Comparison of tumor regression grading system in locally advanced esophageal squamous cell carcinoma after preoperative radio-chemotherapy to determine the most accurate system predicting prognosis
Introduction
Esophageal cancer (EC) is the seventh most common cancer and is the sixth leading cause of cancer death worldwide (1). Esophageal squamous cell carcinoma (ESCC) is the most common type of EC, followed by esophageal adenocarcinoma (EAC). ESCC is more common in Asia and Africa (2). The incidence and type of cancer vary by region of the world. In the western world, the incidence of ESCC has been reduced and that of EAC is higher (3). In Asian countries, the incidence of ESCC is still more than 90%, and is a major health burden (2,4). Smoking and alcohol consumption are the main risk factors for ESCC. Inadequate consumption of vegetable and fruit, and low socioeconomic status are also risk factors (4).
Nowadays, many systematic reviews and meta-analyzes support that preoperative radio-chemotherapy is a standard treatment for locally advanced ESCC. Because the standard treatment improves survival rates, reduces the rate of margin-positive resection, reduces hospital stay, and reduces the rate of return to hospital, compared to surgery alone (5-9). Numerous studies have proposed pathological indicators that predict the prognosis of postoperative patients, such as tumor regression grade (TRG), lymph node status, margin status, post-therapy pathologic stage (10-14). TRG refers to the classification of cancer response to preoperative treatment, which can inform the prognosis. In published studies, many TRG systems were proposed for use in esophageal cancer. Mandard et al. (10) first proposed five-tier grading system. Chirieac et al. (12) suggested three-tier grading system with percentage cut-point and many studies encouraged it (15-19). Schneider et al. (13) included lymph node status into four-tier grading system. Hermann et al. (20) proposed two-tier grading system, considering only if there was residual tumor. The Japan Esophageal Society (JES) system (21) used four-tier grading system.
Although there are many TRG systems, none of them is standardized. This research aimed to compare five TRG systems to find the most accurately predictive one. Five TRG systems included Mandard system (10), Chirieac system (12), Schneider system (13), Hermann system (20) and JES system (21).
Methods
Study population
We made a retrospective review in database of King Chulalongkorn Memorial Hospital. The research protocol has been approved by an institutional review board (IRB) of Research Affairs, Faculty of Medicine, Chulalongkorn University. The number of the approval was IRB No. 682/60. This study collected patient data from hospital medical record system with official permission from director of the hospital and informed consent was not required. The patient data have been secured. Forty-five participants were recruited, which the inclusion criteria included patients with locally advanced ESCC (clinical stage T3-4/N0-N+/M0) from 2006to 2014 and all of them received preoperative radio-chemotherapy followed by esophagectomyI. All participants were diagnosed by computed tomography and endoscopy with biopsy, and treated with preoperative radio-chemotherapy, including 50–64 Gy radiotherapy with cisplatin or carboplatin and 5-fluorouracil (5-FU), followed by transthoracic esophagectomy, at 12 weeks after completion of chemoradiation. The exclusion criteria were surgical specimen with incomplete resection margin and specimen without lymph nodes obtained. Other data, such as demographic characteristic and operative procedure were also obtained from medical records. All the patients conformed a follow-up visit scheduled every 3 months in the first 2 years and then every 6 months for the next 3 years together with physical examination, computerized tomography scan and endoscopy every 3–6 months.
Histomorphological analysis
All resection specimens were processed following the standard protocol (22). The specimens were grossly examined and recorded information about macroscopic appearance, size, location, and relation to proximal margin, distal margin and radial margin. If no gross tumor was identified, lesion such as ulcer, fibrotic area or area covered by mucosa and adjacent mucosa were entirely submitted for microscopic examination to adequately assess residual tumor. Lymph nodes were all submitted for evaluation.
In this study, all participants were evaluated histologically by an experienced gastrointestinal pathologist for tumor type, percentage of viable residual tumor comparing with tumor bed, extension of tumor, margin status and number of involved lymph nodes. Only 37 participants met inclusion criteria and exclusion criteria, and were analyzed in this study.
The viable tumor cells were described as intact cytomorphological integrity. The tumor bed included all areas of regression changes, such as fibrosis, keratin pearls, and foreign-body giant cell reactions (10).
To evaluate percentage of residual tumor was performed by estimating proportion of residual viable tumor cells over tumor bed, which was counted together from all slides with/without residual tumor cells. Only the lymph nodes with viable tumor cells were considered as positive lymph nodes. The lymph nodes with only regression change without viable tumor were negative lymph nodes.
Then, each participant was assigned TRG grade according to each TRG system such as Mandard system (10), Chirieac system (12), Schneider system (13), Hermann system (20), and JES system (21). Mandard system classified by assessing proportion of tumor comparing to the fibrosis. Chirieac system used certain percentage of residual tumor as clearer classifier. Schneider system took percentage of residual tumor and lymph node status into account together. Hermann system graded into presence or absence tumor cells. JES system also classified by certain percentage of residual tumor as Chirieac with different cut-point. The detail of each TRG system is presented in Table 1. In this study, we collapsed Mandard grade 4 and Mandard grade 5 into the same group because none of the case was classified into Mandard grade 5.
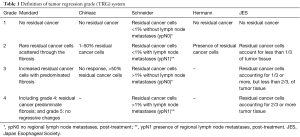
Full table
Statistical analysis
Descriptive statistics was used to describe characteristic of participants. Kaplan-Meier (KM) graphs and log-rank tests were used to describe the median survival time for each TRG system and assess the statistical significance of each TRG system, respectively. Cox proportional hazard regression model was used to assess the relationship between TRG system and mortality. Hazard ratio and 95% confidence interval were estimated from the cox regression model. Proportional hazard assumption was evaluated on the basis of Schoenfeld and log-log plot. The univariate Cox regression analysis was conducted to evaluated confounding factors. The confounding factor was selected based on P value <0.2. Age, sex and location of tumor were considered as potential confounders. The multivariate Cox regression analysis was conducted for each TRG system with confounding factors that meet the criteria in univariate analysis.
The model fit of each TRG grading system was assessed using the Akaike information criterion (AIC) value and pseudo R-squared value (23). The model with the lowest AIC value was considered the best. The higher pseudo R-squared value indicated better model fit. All statistical analyses were two-sided and P value <0.05 was considered a statistical significance, and were conducted with Stata version 15 software (24).
Results
Study population
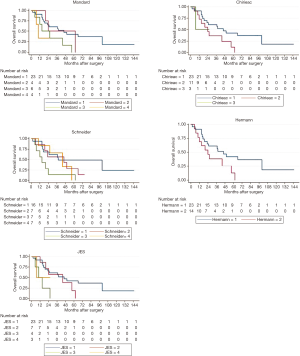
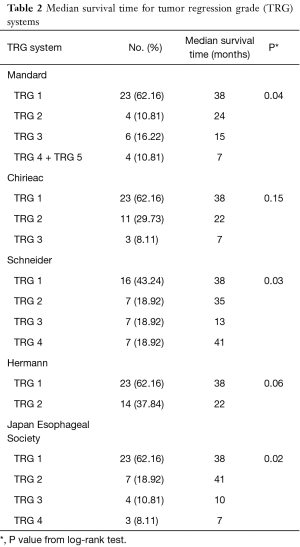
This study had 37 participants diagnosed with locally advanced ESSC. Median age at diagnosis was 58 years (ranging from 41 to 81 years). A majority of participants were male (84%). Approximately 62.2% of participants had cancer located at mid-esophagus, some (35.1%) at lower esophagus and only 1 case (2.7%) at upper esophagus. After receiving radio-chemotherapy, transthoracic esophagectomy with 2-field lymphadenectomy, either with an Ivor-Lewis or McKeown operation, was performed in all participants with a median time before surgery of 13 weeks. The median follow-up time after surgery was 37 months (interquartile range, 16.5 to 65.5 months). Twenty-six participants (70%) died and 19 participants (51%) developed local recurrence within the follow-up period. Classification of the participants into the various TRG systems and median survival time of each TRG system were shown in Table 2.
Full table
Association between TRG system and survival
The KM graphs of all TRG systems displayed overlapped curves as shown in Figure 1. Log-rank test results revealed that Schneider system, JES system and Mandard system were statistically associated with overall-survival (P<0.05), but Hermann system and Chirieac system were not, as shown in Table 2.
The univariate Cox regression analyses showed that age and sex were significant confounding factors, and, subsequently, included in the multivariate analyses. Only multivariate Cox regression analysis for Schneider system adjusted for age and sex was statistically significant (P=0.037).
Model fit
The model fits were ranked according to their AIC values. From multivariate analyses, Schneider system had the lowest AIC value (152.677), followed by Hermann system (153.367), Chirieac system (155.331), JES system (156.088) and Mandard system (156.185) (Table 3). Pseudo R-squared for the Schneider system was the highest (0.367), followed by JES system (0.278), Mandard system (0.275), Chirieac system (0.243), and Hermann system (0.242) (Table 3).

Full table
Discussion
Many indicators predicting prognosis in ESCC patients, who received preoperative radio-chemotherapy, were purposed, including TRG. In this study, we compared five TRG systems in their ability to predict prognosis, including Mandard system, Chirieac system, Schneider system, Hermann system, and JES system.
We found that Schneider system might be the best predictive system with statistically significant multivariate Cox regression analysis after adjusting for sex and age, and significant log-rank test. About the model fit, Schneider system also had the best model fit with lowest AIC value and highest pseudo R-squared value. In our opinion, Schneider system differed from other TRG systems because it included lymph node status for evaluation. Consistently, several studies showed significant association between lymph node status and postoperative survival (10,11,13,15,16,18). The overlapped KM curves might be the evidence from small number of cases and few people in extreme categories.
There were limitations in this study due to small number of participants, more participants in lower TRG grade and less participants in higher TRG grade. However, it is informative since this is the first study to explore which TRG system is the best in predicting prognosis by comparing various systems using multiple measures. In the future, study with more cases should be performed to confirm the findings.
Conclusions
Tumor regression grading system could predict overall survival for the ESCC patient treated with preoperative radio-chemotherapy. Schneider system might be the best model predicting prognosis with statistically significant log-rank test and multivariate Cox regression analysis, best AIC value, and best pseudo R-squared value. However, overlapped KM curves opposed. There were limitations in this study due to small number of participants and small number of participants in some TRGs. More cases collected would be more informative.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research protocol has been approved by an institutional review board (IRB) of Research Affairs, Faculty of Medicine, Chulalongkorn University (approval No. 682/60). This study collected patient data from hospital medical record system with official permission from director of the hospital and informed consent was not required.
References
- Fact Sheets by Cancer. Globocan.iarc.fr. 2018 (cited 20 September 2018). Available online: http://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf
- Kamangar F, Dores G, Anderson W. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Pohl H, Welch H. The Role of Overdiagnosis and Reclassification in the Marked Increase of Esophageal Adenocarcinoma Incidence. J Natl Cancer Inst 2005;97:142-6. [Crossref] [PubMed]
- Kamangar F, Chow WC, Abnet C, et al. Environmental Causes of Esophageal Cancer. Gastroenterol Clin North Am 2009;38:27-57. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival After Neoadjuvant Chemotherapy or Chemoradiotherapy for Resectable Oesophageal Carcinoma: an Updated Meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Guttmann DM, Mitra N, Metz JM, et al. Neoadjuvant Chemoradiation Is Associated with Improved Overall Survival in Older Patients with Esophageal Cancer. J Geriatr Oncol 2018;9:40-6. [Crossref] [PubMed]
- Cao XF, He XT, Ji L, et al. Effects of Neoadjuvant Radiochemotherapy on Pathological Staging and Prognosis for Locally Advanced Esophageal Squamous Cell Carcinoma. Dis Esophagus 2009;22:477-81. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic Assessment of Tumor Regression After Preoperative Chemoradiotherapy of Esophageal Carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Bachmann R, Bachmann J, Hungbauer A, et al. Impact of Response Evaluation for Resectable Esophageal Adenocarcinoma – A Retrospective Cohort Study. Int J Surg 2014;12:1025-30. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy Pathologic Stage Predicts Survival in Patients with Esophageal Carcinoma Receiving Preoperative Chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic Tumor Regression and Lymph Node Metastases Determine Prognosis Following Neoadjuvant Radiochemotherapy for Esophageal Cancer. Ann Surg 2005;242:684-92. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor Stage After Neoadjuvant Chemotherapy Determines Survival After Surgery for Adenocarcinoma of the Esophagus and Esophagogastric Junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Guo K, Cai L, Zhang Y, et al. The predictive value of histological tumor regression grading (TRG) for therapeutic evaluation in locally advanced esophageal carcinoma treated with neoadjuvant chemotherapy. Chin J Cancer 2012;31:399-408. [Crossref] [PubMed]
- Langer R, Ott K, Feith M, et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol 2009;22:1555-63. [Crossref] [PubMed]
- Wu TT, Chirieac LR, Abraham SC, et al. Excellent Interobserver Agreement on Grading the Extent of Residual Carcinoma After Preoperative Chemoradiation in Esophageal and Esophagogastric Junction Carcinoma: A Reliable Predictor for Patient Outcome. Am J Surg Pathol 2007;31:58-64. [Crossref] [PubMed]
- Chao YK, Chang CB, Chuang WY, et al. Correlation Between Tumor Regression Grade and Clinicopathological Parameters in Patients With Squamous Cell Carcinoma of the Esophagus Who Received Neoadjuvant Chemoradiotherapy. Medicine 2015;94:e1407. [Crossref] [PubMed]
- Swisher SG, Hofstetter W, Wu TT, et al. Proposed Revision of the Esophageal Cancer Staging System to Accommodate Pathologic Response (pP) Following Preoperative Chemoradiation (CRT). Ann Surg 2005;241:810-7; discussion 817-20. [Crossref] [PubMed]
- Hermann RM, Horstmann O, Haller F, et al. Histomorphological tumor regression grading of esophageal carcinoma after neoadjuvant radiochemotherapy: which score to use? Dis Esophagus 2006;19:329-34. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2016;14:1-36.
- van Meerten E, van der Gaast A, Tilanus H, et al. Pathological analysis after neoadjuvant chemoradiotherapy for esophageal carcinoma: The rotterdam experience. J Surg Oncol 2009;100:32-7. [Crossref] [PubMed]
- Hosmer DW Jr, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd ed. Hoboken: Wiley, 2008.
- StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.