CT-guided microcoil VATS resection of lung nodules: a single-centre experience and review of the literature
Introduction
Currently in Canada, the majority of lung resections are being performed with video-assisted thoracic surgery (VATS). This surgical technique has decreased the length of post-operative stay and complications for patients undergoing lung resection compared with open thoracotomy (1). Although it has many advantages, one important disadvantage is that it removes the surgeon’s tactile perception, often making it difficult to resect small pulmonary nodules that are difficult to palpate such as those that are deep in the lung parenchyma or have ground glass morphology. As imaging modalities improve, more pathology is found in the lung parenchyma with uncertain significance (2). Often, these patients are referred to surgery for diagnostic resection. Also, an increasing number of ground-glass opacities (GGOs) with no solid component are being identified, which are very difficult to localize intra-operatively even with open surgery because they are not palpable. In order to address this problem of identifying small pulmonary nodules for resection with VATS, many different techniques have been developed for nodule localization such as injection of compounds (methylene blue, radiotracers), hook wire, suture or microcoil insertions under CT guidance. Since October 2008, our center has been using computed-tomography (CT)-guided insertion of microcoils pre-operatively for intra-operative localization, modelled after the technique first described by Mayo et al. This study will present a single institution experience with CT-guided microcoil insertion followed by VATS resection.
Methods
After institutional Research Ethics Board approval was obtained (14-7395-BE), a retrospective chart review was performed that included all patients who underwent CT-guided microcoil insertion followed by VATS resection at a single institution, from the first procedure in October 2008, to January 2014. Requirement for patient consent was waived due to the retrospective nature of the study. No patients were excluded. Data was available for all patients. The data was collected and stored in a secure database. Statistical analysis was performed using SPSS software.
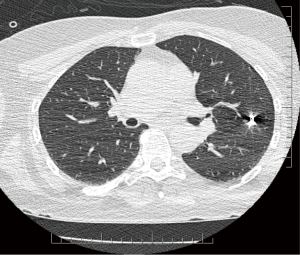
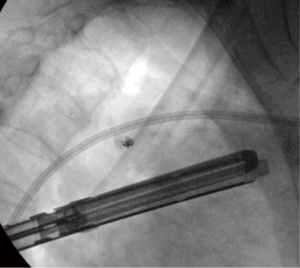
Once patients were identified as potential candidates for this technique (nodules less than 3 cm from the surface of the lung, GGOs, nodules less than 2 cm in size, and nodules that were suspicious for malignancy), a discussion occurred between the surgeon and radiologist to ensure that the CT-guided microcoil insertion was possible. The radiologic and surgical procedures were booked on the same day. CT-guided microcoil insertions were completed in the radiology department. For the first part of our series, one microcoil was placed to mark both the nodule and the visceral pleural surface. If the first coil was found to have migrated or retracted into the chest wall, a second microcoil was placed. This technique was modified due to difficulties placing the end of the microcoil that marked the visceral surface. For the latter part of the series only one microcoil was placed adjacent to the nodule without pleural marking (see Figure 1). The use of intra-operative fluoroscopy to locate the microcoil as well as multiple 3D reconstructions made it possible to avoid placing a visceral pleural surface marker. After microcoil insertion, the patients waited until the operating room was ready, at which time they proceeded to have their surgical resection. Seven surgeons performed the procedures. The VATS was done in the lateral decubitus position. The number of ports was based on surgeon preference. Intra-operative fluoroscopy was used to identify the microcoil, which localized the nodule to be resected (see Figure 2). Resection was then performed with multiple stapler firings. Intra-operative pathologic assessment and frozen section were requested based on surgeon preference and suspicion of malignancy. If frozen section showed primary lung cancer, the patients had an immediate completion lobectomy after the wedge resection. If frozen section was not performed, was not diagnostic, or showed that the nodule was benign, a chest tube was placed and the patient was awoken and taken to the recovery room.
Results
A total of 64 patients were included (33% male, mean age of 61.6±11.4 years). Forty-two patients (65.6%) had a history of smoking, of which 10 were current smokers. Twenty-nine patients (46%) had no history of cancer, 1 patient (1.6%) had a history of lung cancer, 32 (50.8%) of patients had a history of another type of cancer, and 1 patient (1.6%) had a history of lung cancer and another type of cancer. The characteristics of the nodules on CT were as follows: pure GGO 12 (18.8%), semi-solid 22 (34.4%), and solid 30 (46.9%) (see Table 1).

Full table
In terms of procedure performed, 61 (95%) patients had a wedge resection and 3 (5%) patients had a segmentectomy. A total of 3 patients required intra-operative re-resection of the staple line for positive or close margins. Intra-operative pathology was used for confirmation that the nodule was included in the specimen in 24 cases, and frozen section for diagnostic purposes was performed in 19 patients (8 adenocarcinoma, 1 non-small cell lung cancer, 2 malignant, 2 adenocarcinoma in situ, 2 granuloma, 2 benign, and 2 no diagnosis).
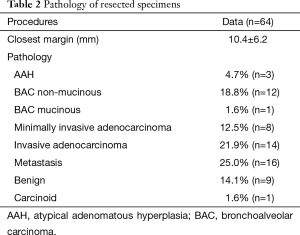
The average time between the CT-guided insertion and start of operation was 136.6±89.0 min, and average operative time was 84.0±53.3 min. For patients who only underwent wedge resection (excluding those who had concomitant lobectomy for separate primary lung cancer, or completion lobectomy for newly diagnosed primary lung cancer) the average operative time was 68.8±27.3 min. Eleven patients went on to have a completion lobectomy, 5 of which were during the same anaesthetic. Some microcoil-guided procedures were combined with other lung resections, with 2 patients having a separate lobectomy and one patient a separate segmentectomy. The intra-operative complication rate was 5% (3 patients), and included an episode of hemoptysis and two conversions to thoracotomy, one because the nodule was too deep to perform a wedge resection. The post-operative complication rate was 8% (5 patients), and included 2 air leaks, 1 hemothorax (drop in hemoglobin), 1 post chest tube removal pneumothorax, and one venous infarction of the lingula after lingula-sparing lobectomy requiring completion lobectomy. The post-operative length of stay ranged from 1–8 days, with an average stay of 2.5 days. A diagnosis was made for all patients (see Table 2).
Full table
Discussion
This retrospective study has confirmed the existing evidence that VATS resection after CT-guided microcoil insertion is a safe and successful procedure for diagnosis and treatment of small pulmonary nodules. For lesions with an established diagnosis of lung cancer, the current standard of care is to perform an anatomic resection, such as a lobectomy or bilobectomy, which is readily done using VATS. The situation becomes somewhat more challenging, though, when a non-anatomic resection, known as a wedge resection, is performed for diagnostic purposes.
As lung cancer screening gains popularity and the quality of imagining modalities improve, the number of small pulmonary nodules detected is increasing (2). Also, as systemic cancer therapies improve, there is becoming an increasing role for surgical resection of pulmonary nodules in patients with a history of cancer, for both diagnostic and therapeutic purposes. Small pulmonary nodules can be classified based on their CT characteristics, which may suggest whether or not they are malignant, but pathologic confirmation is still required due to the overlap in appearance between benign and malignant nodules (3). Within the primary lung adenocarcinoma spectrum, these nodules are classified based on whether or not they have a solid component, and range from pure GGOs, to semi-solid nodules, to solid nodules. The likelihood of invasive malignancy increases with the proportion of the nodule that is solid (3). The most common method of diagnosing lung lesions is CT-guided percutaneous biopsy, a method limited by small nodule size and challenging location (4). An alternative way of diagnosing small pulmonary nodules is by surgical resection, which is often used after percutaneous biopsy is non-diagnostic. Historically thoracotomies were performed, which allowed the detection of the nodules by sight as well as touch, especially useful for all lesions that were not located close to the visceral pleural surfaces. As VATS has become increasingly popular, a move has been made to perform diagnostic resections using this surgical approach, due to its reduced morbidity compared to lobectomy via open thoracotomy. One limitation of VATS, though, is that it removes tactile perception to locate the nodule intra-operatively. Not only is it difficult to identify solid and semi-solid nodules that are peripheral yet deep to the surface of the lung, it is also very difficult to identify pure GGOs as they are typically not palpable. In this study, 18.8% of lesions were pure GGOs, 34.4% were semisolid, and 46.9% were solid. With the use of the pre-operative microcoil placement and intra-operative fluoroscopy, successful diagnosis was made for all patients. Lobectomies were only performed once a diagnosis of lung cancer was made. All diagnoses were made using wedge (n=61) and segmentectomy (n=3).
The procedure for microcoil localization currently involves collaboration with the radiology department. Patients with lesions amenable to wedge resection are identified. In this study, selection criteria included nodules less than 3 cm from the surface of the lung, GGOs, nodules less than 2 cm in size, and nodules that were suspicious for malignancy (e.g., increase in size over serial CT scans or based on morphology). Coordination with radiology in planning the date of the procedure is necessary as patients have the microcoil placed in the radiology department on the morning of their surgical procedure. The fact that two procedures are required in two separate departments presents the potential for long wait times for the patients prior to their surgery. In our study, the time between the completion of the microcoil insertion (time of last CT scan) and start of operation (time patient entered the room) was 136.6±89.0 min. For most patients, therefore, surgery was started just over 2 h after the finished in the radiology department. Recently, a randomized controlled trial was completed by Finley et al., which showed that microcoil insertion prior to VATS wedge resection resulted in a shorter operative time, shorter length of stay, and improved diagnostic rate (5). The operative time reported in our series for all patients was 84.0±53.3, including two patients underwent a separate lobectomy (i.e., lobectomy was performed for a second lesion) and one patient underwent a separate segmentectomy during the same operation as the microcoil-guided wedge resection. Also, five patients went on to have completion lobectomies during the same anaesthetic after frozen section confirmed malignancy. For each of these eight patients, the total OR times included these much longer procedures, thereby increasing the average operative time for the wedge resections by an average of 15.2 min. The average length of stay was 2.5 days, and ranged from 1–8 days. For the patients who had an additional procedure during the same anaesthetic (i.e., completion lobectomy, separate lobectomy or segmentectomy) the average length of stay was 4.5 days. For patients who underwent only the microcoil-guided wedge resection, the average length of stay was 2.2 days. The diagnosis rate was 100% on final pathology. In 19 cases, frozen section was performed to establish a diagnosis intra-operatively, but for others intra-operative pathology was used only to confirm that the nodule was completely resected. Six patients who underwent completion lobectomy during a second anaesthetic were readmitted once the pathology results were available. The choice to perform intra-operative frozen section and completion lobectomy during the same anaesthetic was based on individual surgeon preference, and took into account operating room availability and level of suspicion of malignancy in the lesion.
The largest series reporting on the safety and efficacy of microcoil-guided VATS resection of small lung nodules is from Mayo et al., who studied 69 patients with 75 nodules. They described successful diagnosis of 97% of small lung nodules, with a 3% procedural complication rate (6). In examining the data, though, it is evident that the practice at our institution may differ from that described by Mayo, on important points such as the procedure for microcoil insertion and hospital length of stay. At our centre, coil insertion was performed by multiple chest radiologists and fellows, likely increasing the overall procedure time compared with insertions by a single operator. Bommart et al. recently published a series of 60 patients, and showed that the procedure resulted in smaller volumes of lung resected with a short procedure time and minimal complications (7). Similar findings have been recently published for the technique of pre-operative CT-guided microcoil placement and resection with or without intra-operative fluoroscopy (8-10). Successful use of embolization coils has also been reported in several small series (11-13), Similar techniques have been described, with pre-operative CT-guided insertion of radiopaque markers attached to sutures that lie on the surface of the lung (14,15), placement of metallic clips (16), fragmented coils (17). One series describes intentional creation of a pneumothorax during the coil placement, by inserted a pigtail catheter to instil air into the pleural space. The authors suggest that the presence of the pneumothorax facilities the position of the portion of the coil on the visceral pleural surface (18). With our modified technique, this extra chest tube insertion would be unnecessary as no visceral pleural surface marker is used. Most techniques for microcoil insertion in the literature involve transthoracic percutaneous coil insertion, yet there are also reports of successful CT-guided bronchoscopic marking with metallic coils (19). This technique of pre-operative microcoil insertion followed by VATS resection has also been used successfully in the pediatric population for resection of small pulmonary nodules (20).
Many methods have been described to aid in localization of small pulmonary nodules before VATS resection, including hookwire insertion, intra-operative ultrasound, injection of contrast material and injection of radioactive material.
One of the earliest described techniques is the use of a hookwire. Initially described by Gossot et al. in 1994, the procedure used a breast localization wire to localize the small peripheral pulmonary nodules. Their experience with 21 patients resulted in one failed placement with minimal complications and a mean length of stay of 1 to 6 days (21). The technique of using a breast localization wire has been used in a number of other studies, with rates of successful thoracoscopic resection ranging from 80–100% (22-24). Complication rates ranged from 7.5–51%, and included mostly asymptomatic pneumothoraces (22-24). One major problem with hookwires is the rate of dislodgement, as a component of the wire is external to the chest. This complication has been shown at rates ranging from 0–7.5% (22,23). Average length of stay was 3 days for VATS patients (22). Two commercial hookwire systems have been developed, the first in 1995 by Kanazawa et al. This system attempted to improve upon breast localization designs by making the wire 10 mm in length and attaching a 30-cm nylon suture as the component to be brought externally in order to decrease the chance of wire dislodgement secondary to external disruption (25). The same group published their results in 2002 for 168 patients, with a success rate of 97.6% (4 dislodgements). Procedure time was reported as 25–28 min, and although most patients [110] went to the OR within 1 h, 38 patients waited from 4–14 h between the hookwire insertion and resection (26). Using this system, Miyoshi et al. attempted to determine risk factors for dislodgement, but found none that had a significant impact. Also, they reported that insertion of the hookwire does not negatively impact outcome for patients with malignancy, with no evidence of tumour seeding or dissemination secondary to hook insertion (27). The second commercially available hookwire system has a coiled tip to be placed within the lung parenchyma, with a flexible wire that is brought external and coiled on the chest wall prior to VATS (28). A number of groups have reported results using this system, with success rates ranging from 86–100% and dislodgement rates of 0–9% (28-32). Other hookwire designs have been attempted, with success rates for VATS resection ranging from 88–100% with few complications reported and low rates of dislodgement (33-37).
Intra-operative ultrasound has been well-described for intra-operative localization of small pulmonary nodules. One of the first small series reported in 1996 described successful identification of small pulmonary nodules, with only 7.5 min of extra operative time required yet up to 150 min required from radiology staff to complete the procedure (38). Proponents of this technique hail the lack of procedure-related complications that are often associated with other methods of pre-operative localization (39,40). Despite the success describes by the aforementioned studies, one main drawback to this technique is the need for near-complete deflation of the lung to improve the resolution of the images. Although most studies report the actual ultrasound procedure time to be quite low, the time to deflate the lung can be quite lengthy in patients with emphysematous lungs, and many patients who have small pulmonary nodules fall into this group (41,42). In one series, ultrasonography wasn’t attempted until the lung had been excluded from ventilation for at least 40 min (43). Also, there is a learning curve associated with use of the ultrasound equipment, with some studies reporting the presence of a radiologist in the operating room (38,43,44). Suggestion has been made that nodules can be diagnosed based on their ultrasonographic characteristics, but more work must be done in this area (41,45).
Injection of various types of contrast media is another technique used for pre-operative localization of small pulmonary nodules. Some studies report the injection of a combination of both contrast material and a pigmented material, such as indigo carmine or methylene blue, with successful resection of all nodules (46). In one series, there was a delay up to one day after contrast injection until VATS resection, although this time lag did not affect the success of the procedure (47). The sole use of methylene blue, India ink, lipiodol and barium have also be reported in a few small series (48-52). Similar to the microcoil technique described above, most series involve the percutaneous placement of the contrast material yet some recent reports are using bronchoscopic guidance for pleural dye marking (53,54). Although a potentially promising technique, success rates as low as 81% have been reported due to both diffusion of the dye in the pleural space and lack of visualization of the dye (53). This technique has also been used in children, with one series reporting lung tattooing and VATS resection during the same general anaesthetic (55,56). The main cited limitation of this technique is that of diffusion of the contrast material and inability to accurately localize the pulmonary nodules, although in the studies mentioned above the technique was very successful with minimal complications (48,50). The use of surgical navigation systems with placement of skin fiducials to guide methylene blue injection has also been reported (57). A unique technique that has been described is that of “stamping” the nodule with a dye-containing gauze ball attached to thread, although the utility of this technique is limited because all nodules have to be identified by palpation prior to stamping (58).
A number of studies have investigated the use of radioactive-labelled colloid for pulmonary nodule localization. Most describe the use of 99Tc-labeled albumin, with pre-operative CT-guided injection and intra-operative localization with the use of the gamma ray detector. First described by Chella et al., this technique has been shown to be safe with minimal associated complications (59-62). The largest series of 200 patients reported a successfully VATS resection rate of 99.5% (63). Difficulties with localization were encountered for patients with bullous emphysema who had diffusion of the radiotracer throughout the parenchyma, and with spillage of the radiotracer into the pleural space resulting in very high background counts that required a repeat procedure at a later date in a small percentage of patients (60,63). The procedure time is reported to be as low as 6 min on average (64). Other advantages described include lack of insertion of a foreign body into the parenchyma, thereby removing the inherent associated risks, although a unique side-effect reported was that of allergic reaction to the material (60). Iodine seed (I-125) implantation has also been reported, although this technique was associated with a 21.4% rate of suboptimal placement (65).
Novel techniques have also been developed, including a tactile mechanoreceptor as well as the use of mobile CT for intra-operative localization (66,67).
Our study has a number of limitations. Firstly, there is no comparison group. Prior studies have compared non-localized VATS resection and microcoil-localized VATS resection (5). Although this study does not have a control group, the results are similar to prior reports described above that demonstrated short operative time, minimal post-operative complications, and high rate of successful diagnosis. Also, our study is limited by both the retrospective nature and relatively small number of patients included. As the technique gains acceptance, increasing numbers of patients are being selected for this protocol, which has demonstrated safety and efficacy compared to the pleural marking technique.
Conclusions
Pre-operative CT-guided microcoil localization increases the success rate of VATS wedge resection for small pulmonary nodules. This procedure is safe, with a short length of stay and minimal associated complications. This study has confirmed the results of prior studies using similar techniques. CT-guided microcoil localization should be considered for all patients with small pulmonary nodules that are not on the lung surface prior to VATS resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: After institutional Research Ethics Board approval was obtained (14-7395-BE), a retrospective chart review was performed that included all patients who underwent CT-guided microcoil insertion followed by VATS resection at a single institution, from the first procedure in October 2008, to January 2014. Requirement for patient consent was waived due to the retrospective nature of the study. No patients were excluded. Data was available for all patients. The data was collected and stored in a secure database.
References
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Godoy MC, Truong MT, Sabloff B, et al. Subsolid pulmonary nodule management and lung adenocarcinoma classification: state of the art and future trends. Semin Roentgenol 2013;48:295-307. [Crossref] [PubMed]
- Klein JS, Zarka MA. Transthoracic needle biopsy. Radiol Clin North Am 2000;38:235-66. vii. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [Crossref] [PubMed]
- Bommart S, Bourdin A, Marin G, et al. Impact of preoperative marking coils on surgical and pathologic management of impalpable lung nodules. Ann Thorac Surg 2014;97:414-8. [Crossref] [PubMed]
- Sui X, Zhao H, Yang F, et al. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis 2015;7:1580-7. [PubMed]
- Su TH, Fan YF, Jin L, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015;25:2627-33. [Crossref] [PubMed]
- Hajjar WM, Alnassar S, Almousa O, et al. Thoracoscopic resection of suspected metastatic pulmonary nodules after microcoil localization technique, a prospective study. J Cardiovasc Surg (Torino) 2014.16. [PubMed]
- Yoshida J, Shirota T, Tanimura A, et al. Clinical pathway for impalpable or small lung lesions treated with coil marking and thoracoscopy. Jpn J Thorac Cardiovasc Surg 2001;49:108-12. [Crossref] [PubMed]
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Liu L, Zhang LJ, Chen B, et al. Novel CT-guided coil localization of peripheral pulmonary nodules prior to video-assisted thoracoscopic surgery: a pilot study. Acta Radiol 2014;55:699-706. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Reinschmidt JP, Murray SP, Casha LM, et al. Localization of pulmonary nodules using suture-ligated microcoils. J Comput Assist Tomogr 2001;25:314-8. [Crossref] [PubMed]
- Tyng CJ, Baranauskas MV, Bitencourt AG, et al. Preoperative computed tomography-guided localization of ground-glass opacities with metallic clip. Ann Thorac Surg 2013;96:1087-9. [Crossref] [PubMed]
- Moon SW, Cho DG, Cho KD, et al. Fluoroscopy-assisted thoracoscopic resection for small intrapulmonary lesions after preoperative computed tomography-guided localization using fragmented platinum microcoils. Thorac Cardiovasc Surg 2012;60:413-8. [Crossref] [PubMed]
- Iqbal SI, Molgaard C, Williamson C, et al. Purposeful creation of a pneumothorax and chest tube placement to facilitate CT-guided coil localization of lung nodules before video-assisted thoracoscopic surgical wedge resection. J Vasc Interv Radiol 2014;25:1133-8. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Heran MK, Sangha BS, Mayo JR, et al. Lung nodules in children: video-assisted thoracoscopic surgical resection after computed tomography-guided localization using a microcoil. J Pediatr Surg 2011;46:1292-7. [Crossref] [PubMed]
- Gossot D, Miaux Y, Guermazi A, et al. The hook-wire technique for localization of pulmonary nodules during thoracoscopic resection. Chest 1994;105:1467-9. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Wicky S, Dusmet M, Doenz F, et al. Computed tomography-guided localization of small lung nodules before video-assisted resection: experience with an efficient hook-wire system. J Thorac Cardiovasc Surg 2002;124:401-3. [Crossref] [PubMed]
- Paci M, Annessi V, Giovanardi F, et al. Preoperative localization of indeterminate pulmonary nodules before videothoracoscopic resection. Surg Endosc 2002;16:509-11. [Crossref] [PubMed]
- Kanazawa S, Ando A, Yasui K, et al. Localization of small pulmonary nodules for thoracoscopic resection: use of a newly developed hookwire system. Cardiovasc Intervent Radiol 1995;18:122-4. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [Crossref] [PubMed]
- Partik BL, Leung AN, Müller MR, et al. Using a dedicated lung-marker system for localization of pulmonary nodules before thoracoscopic surgery. AJR Am J Roentgenol 2003;180:805-9. [Crossref] [PubMed]
- Hänninen EL, Langrehr J, Raakow R, et al. Computed tomography-guided pulmonary nodule localization before thoracoscopic resection. Acta Radiol 2004;45:284-8. [Crossref] [PubMed]
- Torre M, Ferraroli GM, Vanzulli A, et al. A new safe and stable spiral wire needle for thoracoscopic resection of lung nodules. Chest 2004;125:2289-93. [Crossref] [PubMed]
- Eichfeld U, Dietrich A, Ott R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6; discussion 316-7. [Crossref] [PubMed]
- Kastl S, Langwieler TE, Krupski-Berdien G, et al. Percutaneous localization of pulmonary nodules prior to thoracoscopic surgery by CT-guided hook-wire. Anticancer Res 2006;26:3123-6. [PubMed]
- Kakuda J, Omari B, Renslo R, et al. CT guided needle localization for video-thoracoscopic resection of pulmonary nodules. Eur J Med Res 1997;2:340-2. [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Role of video-assisted thoracic surgery for the diagnosis of indeterminate pulmonary nodule. Ann Thorac Cardiovasc Surg 2006;12:388-92. [PubMed]
- Hirschburger M, Sauer S, Schwandner T, et al. Extratumoral spiral fixed wire marking of small pulmonary nodules for thoracoscopic resection. Thorac Cardiovasc Surg 2008;56:106-9. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Li W, Wang Y, He X, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: Analysis of 103 patients. Oncol Lett 2012;4:824-8. [PubMed]
- Greenfield AL, Steiner RM, Liu JB, et al. Sonographic guidance for the localization of peripheral pulmonary nodules during thoracoscopy. AJR Am J Roentgenol 1997;168:1057-60. [Crossref] [PubMed]
- Mattioli S, D'Ovidio F, Daddi N, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg 2005;79:443-9; discussion 443-9. [Crossref] [PubMed]
- Khereba M, Ferraro P, Duranceau A, et al. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J Thorac Cardiovasc Surg 2012;144:1160-5. [Crossref] [PubMed]
- Yamamoto M, Takeo M, Meguro F, et al. Sonographic evaluation for peripheral pulmonary nodules during video-assisted thoracoscopic surgery. Surg Endosc 2003;17:825-7. [Crossref] [PubMed]
- Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999;68:218-22. [Crossref] [PubMed]
- Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol 2003;13:2358-64. [Crossref] [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [Crossref] [PubMed]
- Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur J Cardiothorac Surg 2004;26:469-73. [Crossref] [PubMed]
- Wang YZ, Boudreaux JP, Dowling A, et al. Percutaneous localisation of pulmonary nodules prior to video-assisted thoracoscopic surgery using methylene blue and TC-99. Eur J Cardiothorac Surg 2010;37:237-8. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Willekes L, Boutros C, Goldfarb MA. VATS intraoperative tattooing to facilitate solitary pulmonary nodule resection. J Cardiothorac Surg 2008;3:13. [Crossref] [PubMed]
- Magistrelli P, D'Ambra L, Berti S, et al. Use of India ink during preoperative computed tomography localization of small peripheral undiagnosed pulmonary nodules for thoracoscopic resection. World J Surg 2009;33:1421-4. [Crossref] [PubMed]
- Kim YD, Jeong YJ. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol 2011;52:64-9. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014.4. [PubMed]
- Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001;72:296-7. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Narayanam S, Gerstle T, Amaral J, et al. Lung tattooing combined with immediate video-assisted thoracoscopic resection (IVATR) as a single procedure in a hybrid room: our institutional experience in a pediatric population. Pediatr Radiol 2013;43:1144-51. [Crossref] [PubMed]
- Chen W, Chen L, Yang S, et al. A novel technique for localization of small pulmonary nodules. Chest 2007;131:1526-31. [Crossref] [PubMed]
- Kawada M, Okubo T, Poudel S, et al. A new marking technique for peripheral lung nodules avoiding pleural puncture: the intrathoracic stamping method. Interact Cardiovasc Thorac Surg 2013;16:381-3. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifirò G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Boni G, Bellina CR, Grosso M, et al. Gamma probe-guided thoracoscopic surgery of small pulmonary nodules. Tumori 2000;86:364-6. [PubMed]
- Burdine J, Joyce LD, Plunkett MB, et al. Feasibility and value of video-assisted thoracoscopic surgery wedge excision of small pulmonary nodules in patients with malignancy. Chest 2002;122:1467-70. [Crossref] [PubMed]
- Ambrogi MC, Melfi F, Zirafa C, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914-9. [Crossref] [PubMed]
- Bertolaccini L, Terzi A, Spada E, et al. Not palpable? Role of radio-guided video-assisted thoracic surgery for nonpalpable solitary pulmonary nodules. Gen Thorac Cardiovasc Surg 2012;60:280-4. [Crossref] [PubMed]
- Gobardhan PD, Djamin RS, Romme PJ, et al. The use of iodine seed (I-125) as a marker for the localisation of lung nodules in minimal invasive pulmonary surgery. Eur J Surg Oncol 2013;39:945-50. [Crossref] [PubMed]
- Barmin V, Sadovnichy V, Sokolov M, et al. An original device for intraoperative detection of small indeterminate nodules†. Eur J Cardiothorac Surg 2014;46:1027-31. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]