Sarcopenia and nonalcoholic fatty liver disease: a causal relationship
Concurrent with the obesity epidemic, non-alcoholic fatty liver disease (NAFLD) is rapidly becoming the leading cause of chronic liver disease worldwide (1). NAFLD, particularly its histological phenotype non-alcoholic steatohepatitis (NASH), can progress to advanced liver disease, cirrhosis and hepatocellular carcinoma and indication for liver transplantation (2). Although remarkable advances have been made in the understanding of the pathophysiology of NAFLD, the exact mechanism of hepatic fat accumulation and the progression to NASH is not fully understood. Thus, in order to reduce the burden associated with these diseases, it is imperative that all major factors that contribute to the development of NAFLD are identified.
Over the last years, growing interest has been directed to the involvement of skeletal muscle mass in chronic liver disease. The decline in lean body mass, namely sarcopenia, has been recognized as one of the comorbidities associated with liver cirrhosis, and it is observed in up to 60% of patients with end-stage liver disease (ESLD) (3). Sarcopenia is defined as a progressive and generalized loss of skeletal muscle mass, strength, and/or physical performance, and it is associated with a poor prognosis in patients with ESLD (4). Infiltration of fat in muscles, namely myosteatosis, increases with age and adiposity, and is associated with an increased risk of mortality, suggesting that both muscle quantity and quality are important prognostic markers (5). In the past, sarcopenia was only regarded as part of aging; more recently, it was recognized as a progressive disease frequently associated with cardiometabolic disorders including diabetes mellitus, metabolic syndrome and cardiovascular disease (6). Interestingly, several studies have reported the presence of sarcopenia in subjects with NAFLD, and sarcopenia is emerging as a novel risk factor for the development of NAFLD (7-10).
Three recent distinct meta-analyses with overlap of selected articles have estimated the risk of NAFLD among patients with sarcopenia. The first meta-analysis, summarizing the data available through November 2016, included five cross-sectional studies (of whom two in the form of abstract) with 27,804 participants (8). Patients with sarcopenia had a 1.5-fold increased risk of NAFLD compared to those without sarcopenia [odds ratio (OR) =1.54; 95% confidence interval (CI), 1.05–2.26]. However, a high heterogeneity was observed among the studies (I2=83%). The subsequent meta-analysis, evaluating electronic databases through September 2017, included six cross-sectional studies with 19,024 participants (9). Compared with subjects without sarcopenia, those with sarcopenia had a 1.3-fold increased risk of NAFLD (OR =1.29; 95% CI, 1.12–1.49). The heterogeneity among the studies was high (I2=61%). The last meta-analysis included three studies published before August 2017 (10). While all three studies with a total of 3,226 individuals reported data on sarcopenia with liver fibrosis of NAFLD, two of them involving 465 participants examined the association between sarcopenia and NASH. Overall, the meta-analysis found a significant association between sarcopenia and NASH (OR =2.35; 95% CI, 1.45–3.81) as well as between sarcopenia and advanced liver fibrosis (OR =2.41; 95% CI, 1.94–2.98).
Limitations of these studies need to be considered. First, most of the studies included in the meta-analyses used bioelectrical impedance analysis (BIA) to estimate the skeletal muscle mass, while the others used dual-energy X-ray absorptiometry (DEXA). Magnetic resonance imaging (MRI) and computed tomography (CT) are the reference methods for quantifying muscle tissue. DEXA, which is well correlated with MRI and CT, has been proposed to measure body composition, because of its wider availability and lower cost. Compared to these gold-standard techniques, BIA may have a lower accuracy; however, it offers a simpler, more rapid, and safe means to estimate skeletal muscle mass, making it suitable for large-scale screening at the population level. Second, the majority of studies used surrogate indices to identify NAFLD and fibrosis in NAFLD. Only two studies used liver biopsy which is the gold standard to diagnose NAFLD and its complications such as NASH and fibrosis. Third, there were differences in the studied populations. The Asian population has been mainly investigated whereas evidence from other ethnic groups is scarce to date. Fourth, many studies did not adjust for potential confounders such as markers of inflammation, and physical activity. Finally, all studies were cross-sectional in nature. Thus, the temporal relationship between sarcopenia and NAFLD could not be established, making it impossible to interpret any cause-effect relationship.
In this context, the longitudinal study by Kim et al. (11) adds important information about the association of low muscle mass and NAFLD. The researchers evaluated a general population cohort of 12,624 subjects without baseline NAFLD and 2,943 subjects with baseline NAFLD undergoing health check-up examinations, and tested the impacts of relative skeletal muscle mass and its changes over time on the development of incident NAFLD or the resolution of baseline NAFLD. NAFLD was diagnosed by the hepatic steatosis index (HSI), while relative skeletal muscle mass was presented using the skeletal muscle mass index (SMI), estimated by BIA. During the 7-year follow-up period, 1,864 (14.8%) of the 12,624 subjects without baseline NAFLD developed NAFLD. Using Cox proportional hazard analysis, compared to the lowest sex-specific SMI tertile at baseline, the highest tertile was inversely associated with incident NAFLD [adjusted hazard ratio (AHR) =0.44; 95% CI, 0.38–0.51], and positively associated with the resolution of baseline NAFLD (AHR =2.09; 95% CI, 1.02–4.28). In addition, compared to the subjects in the lowest tertile of change in SMI over a year, those in the highest tertile exhibited a significantly reduced risk of development of incident NAFLD (AHR =0.69; 95% CI, 0.59–0.82) as well as resolution of existing baseline NAFLD (AHR =4.17; 95% CI, 1.90–6.17) after adjustment for many covariates including age, gender, waist circumference, diabetes status, hypertension, smoking, regular exercise, and baseline SMI. This new study unquestionably showed that increases in relative skeletal muscle mass over time may have beneficial effects on NAFLD development or resolution of existing NAFLD.
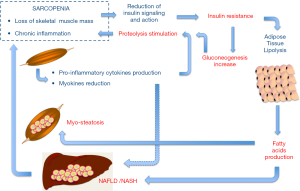
Why skeletal muscle mass influences the development of NAFLD is not completely elucidated (Figure 1). It is acknowledged that NAFLD and sarcopenia share several pathophysiologic processes, particularly insulin resistance and chronic inflammation (12,13). Skeletal muscle is a major insulin-responsive target organ, and loss of muscle mass reduces a key cellular target for insulin, contributing to glucose intolerance and promoting gluconeogenesis, which in turn exacerbates proteolysis and muscle depletion in a vicious cycle. Furthermore, in adipose tissue insulin resistance increases lipolysis with consequent release of free fatty acids to the liver and muscles, ultimately resulting in NAFLD and myosteatosis, respectively.
Chronic inflammation is present in subjects with sarcopenia as evidenced by increased levels of C-reactive protein and pro-inflammatory cytokines. The pro-inflammatory milieu may promote the catabolic stimulation of muscles. Subclinical inflammation and oxidative stress are also important in the development of NAFLD. Thus, inflammation is likely another shared mediator between sarcopenia and NAFLD. Yet, skeletal muscle is considered an endocrine organ that secretes peptides called myokines that can mediate crosstalk between metabolic tissues including the liver (13,14). Among several myokines, interleukin-6 has a protective effect on the development of NAFLD in inflammation–prone animal models, and irisin, an exercise-inducible myokine has been found inversely associated with the degree of fatty liver in obese patients. Thus it is possible that muscle could play a causative role for NAFLD through reduced secretion of various salutary myokines in the context of sarcopenia. Notably, accumulating evidence supports an exercise effect and a physical fitness role per se in the pathophysiology of NAFLD (15). Exercise alone, without any dietary intervention, has been demonstrated to determine a significant decrease of the intrahepatic lipid content, even in the absence of weight change, or minimal weight loss. Physical inactivity also represents a well-established risk factor for sarcopenia. The lack of physical activity favors the decline of lean body mass, triggering a vicious cycle leading to both progressive inactivity and sarcopenia.
Some caveats of the study by Kim and coworkers should be considered: (I) the absence of histological diagnosis of NAFLD; (II) the lack of a reference method for measuring muscle mass; and (III) failure to report the changes in muscle strength and quality, that help to characterize sarcopenia in a functional way. Despite these limitations, the current longitudinal study definitely establishes that sarcopenia is one of the causative factors for development of NAFLD, pinpointing to a broader therapeutic approach to the disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686-90. [Crossref] [PubMed]
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263-73. [Crossref] [PubMed]
- Bhanji RA, Carey EJ, Yang L, et al. The long winding road to transplant: How sarcopenia and debility impact morbidity and mortality on the waitlist . Clin Gastroenterol Hepatol 2017;15:1492-7. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [PubMed]
- Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126-35. [Crossref] [PubMed]
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819-29. [Crossref] [PubMed]
- Bhanji RA, Narayanan P, Allen AM, et al. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055-65. [Crossref] [PubMed]
- Wijarnpreecha K, Panjawatanan P, Thongprayoon C, et al. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J Gastroenterol 2018;24:12-7. [Crossref] [PubMed]
- Pan X, Han Y, Zou T, et al. Sarcopenia Contributes to the Progression of Nonalcoholic Fatty Liver Disease- Related Fibrosis: A Meta-Analysis. Dig Dis 2018;36:427-36. [Crossref] [PubMed]
- Yu R, Shi Q, Liu L, et al. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol 2018;18:51. [Crossref] [PubMed]
- Kim G, Lee SE, Lee YB, et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018;68:1755-68. [Crossref] [PubMed]
- Zhai Y, Xiao Q. The Common Mechanisms of Sarcopenia and NAFLD. Biomed Res Int 2017;2017:6297651. [Crossref] [PubMed]
- Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 2016;229:R67-81. [Crossref] [PubMed]
- Pedersen BK. Muscle as a secretory organ. Compr Physiol 2013;3:1337-62. [PubMed]
- Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157-66. [Crossref] [PubMed]