Does a stoma reduce the risk of anastomotic leak and need for re-operation following low anterior resection for rectal cancer: systematic review and meta-analysis of randomized controlled trials
Introduction
Low anterior resection (LAR) with colorectal or coloanal anastomosis for mid to distal rectal cancer less than 10 cm from the anal verge is associated with a higher risk of anastomotic leak compared to high anterior resection and restorative colonic procedures (1). LAR is associated with higher rates of morbidity and mortality including re-operations, wound infection, conversion to permanent stoma, stenosis and recurrence (1-3). Until recently, there has not been a predictor of anastomotic leak post LAR, but there is now a model based on mass nationwide data that calculates the risk of anastomotic leakage post LAR (4). In general, the Cochrane review in 2011 reported the rate of anastomotic leak following LAR to be 8.8% (38/431) without mechanical bowel preparation (MBP) and 10.4% (43/415) with bowel preparation (5).
Proximal faecal diversion via a loop ileostomy or colostomy is a common strategy used to reduce the risk of anastomotic leak post LAR. The evidence of benefit, however, has been unclear with the initial randomized controlled trials (RCTs) comparing LAR with and without stoma failing to demonstrate any significant benefit. Graffner et al. reported no differences in anastomotic leak rates between 50 patients randomized into the two groups (6). Pakkastie et al. conducted an RCT which demonstrated a trend towards lower leak rate in the stoma group, but the level of significance was not achieved (7). Other studies have reported conflicting results with regards to leak rate, mortality and morbidity (8,9). There is not consensus in the literature regarding routine use of diverting stoma following LAR.
The majority of existing meta-analyses has been significantly limited by the inclusion of studies retrospective and observational in nature (10-12). Those which attempted subgroup analysis for RCTs had small cohort sample sizes for the randomized data, and were not adequately powered to detect differences in complication rates.
To address current limitations in the literature, we have performed an updated meta-analysis including only prospective RCTs with the aim of comparing outcomes following LAR in terms of leak rates, reoperations, mortality rates and complication rates based on defunctioning stoma status.
Methods
Search strategy
Given that this study does not involve humans, animals and uses only previously published and publically available data, ethics approval was waived. The present study was performed according to PRISMA guidelines (13,14). Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to 9th October 2017. To achieve maximum sensitivity of the search strategy and identify all studies, we combined the terms: “stoma”, “ileostomy”, “diversion”, “defunctioning”, “low anterior resection”, “rectal cancer”, “rectal malignancy”, as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible RCTs for the present systematic review and meta-analysis included those in which patients were randomized to either LAR with a diversion/defunctioning stoma versus no diversion/defunctioning. Stoma could be in the form of ileostomy or colostomy. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only either the most recent or most complete reports were included for quantitative assessment. All publications were limited to those involving human subjects and in the English language. Case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. Assessment of risk of bias for each selected study was performed according to the most updated Cochrane statement. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigator.
Statistical analysis
The relative risk (RR) and weighted mean difference (WMD) were used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 = 100% × (Q – df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.3.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Search strategy and appraisal
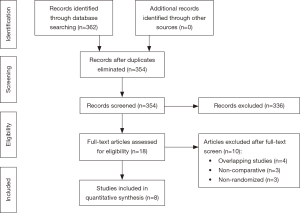
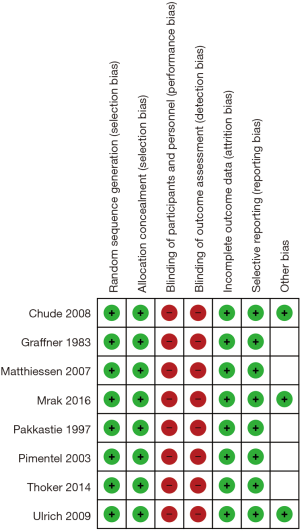
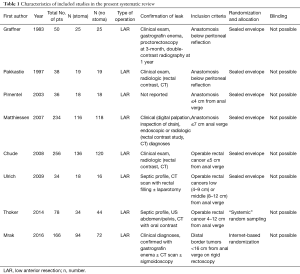
From systematic electronic database searches, a total of 362 references were identified (Figure S1). After exclusion of duplicate references, 354 potentially relevant articles were retrieved. After detailed evaluation of these articles, 18 articles remained for further full-text assessment. After application of selection criteria, 8 RCTs (6,7,15-20) were selected for analysis. The study characteristics of these trials are summarized in Table 1. Pimentel et al. was only published as abstract form but was included in analysis as the abstract reported leak rate and reoperation rates. In total, this systematic review included 892 LAR procedures for rectal cancers, comprising 460 cases with diversion stomas and 432 without temporary diversion stomas. The 8 RCTs were also assessed qualitatively using tools recommended by the Cochrane Collaboration for the risk of bias. A graph and summary of selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias identified is shown in Figure S2. All included studies had high risk of bias for blinding of personnel and outcome assessors.
Full table
Operative characteristics
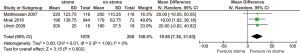
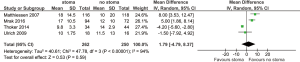
For all study populations, patients underwent a LAR procedure for rectal cancer, with tumor resection and safe stapled anastomosis with complete anastomotic rings and negative air leak test. There was a trend towards less operative blood loss for patients with no stoma diversion compared to those with stoma diversion (WMD 87.67 mL, 95% CI: −9.40, 184.75; P=0.08) (Figure S3) however this did not reach statistical significance. There was a significantly longer operative duration for patients with stoma diversion (WMD 19.50 min; 95% CI: 7.38, 31.63; I2=0%, P=0.002, Figure S4). No significance difference in hospital stay was noted between the groups (WMD 1.79; 95% CI: −4.79, 8.37; I2=94%; P=0.59; Figure S5).
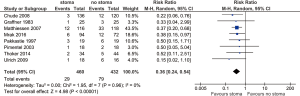
Clinical anastomotic leak
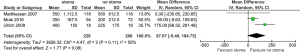
All included studies reported anastomotic leak rates. The pooled rate was significantly lower for those with stoma diversion (6.3% vs. 18.3%; RR 0.36; 95% CI: 0.24, 0.54; I2=0%; P<0.00001) (Figure 1) with no significant heterogeneity noted.
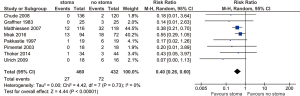
Reoperation for leak
Reoperation rate for leak was reported by all included trials. Pooled analysis demonstrated significantly lower reoperation rate for patients with stoma diversion compared to no stoma (5.9% vs. 16.7%; RR 0.40; 95% CI: 0.26, 0.60; I2=0%; P<0.00001) (Figure 2), with no significant heterogeneity noted.
Perioperative mortality rate
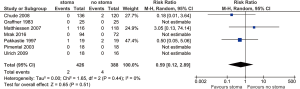
There was no significant difference found in terms of leak-related mortality between stoma vs. no-stoma cohorts (0.47% vs. 1.0%; RR 0.59; 95% CI: 0.12, 2.89; I2=0%; P=0.51) (Figure 3). No significant heterogeneity was noted.
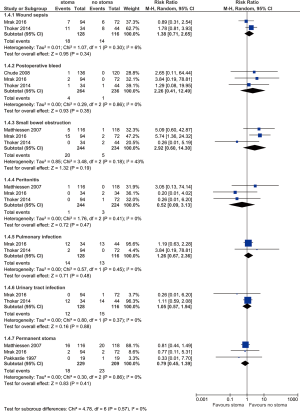
Other complications
Other complications are summarized in Figure 4. No difference is found between stoma vs. no-stoma approaches in terms of wound sepsis (14.1% vs. 12.1%, P=0.34), postoperative bleed (1.5% vs. 0.4%, P=0.35), small bowel obstruction (8.2% vs. 2.1%, P=0.19), peritonitis (0.4% vs. 1.3%, P=0.47), pulmonary infection (10.9% vs. 11.2%, P=0.48), urinary tract infection (9.4% vs. 12.9%, P=0.88), or permanent stoma rates (7.9% vs. 11.0%, P=0.41).
Discussion
The evidence of benefit for performing defunctioning stoma following LAR has been unclear. Various observational studies, systematic reviews and meta-analyses and lower quality studies have reported a wide range of results. Meta-analyses on this topic so far have been of limited sample size or based on lower quality evidence than RCTs. In order to synthesize the highest quality evidence, we performed a meta-analysis of only RCTs comparing stoma diversion versus no diversion for LAR for rectal cancers. Our pooled analysis of 8 RCTs, with more included randomized studies compared to prior meta-analyses (10,11), demonstrated a significant reduction in clinically relevant anastomotic leak rates and re-operation rates in patients with stoma diversion, without significant differences in perioperative mortality or other complications. As such, our findings support the creation of a defunctioning stoma following LAR for mid and low rectal cancers.
The pooled results of the present analysis are consistent with reported outcomes of several prior studies. Matthiessen et al. (15) conducted a study involving 234 LAR patients from 21 hospitals in Sweden, who were randomized to 116 cases with stoma and 118 cases without diverting stoma. The authors reported that the odds of having symptomatic leakage following LAR was 3.4 times higher without ileostomy compared to those with diverting ileostomy. They also reported urgent abdominal reoperation rate of 8.6% in patients with stoma compared to 25.4% in patients without stoma.
A five-year follow-up report of this randomized study also demonstrated that anorectal function at long-term follow-up was not affected by the use of a diverting ileostomy approach (21). In another RCT, Mrak et al. randomized 166 patients with LAR with J-pouch reconstruction and demonstrated significant higher leakage rates and reoperation rates for patients without stoma (19).
Multivariate analysis demonstrated that male gender and absence of stoma were significant independent predictors of anastomotic leakage. Overall, the results of the present meta-analysis and prior published literature support the use of a defunctioning stoma following LAR to lower rates of anastomotic leak and reoperation rates.
Our results should not be applied blindly to clinical practice, given that quality-of-life outcome measures were not included for analysis. A temporary stoma can have significant impact on a patient’s physical and psychological well-being, as assessed using the 36-item Short-Form Health Survey (SF-36) (22). Tsunoda et al. (23) conducted a prospective longitudinal study in 22 patients with rectal cancer who underwent a LAR with loop ileostomy. The authors found significant reduction in European Organization for Research and Treatment of Cancer QLQ-CR38 and QLQ-C30 scores after the LAR procedure compared to baseline preoperative levels, indicating reductions in physical and role functioning. These scores markedly improved following ileostomy closure.
Given the considerable quality-of-life implications, the decision to proceed with a stoma should be a highly individualised one which take into account both the negative impact on role and function and balanced with the benefits of leak risk reduction. A risk model of anastomotic leak following LAR (4) would be useful to weigh up the risk of anastomotic leak with the morbidity and quality of life implications associated with a stoma.
The strengths of the present meta-analysis include the exclusion of all retrospective evidence from the current analysis. Retrospective observational studies are susceptible to an inherent selection bias, in particularly favouring surgery without stoma. This is likely due to surgeons creating stomas in patients who they believe are likely to develop anastomotic leak complication, leading to the selection bias. Indeed there is evidence to demonstrate risk factors for leak complications including male gender, anastomotic height, obesity, steroid use, malnutrition, steroid use and prior irradiation (1,24-31). Thus in retrospective studies surgeons may more often elect to perform a stoma diverting approach in such populations. To minimize the effects of such bias, the present meta-analysis pooled data from only prospectively designed RCT data, with minimal heterogeneity detected between RCTs for the outcomes reported.
The present study is constrained by several limitations. There has been a lack of reported long-term outcomes following stoma diversion or no diversion techniques for LAR, and as such, the long-term morbidity associated with a defunctioning stoma is unclear. Some endpoints could not be pooled for analysis due to lack of reporting in the included studies, such as stoma retraction, obstruction, excoriation, prolapse rates, readmission rates, and stoma-related complications such as peristomal ulcerations/abscess, parastomal hernias, and high output leading to kidney injury. Our meta-analysis also did not address quality-of-life endpoints. Certainly, the creation of a stoma regardless of its temporary or permanent status, would reduce quality of life of patients in this population, particularly if stoma complications were to occur.
Conclusions
The findings of this present meta-analysis of level one RCT evidence demonstrates that a defunctioning stoma following LAR for rectal cancer reduces the risk of anastomotic leak and re-operation rates, without a significant increase in the risk of mortality or serious short-term morbidity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Given that this study does not involve humans, animals and uses only previously published and publically available data, ethics approval was waived.
References
- Law WL, Choi HK, Lee YM, et al. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg 2007;11:8-15. [Crossref] [PubMed]
- Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg 2001;88:400-4. [Crossref] [PubMed]
- Hallböök O, Sjodahl R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg 1996;83:60-2. [Crossref] [PubMed]
- Watanabe T, Miyata H, Konno H, et al. Prediction model for complications after low anterior resection based on data from 33,411 Japanese patients included in the National Clinical Database. Surgery 2017;161:1597-608. [Crossref] [PubMed]
- Güenaga KF, Matos D, Wille-Jorgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011.CD001544. [PubMed]
- Graffner H, Fredlund P, Olsson SA, et al. Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum 1983;26:87-90. [Crossref] [PubMed]
- Pakkastie TE, Ovaska JT, Pekkala ES, et al. A randomised study of colostomies in low colorectal anastomoses. Eur J Surg 1997;163:929-33. [PubMed]
- Machado M, Hallbook O, Goldman S, et al. Defunctioning stoma in low anterior resection with colonic pouch for rectal cancer: a comparison between two hospitals with a different policy. Dis Colon Rectum 2002;45:940-5. [Crossref] [PubMed]
- Matthiessen P, Hallbook O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004;6:462-9. [Crossref] [PubMed]
- Tan WS, Tang CL, Shi L, et al. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 2009;96:462-72. [Crossref] [PubMed]
- Hüser N, Michalski CW, Erkan M, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008;248:52-60. [Crossref] [PubMed]
- Gu WL, Wu SW. Meta-analysis of defunctioning stoma in low anterior resection with total mesorectal excision for rectal cancer: evidence based on thirteen studies. World J Surg Oncol 2015;13:9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [PubMed]
- Matthiessen P, Hallbook O, Rutegard J, et al. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007;246:207-14. [Crossref] [PubMed]
- Chude GG, Rayate NV, Patris V, et al. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology 2008;55:1562-7. [PubMed]
- Ulrich AB, Seiler C, Rahbari N, et al. Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum 2009;52:412-8. [Crossref] [PubMed]
- Thoker M, Wani I, Parray FQ, et al. Role of diversion ileostomy in low rectal cancer: a randomized controlled trial. Int J Surg 2014;12:945-51. [Crossref] [PubMed]
- Mrak K, Uranitsch S, Pedross F, et al. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery 2016;159:1129-39. [Crossref] [PubMed]
- Pimentel JM, Duarte A, Patricio J. The role of a protecting stoma in low anterior resection with TME and colonic J-pouch for rectal cancer; results of a prospective randomized trial. Colorectal Dis 2003;5:83.
- Floodeen H, Lindgren R, Hallbook O, et al. Evaluation of long-term anorectal function after low anterior resection: a 5-year follow-up of a randomized multicenter trial. Dis Colon Rectum 2014;57:1162-8. [Crossref] [PubMed]
- O'Leary DP, Fide CJ, Foy C, et al. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg 2001;88:1216-20. [Crossref] [PubMed]
- Tsunoda A, Tsunoda Y, Narita K, et al. Quality of life after low anterior resection and temporary loop ileostomy. Dis Colon Rectum 2008;51:218-22. [Crossref] [PubMed]
- Karanjia ND, Corder AP, Bearn P, et al. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg 1994;81:1224-6. [Crossref] [PubMed]
- Mäkelä JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum 2003;46:653-60. [Crossref] [PubMed]
- Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg 1973;177:513-8. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Lee WS, Yun SH, Roh YN, et al. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 2008;32:1124-9. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg 1997;185:105-13. [Crossref] [PubMed]
- Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355-8. [Crossref] [PubMed]