Robot-assisted coronary artery bypass grafting improves short-term outcomes compared with minimally invasive direct coronary artery bypass grafting
Introduction
Surgical strategies for coronary revascularization are regarded as safe and efficient procedures that lead to excellent results. However, traditional coronary artery bypass grafting (CABG) using cardiopulmonary bypass (CPB) and ordinary off-pump CABG (OPCAB) with median sternotomy are associated with potential postoperative infection, sternal dehiscence, mediastinitis, and neurologic complications (1-3). Minimally invasive direct coronary artery bypass grafting (MIDCAB) can be performed through a left lateral thoracotomy and is a less invasive method of OPCAB. This procedure reduces morbidity, length of stay, need for blood transfusion, prolonged pain, and prolonged recovery (4,5).
An advanced form of MIDCAB has been developed using a robot-assisted coronary artery bypass graft (RACAB) with the da Vinci surgical system. The robot allows the surgeon to conveniently harvest internal mammary arteries (IMAs) and makes a multi-vessel MIDCAB possible (6). RACAB surgery is not reserved for single-vessel revascularization procedures bypassing the left anterior descending artery (LAD) with the left internal mammary artery (LIMA). When combined with a percutaneous coronary intervention (PCI) in a one-step or staged hybrid procedure, MIDCAB and RACAB surgery have been performed in multi-vessel diseases for integrated revascularization. These surgeries are safe and effective and have low perioperative morbidity, mortality, excellent angiographic LIMA patency, and favorable mid-term major adverse cardiac and cerebrovascular event (MACCE)-free survival (5,7,8).
The purpose of the present study was to compare the mid-term results of left-thoracotomy MIDCAB and RACAB in single- or multi-vessel coronary artery disease with or without a hybrid approach.
Materials and methods
Study patients
The medical records for all of the consecutive MIDCAB and RACAB patients with single- or multiple-vessel coronary artery disease who underwent minimally invasive OPCAB between May 2009 and May 2014 were analyzed using our institutional database. This study was approved by the Regional Ethics Committee of Shanghai Jiao Tong University and all patients signed informed consents.
The patients were further subdivided into MIDCAB or RACAB groups based on the type of surgery. There were 61 MIDCAB operations performed through a left lateral thoracotomy, and 71 RACAB surgeries were conducted on beating hearts using the da Vinci telemanipulation system (Intuitive Surgical, Inc., Sunnyvale, CA, USA). There were two and three patients, respectively, whose operation was converted to CABG by femoral artery—femoral vein bypass because of sudden and unexpected circumstances during surgery.
Surgical technique
All patients were prepared in the supine position with their left chest elevated approximately 30 degrees. After routine induction of general anesthesia, a double-lumen intubation was performed for single right lung ventilation. A Swan-Ganz catheter was placed into the cardiac tissue to monitor hemodynamic indices and continuous cardiac output. An external defibrillation pad was also prepared. All of the patients underwent OPCAB.
A small (6–9 cm) left anterior incision was made at the fourth intercostal space in the MIDCAB group. We avoided injuring the mammary tissue while exposing the operative field. The detailed technique of MIDCAB has been described elsewhere (1,9). The LIMA pedicle was completely harvested under direct vision without the help of thoracoscope. The anastomosis of the LIMA to the LAD was performed through the incision on the beating heart using regular stabilization devices (Genzyme Corporation) and stitched by hand. The anastomoses of the non-LAD lesions were also performed on the beating heart using of “Y” blood vessel graft to the LIMA.
In the RACAB group the LIMA or/and right internal mammary artery (RIMA) was harvested using the da Vinci robot system to facilitate port access to the chest while permitting the chest to be insufflated with CO2. The anastomoses were performed by direct-vision through a minithoracotomy (5–6 cm). The detailed technique of RACAB has been described elsewhere (10,11). If a second graft was necessary, the saphenous vein was harvested from the lower extremity simultaneously. In younger patients (below 60 years old), the RIMA pedicle was then harvested with the right chest elevated approximately 30 degrees. The rest of the procedure was similar to MIDCAB.
The graft blood flow was measured using a coronary ultrasonic flow meter (Transonic, Guidant, Indianapolis, IN, USA) after the anastomoses were completed. The systolic blood pressure was maintained at >100 mmHg. The mean flows (mL/min) and the pulsatility index (PI) were assessed. The criteria for chest closure were a PI <5, and the mean flow was at least >15 mL/min. Protamine was applied to neutralize 80% of the heparin before closing the chest.
Hybrid coronary revascularization (HCR) was performed by PCI of non-LAD lesions after grafting for the LIMA-LAD. The patients received stenting with sirolimus-eluting stents 5–14 days after surgical revascularization according to standard practice. Patients received 100 mg/d aspirin indefinitely and 75 mg/d clopidogrel for 1 year from the day after surgery. All patients were thoroughly informed about the procedure preoperatively and provided written consent.
Clinical follow-up
All of the patient baseline characteristics, operative variables, and postoperative outcomes (early events within 30 days postoperatively) were collected. All patients were monitored for at least 6 hours in the intensive care unit (ICU). The creatine kinase and cardiac troponin I (cTnI) levels were measured immediately after surgery and 12 hours later. A 12-lead electrocardiography was performed directly after the procedure. The number of patient with intercostal pain was counted. The presence of intercostal pain after surgery was defined as an incision pain requiring medication more than once a day for 3 days.
All patients were followed up at for least 12 months. The follow-up information included survival, MACCE-free survival, recurrent angina, freedom from percutaneous re-intervention and reoperation (late events). After 12 months of follow-up the asymptomatic patients received an invasive evaluation and coronary computed tomography angiography (CCTA). The other patients who had relevant signs of ischemia, such as palpitation or chest pain, were evaluated immediately by CTA or coronary angiography to determine if further vascular intervention was required.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 19.0 (IBM Corp, Armonk, NY, USA). All data are presented as continuous or categorical variables. The continuous data are presented as the mean ± standard deviation or median with range. The values were compared with unpaired Student’s t-tests after testing for normal distribution. All categorical data are expressed as a number and percentage. Chi-square test or Fisher’s exact test was used for categorical variables with nominal scales. The Wilcoxon test was used for data with ordinal scales. Kaplan-Meier curves and log-rank test were used to analyze overall survival and freedom from MACCEs. A 2-tailed probability value of P<0.05 was considered significantly different.
Results
Baseline characteristics analysis
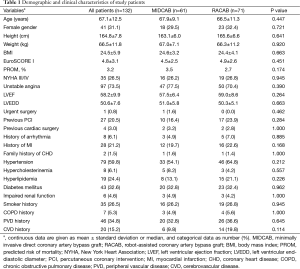
There were 43 males and 18 females in the MIDCAB group, and the mean patient age was 67.9±9.1 years. There were 48 males and 23 females in the RACAB group, and the mean patient age was 66.5±11.3 years. The preoperative patient clinical characteristics are similar between the two groups summarized in Table 1. The patients had a comparable left ventricular function and preoperative hemodynamic status at the time of revascularization. The EuroSCORE II risk model was used to evaluate the patient risk factors, and the two groups had a similar predicted mortality risk (3.5% vs. 2.7%, P=0.174). RACAB was used for more multiple vessel revascularizations (15 vs. 37, P=0.006). There are 46 cases of single LAD lesion in the MIDCAB group and 37 cases in the RACAB group.
Full table
The preoperative ejection fraction (EF) of the MIDCAB group patients ranged from 44% to 71%. The mean EF was 57.5%±6.4%. The EF in the RACAB group ranged from 46% to 73%. The mean EF in the MIDCAB group was 59.0%±8.6%, P=0.264. There was no statistically significant difference between the baseline characteristics of the consecutive MIDCAB and RACAB patients.
Operative data and postoperative 30-day outcomes
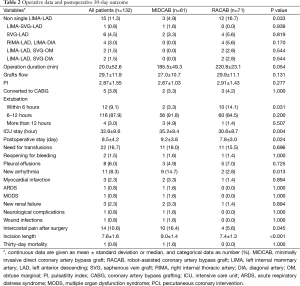
All coronary artery diseases underwent a complete revascularization in the two groups. There were 58 (95.1%) patients with anastomosis of LIMA-LAD in the MIDCAB group and 59 (83.3%) patients in RACAB group. More patients were receiving non-single LIMA-LAD revascularization in the RACAB group (P=0.033). The results are shown in Table 2. The grafted arteries included the left anterior descending coronary artery, ramus, diagonal and obtuse marginal branches 1. The overall conversion rate was 3.8% (two and three patients in the MIDCAB group and RACAB group). One conversion in the MIDCAB group was due to a dissected IMA and the other one was caused by an intramyocardial LAD. One conversion in the RACAB group was due to a dissected LAD and the others were caused by severely calcified LADs. Endarterectomy and CABG were performed in the two calcified LAD lesions after conversion, and the length of calcified vessels was 3–4 cm. There were no converted patients who developed a sternal wound infection.
Full table
The mean surgical duration in the RACAB group was longer than the MIDCAB group, but not significantly different (220.8±23.1 vs. 185.5±49.3 min, P=0.054). The ICU stay in the MIDCAB group (35.2±9.4 hours) was longer than in the RACAB group (30.6±8.7 hours, P=0.004). There were ten patients in the RACAB group who were extubated in the ICU within 12 hours after surgery, while two such patients in MIDCAB group (P=0.031). There were nine (14.7%) MIDCAB patients and two (2.8%) RACAB patients (P=0.013) that developed arrhythmia required intervention in postoperative 30 days. One patient in the MIDCAB group suffered a transient ischemic attack (TIA) and rehabilitated without sequel.
There was one (1.6%) 30-day death postoperatively, an 85-year-old patient due to cardiogenic shock with preoperative EF of 39%, and one patient required revision surgery in the MIDCAB group. There was one revision surgery and no 30-day mortality in the RACAB group. However, these differences were not statistically significant. The incision length was significantly shorter and the patient number with intercostal pain after surgery was significantly lower in RACAB group. These results suggest there is less surgical trauma. The postoperative stay was also shorter in the RACAB group (9.2±3.8 vs. 7.8±3.0 days, P=0.024).
Two-staged hybrid subgroup
The patient demographics and operative data are summarized in Table 3. There were 34 (47.9%) patients from the RACAB group treated with a two-staged hybrid procedure. There were no statistically significant differences between the two sub-groups regarding the mean age, body mass index (BMI), predicted risk of mortality (PROM), and target of PCI. Patients who underwent postoperative PCI in the RACAB group had a significantly shorter interval than the MIDCAB group (7.20±1.75 vs. 5.40±1.90, P=0.041).

Full table
Mid-term follow-up
Follow-up data are shown in Table 4 and were collected till May 2014 (up to 5 years). Median follow-up time was 22 (range, 12–60) months. There were 53 cases (86.9%) in the MIDCAB group and 62 cases (87.3%) in the RACAB group alive during follow-up. The patients lost to follow-up were three cases (4.9%) and five (7.0%) cases, respectively. The postoperative follow-up angiographies were not performed routinely. However, a 1-year follow-up CCTA was performed regularly. Angiographies were performed when individual patients had strong indications for this investigation. The number of patients who developed symptoms of typical myocardial ischemia indicating angiography was relatively small. Therefore, we did not have sufficient data for analysis. The anastomotic patency at 1 year was similar, 96.9% (62/64) and 96.2% (76/79) respectively in the MIDCAB and RACAB group, and all LIMA-LAD were patent.
Full table
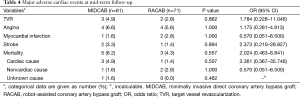
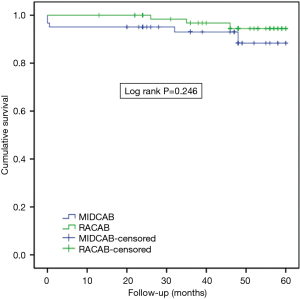
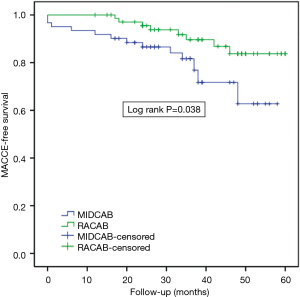
The cumulative survival rates for MIDCAB and RACAB patients were 96.7% and 100% at 1 year, 93.0% and 100% at 2 years and 88.4% and 94.4% at 5 years, respectively (Figure 1). There were no statistically significant differences in terms of cumulative survival (P=0.246, log rank =1.347). There were 13 major adverse cardiovascular events (MACCEs) during the 5-year follow-up. These events included five deaths (three cases died in hospital), two strokes, and three revascularizations of the target vessel in the MIDCAB group. Myocardial infarction (MI) is the commonest cause of midterm death. Two of three in the MIDCAB group were caused by MI and the other one was caused by heart failure. The death in the RACAB group was caused by MI. One of the two patients with stroke in the MIDCAB group was caused by thrombotic stroke due to atrial fibrillation and the other was caused by intracerebral hemorrhage. The stroke in the RACAB group was caused by cerebral infarction. The incidence of adverse events was significantly higher [hazard ratio (HR) =0.72, P=0.038, log rank =4.305] in the MIDCAB group than in the RACAB group (total nine events, three deaths, one strokes, two revascularization of the target vessel) (Figure 2).
Comment
The first CABG was performed in humans by Longmire in 1958 (12). Conventional open-chest CABG has been used for the past decade worldwide (13), and CABG is still the gold standard procedure for patients with multiple coronary artery disease (10). The short-term patency rates of OPCAB is similar to that of CABG, and it is associated with a reduced postoperative length of stay, less blood and blood component usage, and an earlier return to normal lifestyle (14,15). The latest meta-analysis showed that there was no significant difference between on-pump and OPCAB differences in mid-term adverse cardiac and cerebrovascular events or repeat revascularization (16). MIDCAB can also be performed “off-pump” and differs from OPCAB in the type of incision used for the surgery without a median sternotomy. Several published series found that MIDCAB is an effective and safe technique that is associated with shorter hospital stays, lower transfusion rates, reduced wound infection, and faster postoperative physical recovery (17,18).
The da Vinci surgical system has become the most widely used robot-assisted operation system in the world and is used for CABG in cardiac centers. A robotic surgical system has been developed to enhance the surgeon’s ability to harvest both IMAs through the left thoracic cavity using only three thoracic ports in 3D visualization. Thus, performing multi-vessel RACAB is now possible. From May 2009 to May 2014, there were 71 patients treated with the RACAB procedure using the da Vinci surgical system. All of the patients had an excellent short-term result, and 98.6% of patients (70/71) were extubated within 12 hours after surgery. The postoperative data showed that there were no wound infections, postoperative deaths, strokes or neurological complications. There were only three patients requiring conversion to conventional CABG. Only one patient needed mechanical ventilation for more than 24 hours. RACAB is thought to have many advantages over traditional CABG. The advantages include a lower surgical risk, smaller incisions, and a shorter recovery time (18,19). There are several published comparisons of MIDCAB and traditional CABG, but there are no studies examining MIDCAB and RACAB. Additionally, there are no retrospective analyses comparing MIDCAB and RACAB.
The main finding of the present report is that RACAB is safe and feasible. Furthermore, RACAB is associated with recovery advantages, including reduced incision lengths, lower intercostal pain rates (need intervened), shorter ICU and postoperative stay time, and a lower rate of new arrhythmia than MIDCAB. In addition, conventional MIDCAB is limited to single vessel revascularization because the entire length of the LIMA cannot be harvested by this procedure. A surgical robot, such as the da Vinci surgical system, can remove the entire length of both IMAs easily in a less invasive manner. RACAB can be used for multiple vessel revascularizations through three ports without long incisions in the thorax. There were fewer new arrhythmia cases in the RACAB group. This result is likely related to less surgical trauma. There was no significant difference in surgery duration between MIDCAB and RACAB patients reported in this study. However, the faster recovery and shorter ICU and postoperative stay time in the RACAB group implies at least an adequate and possibly superior physical activity in these patients.
HCR is an innovative treatment for multivessel disease that was introduced in the mid-1990s (20). Several studies have demonstrated the long-term patency rate of vein grafts was similar to the PCI of non-LAD vessels (21). In this study, there were 12 MIDCAB and 34 RACAB cases treated using this combination method of grafting for the LIMA-LAD and PCI of the remaining none-LAD lesions. There were more patients receiving RACAB, probably due to their better economic conditions and more reception to concept of new procedure. In all cases, HCR was completed without major complications or mortality. There was one cerebral lacuna infarct in the MIDCAB group and one MI case in the RACAB group. RACAB with or without PCI improved the short-term outcomes compared with MIDCAB. However, the middle term results and mortality outcomes were similar.
Several factors could have affected the outcomes of MIDCAB and RACAB. Off-pump RACAB with robotic harvesting of the LIMA is a reasonable and less invasive procedure than the standard procedures. MIDCAB or RACAB could prolong surgery time compared to traditional CABG (18). In our study, the mean operative times were 185.5±49.3 and 220.8±23.1 min with MIDCAB and RACAB, respectively. Furthermore, the installation and debugging of the da Vinci surgical system during the RACAB procedure could prolong the operative time. Additionally, multiple vessel revascularizations in RACAB could also prolong the operative time. Another criticism of MIDCAB or RACAB was that the surgeon has to be from limited fields and that it is difficult to convert MIDCAB to conventional CABG when the patient is hemodynamically unstable (22). Other potential problems may include difficulty in controlling bleeding from the graft in elderly patients and limited operation fields. Postoperative respiratory complications were more likely to occur in the RACAB group because of continuous one-lung ventilation during surgery (11). Properly identifying the target artery in RACAB is a difficult problem to solve and affected conversion to conventional open thoracic procedures. Cho and his colleague proposed a method to measure and validate perioperative shifts of the heart during RACAB using CT to reduce the conversion rate (23). One study launched by Escoto et al. found that the problem how to localize the target vessels could be resolved with preoperative CT localization (24). In our study, the MACCEs during the 5-year follow-up in the MIDCAB group were significantly higher than in the RACAB group. The difference in freedom from MACCE may be related to the difference in the ratio of concomitant PCI (47.9% vs. 19.7%, P=0.001). But the decreased MACCEs in the RACAB group did not affect overall survival compared to MIDCAB.
Limitations
This study is only a retrospective analysis prospectively collected data rather than a prospective randomized controlled study. However, the medical records for all of the consecutive MIDCAB and RACAB patients were analyzed using our institutional database. The individual patients were not identified, and consent was waived. All RACAB were performed by one group surgeons, but MIDCAB were not. There may be selective bias. But there was no statistically significant difference between the baseline characteristics of the two groups. Another potential limitation of this study is the relative small sample size available for operative and follow-up survival analysis. And the data not analyzed by propensity score were due to the sample population paucity. We did not investigate the difference in complete revascularization between the groups and comparison of outcomes in the subgroup because the relative small sample, which may be more important than comparison in the whole group.
Conclusions
RACAB may be a valuable alternative for patients in need of single or simple multi-vessel CABG. The operation appears to be at least as safe as MIDCAB and is associated with shorter hospital stay, less wound traumas, less intercostal pain and faster postoperative recovery than MIDCAB. RACAB may be more suitable for simple multi-vessel associated with PCI. Although the mid-term mortality outcomes are similar, RACAB improves short-term outcomes and mid-term MACCE-free survival compared with MIDCAB.
Acknowledgements
Funding: This work was funded by the Biomedical Engineering crossover Study Foundation of Shanghai Jiao Tong University (YG2012MS21) and National Natural Science Foundation of China (81200093, 81571826).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Srivastava SP, Patel KN, Skantharaja R, et al. Off-pump complete revascularization through a left lateral thoracotomy (ThoraCAB): the first 200 cases. Ann Thorac Surg 2003;76:46-9. [Crossref] [PubMed]
- Gulielmos V, Eller M, Thiele S, et al. Influence of median sternotomy on the psychosomatic outcome in coronary artery single-vessel bypass grafting. Eur J Cardiothorac Surg 1999;16 Suppl 2:S34-8. [Crossref] [PubMed]
- Dullum MK, Block J, Qazi A, et al. Xyphoid MIDCAB: report of the technique and experience with a less invasive MIDCAB procedure. Heart Surg Forum 1999;2:77-81. [PubMed]
- Magee MJ, Mack MJ. Robotics and coronary artery surgery. Curr Opin Cardiol 2002;17:602-7. [Crossref] [PubMed]
- Rogers CA, Pike K, Angelini GD, et al. An open randomized controlled trial of median sternotomy versus anterolateral left thoracotomy on morbidity and health care resource use in patients having off-pump coronary artery bypass surgery: the Sternotomy Versus Thoracotomy (STET) trial. J Thorac Cardiovasc Surg 2013;146:306-16.e1-9.
- Srivastava S, Gadasalli S, Agusala M, et al. Use of bilateral internal thoracic arteries in CABG through lateral thoracotomy with robotic assistance in 150 patients. Ann Thorac Surg 2006;81:800-6; discussion 806. [Crossref] [PubMed]
- Katz MR, Van Praet F, de Canniere D, et al. Integrated coronary revascularization: percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 2006;114:I473-6. [Crossref] [PubMed]
- Reser D. Mid-Term Outcomes of Minimally Invasive Direct Coronary Artery Bypass Grafting. Thorac Cardiovasc Surg 2015;63:313-8. [PubMed]
- Diegeler A, Thiele H, Falk V, et al. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med 2002;347:561-6. [Crossref] [PubMed]
- Takemura H. Robot-assisted coronary artery bypass. Circ J 2014;78:313-4. [Crossref] [PubMed]
- Liu TJ, Shih MS, Lee WL, et al. Hypoxemia during one-lung ventilation for robot-assisted coronary artery bypass graft surgery. Ann Thorac Surg 2013;96:127-32. [Crossref] [PubMed]
- Longmire WP Jr, Cannon JA, Kattus AA. Direct-vision coronary endarterectomy for angina pectoris. N Engl J Med 1958;259:993-9. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2012;143:4-34. [Crossref] [PubMed]
- Cooley DA. Con: beating-heart surgery for coronary revascularization: is it the most important development since the introduction of the heart-lung machine? Ann Thorac Surg 2000;70:1779-81. [Crossref] [PubMed]
- Polomsky M, Puskas JD. Off-pump coronary artery bypass grafting--the current state. Circ J 2012;76:784-90. [Crossref] [PubMed]
- Takagi H, Watanabe T, Mizuno Y, et al. A meta-analysis of large randomized trials for mid-term major cardio- and cerebrovascular events following off-pump versus on-pump coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2014;18:522-4. [Crossref] [PubMed]
- Lapierre H, Chan V, Sohmer B, et al. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump: a case-matched study. Eur J Cardiothorac Surg 2011;40:804-10. [PubMed]
- Wang S, Zhou J, Cai JF. Traditional coronary artery bypass graft versus totally endoscopic coronary artery bypass graft or robot-assisted coronary artery bypass graft--meta-analysis of 16 studies. Eur Rev Med Pharmacol Sci 2014;18:790-7. [PubMed]
- Ishikawa N, Watanabe G, Tomita S, et al. Robot-assisted minimally invasive direct coronary artery bypass grafting. ThoraCAB. Circ J 2014;78:399-402. [Crossref] [PubMed]
- Angelini GD, Wilde P, Salerno TA, et al. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 1996;347:757-8. [Crossref] [PubMed]
- Green KD, Lynch DR Jr, Chen TP, et al. Combining PCI and CABG: the role of hybrid revascularization. Curr Cardiol Rep 2013;15:351. [Crossref] [PubMed]
- Borst C, Gründeman PF. Minimally invasive coronary artery bypass grafting: an experimental perspective. Circulation 1999;99:1400-3. [Crossref] [PubMed]
- Cho DS, Linte C, Chen EC, et al. Predicting target vessel location on robot-assisted coronary artery bypass graft using CT to ultrasound registration. Med Phys 2012;39:1579-87. [Crossref] [PubMed]
- Escoto A, Trejos AL, Patel RV, et al. Anatomy-based eligibility measure for robotic-assisted bypass surgery. Innovations (Phila) 2014;9:349-53; discussion 353.


