Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma—2 case reports and a brief review
Introduction
Molecular analysis in patients with cholangiocarcinoma has been studied increasingly and has shown marked diversity in the frequency of targetable oncogenic driver mutations. One such aberration is mutation in BRAF (v-Raf murine sarcoma viral oncogene homolog B) which is one of the downstream signals in MAPK (Mitogen-activated protein kinases) pathway. Here we report 2 cases of BRAF mutant intrahepatic cholangiocarcinoma (ICC) refractory to initial chemotherapy treated with dual BRAF and MEK inhibition exhibiting excellent durable clinical and radiological response.
Case presentation
Case 1
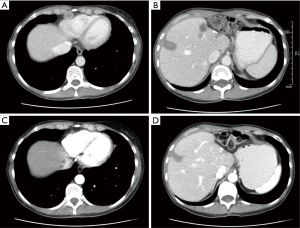
A 49-year-old female presented with abdominal bloating and increasing discomfort in June 2014. She was evaluated by imaging and was found to have a large left lobe liver tumor. Fine needle aspiration cytology (FNAC) was consistent with adenocarcinoma. Her CA19-9 (carbohydrate antigen 19.9) and carcinoembryonic antigen (CEA) serum tumor markers and liver function tests were unremarkable. A [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan showed a left lobe liver mass with no evidence of extra hepatic disease. Colonoscopy was unremarkable. She underwent a left hepatectomy with dissection of celiac nodes and cystic duct in July 2014. Post operative histopathological examination revealed a 7.3-cm moderately differentiated ICC, with direct tumor invasion to local adjacent extra hepatic structures, perineural invasion, and with 3 out of 4 positive lymph nodes (Tumor Node Metastasis stage pT3N1—Stage IVA). No loss of mismatch repair protein (MMR) expression was noted by immunohistochemistry. In view of her high risk of disease recurrence, she received five 3-week cycles of gemcitabine and cisplatin adjuvant chemotherapy. Repeat imaging after cycle 5 (4 months) revealed a new 1 cm right liver lobe lesion with new periportal and portacaval nodes and a pericardial lymph node enlargement. She underwent radiofrequency ablation (RFA) to the liver lesion and was switched to second line single-agent capecitabine. Repeat computerized tomography (CT) scan following 3 cycles of capecitabine (2 months) revealed new liver lesions and progressive lymph node enlargement in the pericardial region (Figure 1A,B). Foundation One comprehensive genomic analysis was performed on her original resection specimen and confirmed a BRAF V600E mutation. She was started on dabrafenib 150 mg PO BID (twice a day) and trametinib 2 mg PO QD (once a day) in May 2015. Follow-up imaging studies confirmed a partial response after 6 weeks of treatment and a complete clinical response after 5 months of treatment (RECIST 1.1) (Figure 1C,D). At 9 months of treatment, CT imaging confirmed recurrence of disease in the pericardial node and periportal/portacaval lymph nodes and new right pleural disease. Treatment with dabrafenib and trametinib was discontinued.
Case 2
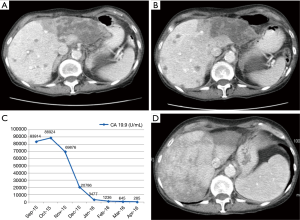
A 71-year-old lady presented with vague right sided abdominal discomfort of 3 to 4 months duration with associated anorexia and weight loss in August 2015. Her past medical history was significant for cardiac dysrythmia requiring pacemaker placement and mild chronic obstructive pulmonary disease (COPD). CT scan of the abdomen and pelvis showed a 7.8 cm infiltrative mass in the left lobe of liver and other too numerous to count smaller metastases in both lobes of liver (August 2015, Figure 2A). CA19.9 levels was elevated at 84,000 U/mL. Her liver enzymes and other biochemical parameters were unremarkable. Colonoscopy and Esophagogastroduodenoscopy (EGD) were unremarkable. FNA biopsy was consistent with ICC, with CK7+, CK19.9+, CK20-, CDX.2-, and TTF1-. She received gemcitabine and cisplatin chemotherapy for 3 cycles followed by re-staging CT scans, which confirmed progressive disease in the liver (November 2015, Figure 2B). Foundation One was performed on her initial diagnostic biopsy and confirmed a BRAF V600E mutation. She was started on dabrafenib 150 mg PO BID and trametinib 2 mg PO QD starting November 2015. Her CA19.9 levels dropped dramatically in the next six weeks (Figure 2C). Her last scan was done 5 months after initiation of BRAF/MEK inhibitors (April 2016, FIG2d) and confirms a partial response to treatment. As of this report, the patient continues on treatment with ongoing clinical benefit.
Discussion
Cholangiocarcinoma (CCA) is one of the most fatal malignancies in the field of pancreatico-hepatobiliary tumors. Based on the SEER database, the 5-year overall survival is 15% and 2% for stage I (localized) and stage IV disease, respectively (1). The median survival of metastatic CCA is approximately one year with currently approved systemic therapies (2). There is an unmet need for new strategies and treatment options for metastatic CCA. One such strategy relies on the stratification of cholangiocarcinoma into distinct molecular subgroups, with matching targeted therapies. These molecular subgroups include BRAF, ERBB2, IDH1 & 2 and FGFR aberrations (3). These molecular aberrations have been shown to be targetable, and are the subject of several ongoing or planned clinical trials. In this reports, we focus on two cases of ICC with BRAF mutation and demonstrate impressive clinical activity for a combination of BRAF and MEK inhibitors, dabrafenib and tramatenib.
BRAF mutations are more common in ICC than extrahepatic cholangiocarcinoma or gallbladder cancer (4,5). The frequency of BRAF mutations in ICC have ranged between 1% to 22% among various cases series or population studies. The frequency appears to be underestimated when assessed by immunohistochemistry studies in comparison to PCR (6,7). In addition, the variations in the reported frequency may be related to differences in PCR techniques and Next Generation Sequencing (NGS) as well as distinct differences in the study populations. Irrespective of the true frequency, BRAF mutant cholangiocarcinoma appears to be a distinct molecular subtype of biliary cancers that can be associated with aggressive behavior and chemotherapy resistance, as demonstrated in our cases. The targeting of this subgroup of patients with single agent BRAF inhibitors has been associated with modest clinical responses and short duration of disease control. In a study by Hyman et al., single agent vemurafenib was associated with a 12% objective response rate in BRAF mutant cholangiocarcinoma (1 out of 8 patients had PR) (8). Several other solid tumors with BRAF mutation have shown a benefit from combining BRAF and MEK inhibitors. Randomized phase III clinical trials have shown superiority of BRAF plus MEK inhibitors to BRAF inhibitors alone in BRAF mutant melanoma (9). In addition, a study of dabrafenib plus tramatenib shows more favorable responses and progression free survival in comparison to another study with vemurafenib alone in patients metastatic BRAF mutant colorectal cancer (10).
In view of improved efficacy of dual BRAF and MEK inhibition in melanoma and colorectal cancer, we elected to treat our patients with BRAF mutant ICC with a combination of dabrafenib and tramatenib. Both cases were characterized by refractoriness to first-line standard chemotherapy with gemcitabine and cisplatin and with aggressive clinical behavior. Dramatic and durable responses to dabrafenib and tramatenib were recorded and included a complete clinical response and an ongoing partial response. Our results are in line with 3 additional case reports of dabrafenib and tramatenib in BRAF mutant ICC. These include 2 cases with a clinical benefit from MDACC (not detailed) and one case with a PR lasting more than 8.5 months from Penn University (11,12). While ideal, the conduct of future conclusive first-line phase III randomized clinical trials of dual BRAF/MEK inhibitors against gemcitabine plus cisplatin will be challenging. However, several Basket studies are currently evaluating combinations of BRAF and MEK inhibitors in non-melanoma and non-colorectal cancer BRAF-V600E mutated tumors (NCT02034110, NCT01713972, NCT01902173). Additional data from these prospective clinical trials will help confirm the activity of dual MEK/BRAF inhibition as a standard approach in BRAF-mutant cholangiocarcinoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Survival statistics for bile duct cancers. Available online: http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-survival-by-stage
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol 2015;33:1845-8. [Crossref] [PubMed]
- Robertson S, Hyder O, Dodson R, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol 2013;44:2768-73. [Crossref] [PubMed]
- Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol 2014;21:3827-34. [Crossref] [PubMed]
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003;52:706-12. [Crossref] [PubMed]
- Goeppert B, Frauenschuh L, Renner M, et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol 2014;27:1028-34. [Crossref] [PubMed]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol 2014;32:3697-704. [Crossref] [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023-31. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 2014;8:479. [Crossref] [PubMed]


