as 40%, approximately 55% of patients have pathologic evaluation of at least one regional LN (27,33). According to our experience, lymphadenectomy should be performed regardless whether local positive lymph nodes are detected or not by preoperative examinations or during surgery, because it can help identify the clinical stage and guide postoperative therapy although the postoperative pathological positive rate is not high. Nevertheless, there are certain risks that should be weighed against the benefits to perform portal lymph node dissection such as common bile duct devascularization, knowing that it is likely to cause complications. Meanwhile, lymph node metastasis and hepatic venous invasion are also negative prognostic factors for resected ICC.
Surgical resection
The surgical treatment for ICC by radical hepatic resection is to achieve negative resection margins (48). Anatomic resection with wide margins is recommended for ICC if adequate functional liver remnant remains (49). Major liver resections, such as hemi-hepatectomy or extended hepatic resection, as well as resections and reconstruction of the bile drainage may be needed in a considerable proportion of ICC patients [over 70% for hemi-hepatectomy or extended hepatic resection (43) and over 20% for biliary resection and reconstruction (27)]. Laparoscopic or robotic liver resection might be an option for suitable patients with T1 or T2 stage ICC, which is associated with a less need for Pringle maneuver and less blood loss (50). Margin-free resections are very challenging in patients with locally advanced tumors or large tumors (33). Nevertheless, resection should not be attempted in patients with unresectable disease based on stage assessment. ICC is considered unresectable in the presence of intrahepatic or distant metastases, invasion or encasement of major vessels, or extensive regional lymph nodes (51).
For non-cirrhotic patients whose predicted residual liver volume after resection is less than 25% or cirrhotic patients whose residual liver volume is less than 40%, selective embolization of the target branches of the portal vein can promote remnant liver proliferation, thus achieving a lower incidence of postoperative complications and mortality.
Although surgical resection remains the gold standard for ICC treatment, local ablation, including radiofrequency ablation and microwave ablation, can achieve good clinical efficacy in the treatment of small tumors or recurrent multiple tumors. So far no prospective controlled study has shown significant difference in the treatment of ICC between minimally invasive procedures and routine liver resection. Local ablation is mainly for unresectable or recurrent tumors and should follow the indication of percutaneous ultrasound-guided ablation. It is reported that PRFA operated by experienced experts can obtain better or equivalent results of surgical resection, with damage rate as high as 90% after the initial treatment, 1-year OS rate of 80–100%, and 5-year OS rate of 20% (52,53). Prognostic factors of RFA include tumor size, lymph node invasion and tumor differentiation. Tumor diameter less than 5cm is an important factor for the effective rate and good survival rate of ablation.
Neoadjuvant chemotherapy can reduce the tumor stage and increase the surgical resection rate for ICC with local progression and regional lymph node metastasis. Chemotherapy regimens like cisplatin plus gemcitabine for treatment of pancreatic cancer have a certain effect on metastatic patients and can prolong the postoperative survival rate, though there is no prospective study to verify its effect on down-stage surgery (54-57).
Postoperative outcomes
The 5-year OS rate of ICC patients after R0 resection is 15–40% and 80% of them has intrahepatic recurrence (58,59),which is worse than that of HCC. The reason may lie in the histological difference between HCC and ICC. The invasion of lymph nodes, nerves, extrahepatic portal vein, perihilar vein and lymph node metastasis accounts for 85%, 80%, 58%, 40% and 37% respectively, similar to that of perihilar cholangiocarcinoma and distal cholangiocarcinoma (60). In addition, the proportion of intrahepatic portal vein invasion, hepatic vein invasion and intrahepatic metastasis is similar to that of HCC (27,32,61). The rate of postoperative complications is 11–58%, including bile leakage, hepatic dysfunction, abdominal infection, and portal vein embolism. The rate of perioperative mortality is 1.2–7%, commonly due to liver failure, septic shock, and multiple organ dysfunction. In conclusion, ICC is characterized by features of both HCC and cholangiocarcinoma, which lead to worse prognosis (29,38,62).
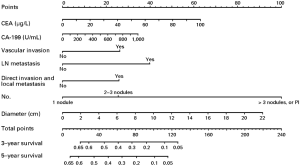
Long-term outcomes vary among ICC patients who receive curative resection and are related to the location and extent of the primary lesion, surgical margin status, and potential complications. Some studies have reported improved survivals over time for the past few years, and pointed out that this change might result from the improved non-surgical therapies or more careful selection of candidates for surgical resection (63). The postoperative outcome of ICC patients mainly depends on the disease stage (especially the status of lymph node involvement and vascular invasion) rather than tumor size, as well as the surgical margin status (64,65). The postoperative 5-year OS rate is generally about 40% (66,67), but a better survival rate as high as 63% could be achieved in patients with negative margins (R0 resections) and negative lymph node involvement (30,68,69). The stage-stratified 5-year survival based on the 7th edition of the AJCC staging system in a French study of 163 patients underwent potentially curative surgery was reported to be 32% for all patients enrolled, 62% for stage I (T1N0), 27% for stage II (T2N0), and 14% for stage III (T3N0, T1-3, N1) (70). Portal vein tumor thrombus was demonstrated to be an independent risk factor for OS (HR 1.783; 95% CI: 1.28–2.49) in ICC patients underwent liver resection (71). A retrospective study reviewed the data from 74 ICC patients treated with surgical resection and found that normal postoperative CA19-9 level could be a predictor of longer survival outcomes, compared with those with persistently high CA19-9 level (72). A propensity score matching analysis showed that the use of nomograms is a good way to predict survival after resection. One nomogram has been developed for ICC, including tumor (T) and nodal (N) classifications, tumor size, the number of tumor nodules, preoperative level of serum tumor markers, and vascular invasion (24) (Figure 1). It is reported by a multicenter retrospective study that the long-term prognosis after hepatectomy for elderly people is similar to that for young people, but the postoperative complication rate is significantly higher.
Recurrence is an important negative factor affecting the prognosis of ICC patients. It is reported that despite curative resection of ICC, recurrence may occur in 79% patients at 5 years (28). Local recurrence is the most common pattern after R0 resection (73), although the other patterns such as intrahepatic, nodal, or extrahepatic distant (intraperitoneal) recurrences/metastases were also observed (74). A study utilizing an international database investigated 563 patients underwent curative-intent resection for ICC with a median follow-up of 19 months and found that the most common recurrence site was intrahepatic only (59.8%), extrahepatic only (14.5%), or both intra- and extrahepatic (25.7%) (75). Unlike HCC in which recurrences mainly occur in the liver, the profile of ICC recurrence is consistent with its characteristics as a systemic disease (32,58,76). Patients with recurrent ICC were found to be associated with worse outcomes. The median survival from the time of recurrence is about 11.1 months in patients treated with different therapies vs. 26.7 months in patients underwent resection of the recurrent tumors (75). Intrahepatic metastasis is the most common event in metastatic ICC patients. Surgery remains optional if the metastatic lesion is solitary and patients are at affordable general condition. Despite small patients size in the publication, 9–30% post-operation recurrences can be successfully re-resected (75,77,78). In our experience, with close post-operation monitoring, a significant proportion of recurrences can be detected at early stages and liver re-resection is safe for patients with RICC after initial liver resection, showing relatively good outcomes in selected patients. With the development and progress in chemotherapy, ablation, embolization, radiation and other treatment choices for ICC, improved survival has been seen in patients with recurrent diseases after surgery who have undergone adjuvant chemotherapy or multimodal treatments including locoregional therapies such as radiofrequency ablation, transarterial Yttrium 90 microsphere radiotherapy, and TACE (79,80).
Extended hepatectomy
Based on the above considerations, complete surgical resection is a vital part of measures to ensure a favorable prognosis. For ICC patients, it means an anatomical hepatectomy or a combined resection with vascular structures and surrounding organs. It has been reported that large tumor size, intrahepatic metastasis, lymph node metastasis, and vascular invasion are associated with poor prognosis of patients after surgical resection of ICC. The association between resection margin width and prognosis (a narrow margin is associated with a poor prognosis) is more evident in ICC patients than in HCC patients. Therefore, R0 resection with preservation of an adequate margin presents a significant challenge for surgeons in ICC patients with a large tumor burden, although a multi-centre study demonstrated the safety and efficacy of extended hepatectomy in patients with advanced ICC, larger tumors or multiple lesions (81). Pawlik et al. reported surgical excision of ICC in a cohort of 557 patients. They assigned 215 patients to Group A with lesion size <7 cm and a solitary tumor, and the remaining 342 patients to Group B with advanced tumor, lesion size >7 cm, and/or more than two lesions. The result showed that the proportion of patients receiving extended hepatectomy in Group B was higher than that in Group A (16.9% vs. 30.4%; P<0.001). In addition, their postoperative pathology demonstrated that vascular invasion, direct invasion of contiguous organs, and nodal metastasis were more common in Group B than in Group A. The incidence of postoperative complications and hospital mortality were similar between the two groups. Patients in group A showed improved 5-year OS and disease-free survival (DFS) as compared with patients in Group B. For patients with multiple lesions (more than 3) or lymph node metastasis, especially those with truncus coeliacus and paraaortic lymph node metastasis, the indication of extended hepatectomy should be carefully assessed, and adjuvant therapies like chemotherapy or transarterial treatment may be considered before further treatment (28). Therefore, even though the effect of resection margins on prognosis of ICC remains controversial (82-84), we recommend that anatomical hepatectomy should be conducted for patients with adequate FLR. When gross liver resection is not feasible because of inadequate FLR or remnant liver function, a resection margin of 1 cm should be the goal.
Combined vascular resection is also one of the strategies to achieve a R0 resection and has been conducted in 9–14% of radical hepatectomy patients (27,29,30). Vascular resection in combination with hepatectomy increases not only the probability to achieve negative surgical margins (28) but also the incidence of postoperative major complications. Therefore, hepatectomy in combination with inferior vena cava and portal vein partial resection plus reconstruction or hepatic artery resection may be considered in adequately assessed cases to achieve a R0 resection. Combined organ resection comprises hepatectomy in combination with resection of adjacent organs like the gall bladder, extrahepatic biliary tract, diaphragm and pancreas and may be performed in patients with invaded biliary junctions, and patients underwent hepatectomy in combination with extrahepatic biliary tract resection and reconstruction (27,85-88). Further clinical data are needed to confirm the long-term efficacy in such patients.
Surgery-centered multidisciplinary team (MDT)
With actively evolving tumor treatment modalities and concepts, and knowing that optimal efficacy can rarely be achieved by a single treatment regimen, MDT as a collaborative health care model has been increasingly recognized. Unlike traditional health care models, the MDT model is characterized by patient-centered and MDT-dependent integration of multiple care models, through which optimal diagnosis and treatment regimens for ICC patents are performed to improve survival. In surgically resected ICC patients, the role of MDT is established throughout the course of treatment. For patients who could not achieve a negative margin, postoperative radiation therapies have been reported to prolong survival (89,90). For instance, in a cohort of 38 patients with tumors removed from the surface of vessels leaving almost no resection margin, DFS (12.5 vs. 5.5 m; P=0.081) and OS (21.8 vs. 15 m; P=0.049) were improved through post-operative intensity modulated radiotherapy (IMRT) (90). Currently, post-operative treatment regimens of combined modality therapy involving multiple disciplines are decided based on the pathological characteristics, the extent of local invasion and pathological stage classification of the neoplasm. Exploration of the anti-recurrence or early recurrence effect of post-operative TACE, radiotherapy, chemotherapy and ablation is ongoing, with some reports showing survival to be improved in patients with positive margins or lymph nodes and early recurrence (79,80,89-91), although the results require further investigation with prospective randomized control studies due to the relatively small sample size and low evidence grades at present.
For downstage resection of ICC, or stage-two surgical resection following neoadjuvant chemotherapy in patients with locally advanced tumors, it has been shown from the perspective of net health benefit (NHB) that for patients with large ICC tumors or vascular invasion, upfront hepatic resection was more cost-effective than systemic chemotherapy (92). But initial systemic chemotherapy followed by possible curative hepatic resection is more cost-effective than upfront hepatic resection for patients with multi-focal ICC (92). ICCs are seldom sensitive to chemotherapy with a low response rate, although it is reported that cisplatin and gemcitabine may improve survival (54). Further prospective studies are required.
Liver transplantation (LT)
Due to the lack of standard indications and highly controversial outcomes, LT is not recommended as a routine procedure for ICC (58). LT for ICC is no longer performed in many centers due to unsatisfactory long-term survival and high recurrence rates. However, some recent studies reported that ICC patients with small solitary tumors had acceptable or even excellent long-term survival after LT (93,94). Without concomitant chemotherapies, the 3-year survival of ICC patients underwent LT ranges between 50% and 65% (95,96), while patients underwent systematic adjuvant or neoadjuvant therapy achieved better long-term survival (97). Commonly reported adverse prognostic factors for LT include perineural invasion, multifocality, infiltrative tumor growth, lymphovascular invasion (98,99), and a history of PSC (100). Meanwhile, recent studies (99,101) found that certain ICC patients, particularly those with small solitary tumors or well-differentiated ICCs, could have more favorable long-term survival after LT. In contrast, moderately-differentiated ICC might be associated with a high recurrence rate and poor survival (102). In summary, LT is not completely ineffective, but its controversial indications and low cost-effectiveness may limit its use in treatment of ICC.
In summary, ICC is among the most common hepatic malignancies second to HCC and the number of ICC patients is increasing. A variety of risk factors including infections, environments and metabolism have been established for ICC. Surgical resection is a well-defined and the only curative option for eligible patients at present, while other treatment strategies like local-regional and systemic therapies can provide more alternatives for unresectable cases for prevention and treatment of recurrences.
Acknowledgements
Funding: This study was supported by the State Key Project on Infectious Diseases of China (2012ZX10002–016 to F Shen) and the National Natural Science Foundation of China (81071867, 81201939 to K Wang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 2012;122:2911-5. [Crossref] [PubMed]
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37:806-13. [Crossref] [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [Crossref] [PubMed]
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77-82. [Crossref] [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [Crossref] [PubMed]
- Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol 2000;19:156-76. [Crossref] [PubMed]
- Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6. [Crossref] [PubMed]
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. [Crossref] [PubMed]
- Lipsett PA, Pitt HA, Colombani PM, et al. Choledochal cyst disease. A changing pattern of presentation. Ann Surg 1994;220:644-52. [Crossref] [PubMed]
- Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76. [Crossref] [PubMed]
- Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221-8. [Crossref] [PubMed]
- Brown KM, Parmar AD, Geller DA, et al. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2014;23:231-46. [Crossref] [PubMed]
- Valls C, Guma A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging 2000;25:490-6. [Crossref] [PubMed]
- Kim SA, Lee JM, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern-correlation with clinicopathologic findings. Radiology 2011;260:148-57. [Crossref] [PubMed]
- Ciresa M, De Gaetano AM, Pompili M, et al. Enhancement patterns of intrahepatic mass-forming cholangiocarcinoma at multiphasic computed tomography and magnetic resonance imaging and correlation with clinicopathologic features. Eur Rev Med Pharmacol Sci 2015;19:2786-97. [PubMed]
- Ringe KI, Wacker F. Radiological diagnosis in cholangiocarcinoma: Application of computed tomography, magnetic resonance imaging, and positron emission tomography. Best Pract Res Clin Gastroenterol 2015;29:253-65. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 2001;92:2374-83. [Crossref] [PubMed]
- Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 2011;22:1644-52. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. New York: Springer, 2002.
- Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB (Oxford) 2015;17:244-50. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Hechtman JF, et al. Outcomes following resection of intrahepatic cholangiocarcinoma. HPB (Oxford) 2015;17:344-51. [Crossref] [PubMed]
- Ali SM, Clark CJ, Zaydfudim VM, et al. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:2023-8. [Crossref] [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern and outcomes. J Am Coll Surg 2001;193:384-91. [Crossref] [PubMed]
- Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subjects of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 2006;20:721-5. [Crossref] [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Poultsides GA, Zhu AX, Choti MA, et al. Intrahepatic cholangiocarcinoma. Surg Clin North Am 2010;90:817-37. [Crossref] [PubMed]
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009;69:259-70. [Crossref] [PubMed]
- Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep 2013;29:1259-67. [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651-8. [Crossref] [PubMed]
- Lang H, Sotiropoulos GC, Sgourakis G, et al. Operations for intrahepatic cholangiocarcinoma: Single-institution experience of 158 patients. J Am Coll Surg 2009;208:218-28. [Crossref] [PubMed]
- Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, et al. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB (Oxford) 2011;13:612-20. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: Prognostic factors after surgical resection. World J Surg 2009;33:1247-54. [Crossref] [PubMed]
- Saxena A, Chua TC, Sarkar A, et al. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: A 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg 2010;14:1128-38. [Crossref] [PubMed]
- Gomez D, Morris-stiff G, Toogood GJ, et al. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2008;97:513-8. [Crossref] [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Bagante F, Spolverato G, Cucchetti A, et al. Defining when to offer operative treatment for intrahepatic cholangiocarcinoma: A regret-based decision curves analysis. Surgery 2016;160:106-17. [Crossref] [PubMed]
- Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 2016;18:79-87. [Crossref] [PubMed]
- Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol 2015;50:913-27. [Crossref] [PubMed]
- Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736-750.e4. [Crossref] [PubMed]
- Lafaro K, Grandhi MS, Herman JM, et al. The importance of surgical margins in primary malignancies of the liver. J Surg Oncol 2016;113:296-303. [Crossref] [PubMed]
- Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40. [Crossref] [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma–a population-based study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Fu Y, Yang W, Wu W, et al. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol 2012;23:642-9. [Crossref] [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Fukuzawa K, et al. Gemcitabine combined with 5-fluorouracil and cisplatin (GFP) in patients with advanced biliary tree cancers: A pilot study. Anticancer Res 2006;26:771-5. [PubMed]
- Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: A prospective multicentre phase II trial. Br J Cancer 2008;98:309-15. [Crossref] [PubMed]
- Wiazzane N, Chauffert B, Ghiringhelli F, et al. Retrospective analysis of survival benefits of chemotherapy for metastatic or non-resectable intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol 2013;37:614-8. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Shirai K, Ebata T, Oda K, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2008;32:2395-402. [Crossref] [PubMed]
- Ellis MC, Cassera MA, Vetto JT, et al. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. HPB (Oxford) 2011;13:59-63. [Crossref] [PubMed]
- Jonas S, Thelen A, Benckert C, et al. Extended liver resection for intrahepatic cholangiocarcinoma: A comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg 2009;249:303-9.
- Yamamoto M, Takasaki K, Yoshikawa T, et al. Extended resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg 1999;6:117-21. [Crossref] [PubMed]
- Carpizo DR, D'Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2009;18:289-305. viii-ix. [Crossref] [PubMed]
- Sasaki A, Aramaki M, Kawano K, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. Br J Surg 1998;85:1206-9. [Crossref] [PubMed]
- Puhalla H, Schuell B, Pokorny H, et al. Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg 2005;189:173-7. [Crossref] [PubMed]
- Maithel SK, Gamblin TC, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013;119:3929-42. [Crossref] [PubMed]
- Paik KY, Jung JC, Heo JS, et al. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol 2008;23:766-70. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Clarke M. The QUORUM statement. Lancet 2000;355:756-7. [Crossref] [PubMed]
- Lu CD, Wang K, Zhang CZ, et al. Outcomes of intrahepatic cholangiocarcinoma with portal vein tumor thrombus following hepatic resection. J Gastroenterol Hepatol 2016;31:1330-5. [Crossref] [PubMed]
- Yoo T, Park SJ, Han SS, et al. Postoperative CA19-9 Change Is a Useful Predictor of Intrahepatic Cholangiocarcinoma Survival following Liver Resection. Dis Markers 2015;2015:298985.
- Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256-63. [Crossref] [PubMed]
- Zhu AX, Knox JJ. Adjuvant therapy for intrahepatic cholangiocarcinoma: the debate continues. Oncologist 2012;17:1504-7. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol 2016;23:235-43. [Crossref] [PubMed]
- Yamamoto M, Takasaki K, Otsubo T, et al. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2001;8:154-7. [Crossref] [PubMed]
- Zhang SJ, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:3596-602. [Crossref] [PubMed]
- Souche R, Addeo P, Oussoultzoglou E, et al. First and repeat liver resection for primary and recurrent intrahepatic cholangiocarcinoma. Am J Surg 2016;212:221-9. [Crossref] [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2011;80:e221-5. [Crossref] [PubMed]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: Survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol 2015;22:2218-25. [Crossref] [PubMed]
- Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-94. [Crossref] [PubMed]
- Murakami S, Ajiki T, Okazaki T, et al. Factors affecting survival after resection of intrahepatic cholangiocarcinoma. Surg Today 2014;44:1847-54. [Crossref] [PubMed]
- Spolverato G, Yakoob MY, Kim Y, et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survivalafter surgery for cholangiocarcinoma: A 30-year population-based SEER database analysis. J Gastrointest Surg 2007;11:1488-96; discussion 1496-7. [Crossref] [PubMed]
- Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year,single-center experience with the surgical management of intrahepatic cholangiocarcinoma: Operative results and long-term outcome. Surgery 2008;143:366-74. [Crossref] [PubMed]
- Hanazaki K, Kajikawa S, Shimozawa N, et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: Univariate and multivariate analysis. Hepatogastroenterology 2002;49:311-6. [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Song S, Kim K, Chie EK, et al. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: Implications for adjuvant radiotherapy. Clin Transl Oncol 2015;17:825-9. [Crossref] [PubMed]
- Jia AY, Wu JX, Zhao YT, et al. Intensity-modulated radiotherapy following null-margin resection is associated with improved survival in the treatment of intrahepatic cholangiocarcinoma. J Gastrointest Oncol 2015;6:126-33. [PubMed]
- Li J, Wang Q, Lei Z, et al. Adjuvant Transarterial Chemoembolization Following Liver Resection for Intrahepatic Cholangiocarcinoma Based on Survival Risk Stratification. Oncologist 2015;20:640-7. [Crossref] [PubMed]
- Cillo U, Spolverato G, Vitale A, et al. Liver Resection for Advanced Intrahepatic Cholangiocarcinoma: A Cost-Utility Analysis. World J Surg 2015;39:2500-9. [Crossref] [PubMed]
- Facciuto ME, Singh MK, Lubezky N, et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation 2015;99:151-7. [Crossref] [PubMed]
- Hashimoto K, Miller CM. Liver transplantation for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2015;22:138-43. [Crossref] [PubMed]
- Fu BS, Zhang T, Li H, et al. The role of liver transplantation for intrahepatic cholangiocarcinoma: a single-center experience. Eur Surg Res 2011;47:218-21. [Crossref] [PubMed]
- Sotiropoulos GC, Kaiser GM, Lang H, et al. Liver transplantation as a primary indication for intrahepatic cholangiocarcinoma: a single-center experience. Transplant Proc 2008;40:3194-5. [Crossref] [PubMed]
- Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9. [Crossref] [PubMed]
- Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar andperipheral cholangiocarcinoma. Ann Surg 2004;239:265-71. [Crossref] [PubMed]
- Sapisochin G, Rodriguez de Lope C, Gastaca M, et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7. [Crossref] [PubMed]
- Hong JC, Petrowsky H, Kaldas FM, et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J Am Coll Surg 2011;212:514-20. [Crossref] [PubMed]
- Sapisochin G, de Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg 2014;259:944-52. [Crossref] [PubMed]
- Takahashi K, Obeid J, Burmeister CS, et al. Intrahepatic Cholangiocarcinoma in the Liver Explant After Liver Transplantation: Histological Differentiation and Prognosis. Ann Transplant 2016;21:208-15. [Crossref] [PubMed]


