Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP)
Introduction
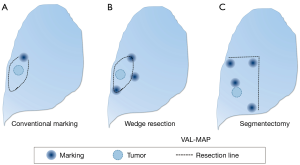
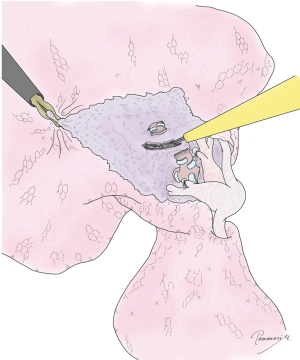
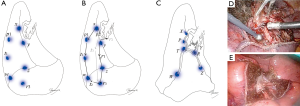
Virtual assisted lung mapping (VAL-MAP) is a novel preoperative lung-marking technique that utilizes three-dimensional (3D) virtual images based on thin-slice CT (1). In contrast with conventional lung-marking techniques, which usually involve the placement of visible markings near an invisible or hardly palpable tumor (2), lung mapping provides “geometric information” on the lung’s surface by placing multiple dye markings bronchoscopically (1,3) (Figure 1). Because of the very low risk of pneumothorax with this technique (3–4%), and the anatomical liberty available during bronchoscopic marking (3,4), the “map” drawn with VAL-MAP not only helps to identify the tumor, but also helps to determine oncologically appropriate resection lines. More specifically, by creating this map on the lung, surgeons can follow their pre-marked ideal resection lines, and ultimately minimize lung tissue removed in addition to obtaining oncologically satisfactory resection margins.
The unique features of VAL-MAP can be applied not only to lung wedge resections, but also to segmentectomies (Figure 1C). It is increasingly clear that segmentectomies provide comparable outcomes to lobectomies for early-stage lung cancers, particularly in those with ground-glass nodules (GGNs) (5,6). In contrast, a potential challenge of segmentectomies in GGN cases is the difficulty of palpating ground-glass components during operations. This is especially true in thoracoscopic surgeries, and without visibility of ideal resection lines. VAL-MAP allows the surgeon to designate appropriate resection lines, determined in 3D images, before the operation. The technique described in this article allows for accurate anatomical segmentectomies, including even complex ones, such as combined subsegmentectomies and extended segmentectomies.
Another challenge in segmentectomies is the creation of an intersegmental plane, and associated postoperative air leakage. Electrocautery is often used to develop an intersegmental plane, typically referring to demarcation lines made by inflation–deflation lines. This conventional technique allows surgeons to easily imagine the three-dimensional structure of the segment to be resected while creating intersegmental planes (7). However, despite multiple strategies reported and adequate risk-factor assessment (8-10), management of air leakage remains a major challenge. Furthermore, it is often associated with prolonged hospital stays, increased cost (11), and even empyemas (12). To avoid these complications, an appropriate stapling technique is required. Indeed, for a relatively simple segmentectomy such as lingular segmentectomy or segmentectomy of the superior segment of lower lobe (S6), stapling has been commonly applied. The strategy of using VAL-MAP, described in this article, enables surgeons to apply staples during even the most challenging of segmentectomies, such as S9 and S10 segmentectomies, and complex surgeries such as S6b + S8a + S9a combined subsegmentectomies.
From marking to mapping
Small lung nodules, including GGNs, are often indicated for surgical resection. However, they can be challenging to palpate, especially during thoracoscopic surgery. This challenge has led thoracic surgeons to devise methods of lung marking, such as CT-guided needle-mediated placement of markers, such as hook-wires (13) and dye injections (14,15). However, complications, including pneumothorax (30–50%), dislodgement of hook wires (up to 9%) (2), and potentially fatal air embolisms (1–2%) (16-19), remain drawbacks. In addition, location of percutaneous marking is anatomically limited, especially in the apex, interlobar fissure, and the areas facing the diaphragm or mediastinum. Another approach is to use a bronchoscope to place a marking such as a microcoil (20), or to inject dye (21,22) or other radiopaque material (23,24). These bronchoscopy-mediated techniques are reportedly and theoretically safer; however, they have not been widely used because they require real-time CT guidance for accurate marking (22).
Virtual bronchoscopies have made bronchoscopy-mediated lung markings possible without real-time CT imaging (25). Regular fluoroscopy is sufficient to ensure the tip of a dye-injection catheter has reached the visceral pleura. To confirm the actual location of a marking, CT can be used after mark placement. Although dye such as indigo carmine or indocyanine green is not radiopaque, the artifacts made by catheter passage are visible in CT within a few hours.
Because of the low risk of pneumothorax in bronchoscopy-mediated lung marking, it is easy to perform multiple markings at the same time. This subsequently resulted in the new concept of “lung mapping”, rather than marking, in VAL-MAP, which adds “geometric information” to the lung surface (1,3). The multiple markings are complementary to each other, and therefore, even if one or two marks fail, the lung map remains satisfactory.
Moreover, the multiple markings of VAL-MAP help surgeons determine the appropriate resection lines with respect to surgical margins. Therefore, compared with conventional single-marking techniques, VAL-MAP allows surgeons to complete a more oncologically-satisfactory surgery.
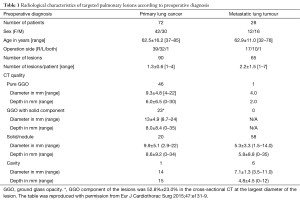
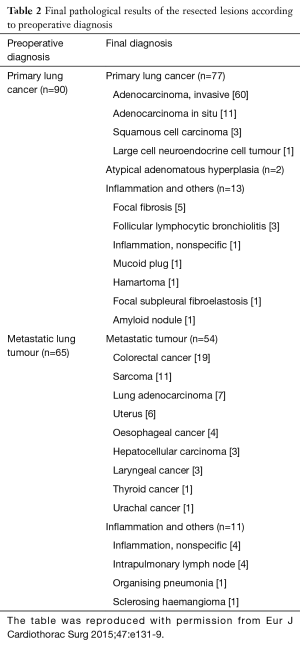
In our previous retrospective study reporting the outcomes of 100 consecutive cases of VAL-MAP, these feature of VAL-MAP led to a successful resection ratio of up to 99% (26). The patient characteristics and pathological outcomes of this study are shown in Tables 1,2. Complications related to VAL-MAP in this study were bulla formation in four patients; minor pneumothoraces in four patients without symptoms or a need for treatment; and suspected minor intra-alveolar bleeding in one patient. Other potential complications in VAL-MAP include fever and/or pneumonia after bronchoscopy, changes in vital signs and related complications during the bronchoscopic procedure, and allergic reactions associated with medications to be used in the procedure.
Full table
Full table
In summarizing the technique, VAL-MAP is a novel bronchoscopy-mediated multiple dye-marking technique utilizing virtual images, composed of several steps (Figure 2). The first step is designing the lung map and determining multiple (3–4 for wedge resection; 3–6 or even more for segmentectomy, see later discussion) target bronchi, using virtual bronchoscopy based on thin slice CT images (enhanced CT is needed only to visualize hilar vasculature in segmentectomy; plain CT is sufficient for VAL-MAP design). It usually takes 10 to 60 minutes to design the map and determine target bronchi, depending on the number of markings, the familiarity of the surgeon and/or bronchoscopist with the 3D software as well as the type of the software to be used.
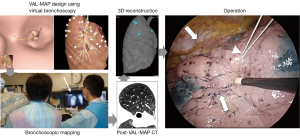
In the second step, an actual bronchoscope is inserted, using the guidance of virtual bronchoscopy after sedation on either the day of surgery or the day prior. For each target bronchus, a metal-tip catheter (PW-6C-1, Olympus, Tokyo, Japan) is inserted, and the dye (1 mL of indigo carmine, a blue dye per mark) is injected, while confirming the location of the catheter tip under fluoroscopy. In our previous study (26), the total bronchoscopy time (from insertion of the bronchoscope to the end of mapping) was 20.1±5.65 minutes, and the time from identification of the first target bronchus to the end of mapping was 15.0±5.1 minutes (3.7±1.0 minutes/mark). There are some technical tips in dye injection that was detailed in previous reports (3,26). Videos showing the principle of the procedure as well as tips in dye injection are accessible online though one of our previous publications (26).
Third, after VAL-MAP, another CT scan (no contrast needed; 1 to 1.25 mm thickness) is taken to visualize the actual location of dye markings. The CT scan is usually taken immediately after the mapping procedure, but 2–3 hours are acceptable to visualize the water density of the dye. Fourth, this CT images reflecting the actual location of markings is reconstructed into 3D images. The location of markings may vary from the original plan’s locations, and this can be adjusted at this stage. Fifth, during surgery, the markings will be identical to those seen in post-VAL-MAP 3D images. For clear visibility of markings, surgery on the same day of the marking procedure or the next day is recommended (3).
Segmentectomy utilizing VAL-MAP
The need for segmentectomies has increased for lung cancer surgeries, particularly for relatively small lesions with ground-glass appearance on imaging, and for metastatic tumors located relatively deep in the lung parenchyma. A potential concern of applying classical segmentectomies to these tumors is local recurrence, which results from insufficient resection margins (27,28). In general, resection margins larger than the diameter of the tumor, or larger than 2 cm, are ideal (28,29). Thus, it is important to apply a strategy to guarantee sufficient resection margins during segmentectomy.
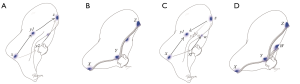
By applying VAL-MAP to segmentectomies, we found that the geometric information on the lung surface enables surgeons to determine oncologically appropriate resection lines, even beyond conventional anatomical segments (such as in a complex segmentectomy). Although the map per se is on the lung surface, virtual 3D images, as shown in Figure 3A, also provide accurate hilar anatomy preoperatively (30,31). Thus, by connecting the hilum to the predetermined resection lines on the lung surface, three-dimensional intersegmental planes can be drawn on 3D images (Figure 3B).
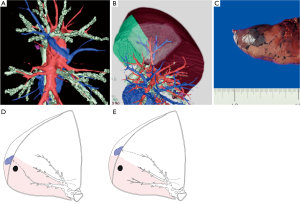
If an appropriate marking technique is applied, VAL-MAP results in the clear staining of a single secondary lobule, which, in principle does not expand between segments (Figure 3C).
Multiple markings via VAL-MAP can be placed along the intersegmental resection lines in a segmentectomy. If a bronchus reaching the outermost area of the target segment is selected, the marking is likely to be very close to the intersegmental line (Figure 3D). Thus, if such a marking is made on the inside of the target segment, the outer staining should exactly match the anatomical intersegmental line. Conversely, if an ideal bronchus reaching close to the intersegmental line is not found, a bronchus coming from the adjacent segment can be used instead. In this case, the inside of the staining is the intersegmental line (Figure 3E). In another case, a surgical margin at the exact anatomical intersegmental plane may be concerning. Therefore, a marking placed on the adjacent segment may be a good landmark to obtain sufficient resection margins by cutting into the adjacent segment [such as in an extended segmentectomy (32)]. In either case, depending on the type of segmentectomy, usually 3–6 markings along the intersegmental line provide sufficient geometric information to complete an anatomical segmentectomy.
Segmentectomies are often applied to pure or mixed GGNs suspicious for lung cancer, for which wedge resection is considered oncologically insufficient, but lobectomy may be reserved. When the extension of the GGN component makes surgical margin concerning, VAL-MAP is especially indicated. Another indication of VAL-MAP for segmentectomies is in the case of a centrally located nodular lesion suspicious for a metastatic lung tumor. Here, pure anatomical segmentectomy is not oncologically necessary; the resection of central structures in combination with a “wide wedge” resection, which guarantees resection margins, may be sufficient.
Five critical steps of stapler-based VAL-MAP segmentectomy
There are five key steps for stapler-based VAL-MAP segmentectomies. Each step is correlated with the others, and plays a critical role in the completion of a successful thoracoscopic segmentectomy.
- Step 1: placing “standing” marking stitches along resection lines;
- Step 2: cleaning hilar anatomy;
- Step 3: confirming hilar anatomy;
- Step 4: going 1 cm deeper;
- Step 5: step-by-step stapling technique.
Step 1: placing “standing” marking stitches along resection lines
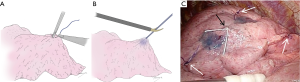
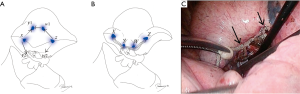
At the very beginning of the operation, several “standing” stitches are placed along the resection lines, typically around the corner and, if necessary, in between them (Figure 4). In segmentectomies with linear stapling, three stitches are usually adequate. In segmentectomies with V- or U-shaped stapling, at least four stitches are placed. In segmentectomies with 3D stapling involving the diaphragmatic aspect of the basal segment, 5–7 stitches are ideal. Semi-firm monofilament stitches, such as 3-0 PDS®, are ideal, because they stand out of the lung at corner and intermediate points, if necessary. The stitches should be cut somewhat long (1 cm or greater) for better visualization from a distance or from a tangential direction. This is especially true at the time of stapling. As an additional option, a stitch in a different color (such as white silk) can be placed near the targeted tumor, to draw attention to the surgical margin during later stapling.
Step 2: exposure of “clean” hilar anatomy
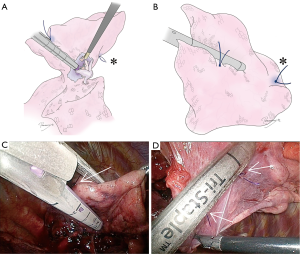
To accurately identify hilar anatomy, and then accurately conduct a segmentectomy, it is critical to expose “clean” anatomy as in a textbook or 3D image constructed from a CT scan. There are several technical tips for good exposure. First, sharp dissection in combination with appropriate blunt dissection is important. If only blunt dissection is used, it is difficult to reach the ideal perivascular or peribronchial layer for smooth dissection. Second, lymph nodes between these hilar structures should be cleanly dissected with blunt and sharp dissection (Figure 5). This is important not only for lymph node sampling and decision making but for exposure of hilar structures. Third, taping and proper retraction of structures should not be avoided, even if the structure is the one to be preserved (Figure 5). In segmentectomy, identification and confirmation of each hilar structure are critical. As such, the structure upfront needs to be preserved until the structure behind is dissected and the anatomy is confirmed. It is important to tape the structure upfront and then retract to it for further dissection (Figure 5). Although this is a very basic surgical technique, it is often forgotten or ignored in complete thoracoscopic operation. It is important to keep in mind that appropriate retraction of a structure could make the dissection procedure extremely easy.
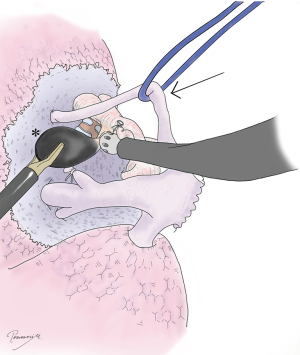
Step 3: confirmation of hilar anatomy
Before resecting hilar structures, it is highly important to confirm the anatomy. Branching of blood vessels should be examined in light of pre-operative 3D images. Note that the 3D image may be a mistake, or may not pick up a tiny branch. Despite this, the anatomy in the operating field should be consistent with the 3D image. To confirm the anatomy, VAL-MAP markings and stitches placed during step 1 (arrow in Figure 6A) are helpful. Intraoperative bronchoscopy from the anesthetist’s side is also critical to confirm the bronchial and related anatomy, and this should be performed routinely. The anatomy of the bronchus from the inside should be checked, and the bronchial branch to be closed should be confirmed by virtual bronchoscopy before the operation. The light from the bronchoscope (arrow in Figure 6B) is helpful. Manipulation in the operating field, such as clumping and declumping of a bronchus, can also be observed by bronchoscopy.
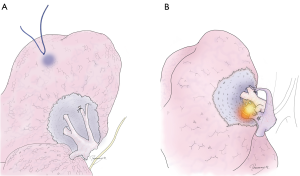
Step 4: going 1 cm deeper
Once all the targeted hilar structures are resected, it is important not to rush to stapling. Using electrocautery or an energy device, the lung tissue between the peripheral edges of the resected structures and the structures to be preserved is cut approximately 1 cm deep (the width of a stapler). It is important to apply appropriate traction on the tissue. This process is common in conventional segmentectomies, and therefore, if the cutting line (interrupted line in Figure 7) is on the right intersegmental plane, an intersegmental vein may also be exposed if it is not previously cut.
Step 5: step-by-step stapling technique
Finally, the targeted segment is stapled out. This is key to the stapler-based VAL-MAP-assisted segmentectomy, and is described in detail in the next section.
The stapling technique
The principle of the stapling technique
Stapler-based segmentectomies can be applied to all types of segmentectomies, including those involving basal segments of lower lobes, which are among the most challenging. The principle of stapling is as follows. First, stapling is guided by the “standing” stitches placed at step 1. Because the stitch markings are standing out of the lung surface, they are easily visible during stapling in the limited vision of thoracoscopic operation (Figure 8). Even when the tip of a stapler does not reach the stitch marking, that ahead of the stapler tip indicates the right orientation of the stapler. Second, when a stapler reaches the hilum, the stapler is placed in the pocket created at step 4, “going deeper by one centimeter”, to avoid cutting into the adjacent segment or involving the hilar structures of the targeted segment. If the pocket is not sufficiently deep for stapling, step 4 (going 1 cm deep) can be repeated at this stage by electrocautery (Figure 8).
Third, the lung should be stapled from the periphery to the center. If multiple intersegmental planes need to be cut, peripheral staplings should be done before going into the central or hilar stapling. In other words, stapling should be started from the peripheral parts of the lung (such as for the easier parts) and continued to reach the most difficult hilar stapling in a step-by-step manner, especially in those necessitating three dimensional stapling.
At the time of stapling, it is highly important to confirm the position of the both sides of a stapler (the anvil and the cartilage) by twisting the stapler, changing the angle of the thoracoscope (Figure 8), and, if necessary, placing the thoracoscope from different ports. Depending on the difficulty in the stapling technique, we divide segmentectomies into three levels: linear stapling, V-shaped or U-shaped stapling, and three-dimensional stapling. Selection of staplers depends on the surgeon’s preference. In general, the tissue close to the hilum tends to be thick so that a staple cartilage with sufficient height and strength (e.g., black or green cartilage) should be selected.
Segmentectomy completed by linear stapling
This is the easiest type of segmentectomy regarding stapler use. The staple lines made basically in a straight fashion (Figure 9A,B). A principle of moving from the outside to the center should always be kept in mind. The linear stapling segmentectomy includes that of S6, basal segment, left lingular (S4+5) segment, left superior (S1+2 and S3) segment and left S1+2. If these segmentectomies are extended into an adjacent subsegment (such as left S1+2 + S3a and S6 + S8a), the intersegmental line is not completely straight but can easily be applied by somewhat bending the line along the stitch markings as described above.
Despite easy stapler application, full attention should be paid to the appropriate surgical margin. For example, in a S6 segmentectomy, S6 is sometimes unexpectedly large, and a GGN is often located close to the border between S6 and the basal segment. Once completing resecting the hilar structure of S6, the stapler could be carelessly applied, because it is not technically difficult. However, the peripheral resection line could be placed a different way, and without VAL-MAP or other marking techniques there is a danger of insufficient resection margins.
Although the stapler can be applied in a linear manner in this group, the stapling line can also be made in a “Mercedes-Benz mark” fashion. If the intersegmental line needs to be stapled more than twice, one stapling could be placed from a different angle, resulting in a “Mercedes-Benz mark”-like staple line (Figure 9C,D), which refers a style of incision in which an upward midline extension is occasionally required. This typically applies to dividing an intersegmental plane between S6 and the basal segment, and the superior and lingular segments of the left lung, where the intersegmental plane is relatively large. By stapling in a Mercedes-Benz mark-like fashion, the remaining lung might be expanded better at the time of inflation.
Segmentectomy completed by V- or U-shaped stapling
In this group of segmentectomies, there are two or three intersegmental planes, and thus, the staple line ends up with a V-shape or U-shape. This group includes segmentectomies of right S1 and left S3. Combined subsegmentectomies such as right S2b+3a and S6b+8a+9a are also in this group. In this type of segmentectomy, two-directional stapling from the periphery to the hilum is effective and is not technically difficult (Figure 10). In most S3 segmentectomies, however, the first stapling along the S3–S4 intersegmental plane is continued toward the apex almost straightly (as in linear stapling segmentectomy) if the anterior part of the S3 segment is pulled toward the caudal direction appropriately. If this is possible, two-directional stapling is not necessary. In right S1 segmentectomies, a similar stapling technique is often applicable depending on the angle of the V-shape.
Segmentectomy completed by three-dimensional stapling
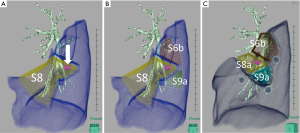
In this most challenging group of segmentectomies, the intersegmental planes make a cuboid-shaped segment primarily because of the plane phasing the diaphragm. However, by following the principles of the stapler-based VAL-MAP segmentectomies, this type of segmentectomy can be completed in a reproducible manner. This group of segmentectomies includes segmentectomies of S7, S8, S9, and S10, and their variations such as S9+10b. Those not facing the interlobar fissure (such as S9 and S10) are especially challenging. The challenge problem arises mostly from difficulty imagining how the cuboidal segment can be folded by multiple stapling. One of the most challenging series of stapling in S9 segmentectomies is summarized in Figure 11. Before operation or even during the operation’s stapling process, it is difficult to imagine how the lung is cut and folded. However, by following the principle of “staple from the periphery to the center” and following “standing” marking stitches step by step, the resection lines come together.
In the example of S9 segmentectomies in Figure 11, the S6–S9 intersegmental plane is the one made first. The S8–S9 intersegmental plane can also be made early. Importantly, these staplings toward the hilum facilitate visualization of the deep hilar structure that is otherwise hidden deep in the lung. Stapling should not be limited to only after transection of all hilar structures to be resected; if a stapling facilitates hilar visualization, it should be done earlier. Two staplings on the diaphragm side should then be managed. Once the relatively peripheral staplings are completed, the remaining parts are stapled peripherally to centrally along the stitch markings to complete the segmentectomy.
After removing the specimen
Once stapling is completed and the specimen is removed, hemostasis and management of air leakage are the final steps to complete the operation. If a bronchus is tied and cut, we usually start from reinforcing the stump with 4-0 monofilament stitch such as proline®. Next, we routinely use soft coagulation which coagulates proteins with relatively low temperature. By coagulating the lung tissue without being covered by the pleura, excellent sealing of air leakage is obtained. Because the lung tissue close to the hilum causes only minor air leakage probably as a result of the scant bronchioles, soft coagulation alone is often sufficient to eliminate air leakage. If not, fibrin glue and/or other biomaterials could be considered.
Usually, once the specimen is out, hemostasis is mostly obtained. Oozing along the staple lines, particularly the peripheral ones are often observed. Soft coagulation is useful to obtain excellent hemostasis as well. Finally, if a bronchial stump is in contact with a pulmonary artery, it might be better to place some material (fat pad or cellulose material such as Surgicel®) between the stump and artery. This typically happens in S6 segmentectomies.
Conventional segmentectomy techniques vs. VAL-MAP segmentectomy
Development of an inflation-deflation line has been a popular strategy to identify the intersegmental plane (8), while the development of the line sometimes appears technically challenging owing to the inflation of adjacent segments or interfered inflation as a result of airway secretion. Importantly, there is no way to confirm that the created inflation-deflation line is the one that was planned before the operation. Moreover, inflation of the lung significantly interferes with visualization under thoracoscope. Another reported strategy is to inject dye such as indocyanine green from a targeted bronchus (33). Although the interference of the operation field under thoracoscope may be better than the inflation-deflation technique, the dye may diffuse heterogeneously in the segment and “reproducibility” remains a concern. Namely, there is no way to confirm the anatomy indicated by the injected dye during operation is correct.
In contrast, the map drawn on the lung by VAL-MAP is correlated with CT images, especially that taken after VAL-MAP (26). Surgeons can tell the distance, for example, from the edge of the tumor to a marking or a resection line connecting two markings on 3D images before the operation. Furthermore, because VAL-MAP provides with geometric information on the lung, surgeons can adjust their operation plan after VAL-MAP using the 3D images that reflect the actual localization of each marking and the tumor (4,26).
This is particularly true in surgeries with anatomical complexity beyond conventional anatomical segmentectomies, such as resection of combined subsegments and extension of a segmentectomy into the adjacent segment (such as extended segmentectomies) (32). Indeed, because cancer is not a disease that develops following anatomical lung segments, conventional anatomical segmentectomies are often unsatisfactory from an oncological point of view. On the one hand, resection of an anatomical segment results in the removal of abundant extra tissue far from the tumor; conversely, the tumor may be located close to the intersegmental plane, resulting in concerning resection margins (Figure 12A). Complex segmentectomies beyond such conventional segmentectomies freely allow surgeons to determine resection lines to obtain sufficient resection margins. Instead of adherence to pure anatomical segments, a combination of an anatomical segment plus part of an adjacent segment or a whole subsegment (Figure 12B) provide sufficient resection margins. Furthermore, a combination of subsegments may result in satisfactory resection margin without taking extra lung tissue (Figure 12C). Recent radiology workstations and related handy software such as Synapse Vincent® (Fujifilm Medical, Tokyo, Japan) enable surgeons to design and display ideal (sub)segments to be targeted in relation to detailed hilar structures (Figure 3B). As such, in combination with recent technologies, stapler-based VAL-MAP-assisted navigational segmentectomies provide surgeons the opportunity to freely design an oncologically satisfactory lung-preserving operation.
Limitations of VAL-MAP
Although stapler-based VAL-MAP-assisted navigational segmentectomies have been successful in our recent clinical trial, the solid scientific data to support the technique remains to be produced. First, our preliminary data suggest excellent pulmonary function of patients who underwent this operation; this needs to be further confirmed. Second, complications associated with conventional segmentectomy, especially prolonged air leak, appear to make the stapler-based segmentectomy far superior to conventional electrocautery-based segmentectomies; more relevant data is needed. Third, the oncological benefit regarding local recurrence and patient survival need to be further examined. We are starting a nation-wide study using VAL-MAP, the primary endpoint of which is the acquisition of sufficient resection margins. This endpoint should be correlated with the local recurrence ratio (28,34) and should be an important step for the evaluation of VAL-MAP.
Technically, VAL-MAP is still on the way of further development. For example, current dye markings are limited owing to difficult visibility on smokers’ black lungs, especially if the Brinkman Index is over 500 (35). A new technique to overcome this limitation is in development (3).
In summary, the stapler-based VAL-MAP-assisted navigational segmentectomy is a novel technique that allows surgeons to design ideal anatomical lung resection in a reproducible way. This technique is expected to reduce the burden on surgeons and patients, such as concern about surgical margins and complications such as air leakage. Further study is needed to support the value of this new technique, and further development of VAL-MAP is desirable.
Acknowledgements
The authors thank to all the surgeons and respirologists who have participated in a multi-center study using VAL-MAP, particularly to those in Kyoto University and the University of Tokyo, where the technique of stapler-based VAL-MAP-assisted segmentectomy was developed.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Sato M. Virtual assisted lung mapping: navigational thoracoscopic lung resection. Cancer Res Front 2016;2:85-104. [Crossref]
- Sato M, Aoyama A, Yamada T, et al. Thoracoscopic wedge lung resection using virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2015;23:46-54. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Jones DR, Stiles BM, Denlinger CE, et al. Pulmonary segmentectomy: results and complications. Ann Thorac Surg 2003;76:343-8; discussion 348-9. [Crossref] [PubMed]
- Isowa N, Hasegawa S, Bando T, et al. Preoperative risk factors for prolonged air leak following lobectomy or segmentectomy for primary lung cancer. Eur J Cardiothorac Surg 2002;21:951. [Crossref] [PubMed]
- Wood DE, Lauer LM, Layton A, et al. Prolonged length of stay associated with air leak following pulmonary resection has a negative impact on hospital margin. Clinicoecon Outcomes Res 2016;8:187-95. [Crossref] [PubMed]
- Yamamoto S, Endo S, Minegishi K, et al. Polyglycolic acid mesh occlusion for postoperative bronchopleural fistula. Asian Cardiovasc Thorac Ann 2015;23:931-6. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Magistrelli P, D'Ambra L, Berti S, et al. Use of India ink during preoperative computed tomography localization of small peripheral undiagnosed pulmonary nodules for thoracoscopic resection. World J Surg 2009;33:1421-4. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araújo LM, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Matsuura Y, Watari M. Two cases of air bubble in intracardiac cavity after computed tomography-guided lung puncture. Jpn J Chest Surg 2010;24:967-71. [Crossref]
- Kondo T, Tokunaga Y, Saito M, et al. Two cases of air embolism during percutaneous pulmonary marking under computed tomography guidance. Jpn J Chest Surg 2012;26:729-33. [Crossref]
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [Crossref] [PubMed]
- Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001;72:296-7. [Crossref] [PubMed]
- Endo M, Kotani Y, Satouchi M, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest 2004;125:1747-52. [Crossref] [PubMed]
- Asano F, Matsuno Y, Ibuka T, et al. A barium marking method using an ultrathin bronchoscope with virtual bronchoscopic navigation. Respirology 2004;9:409-13. [Crossref] [PubMed]
- Okumura T, Kondo H, Suzuki K, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg 2001;71:439-42. [Crossref] [PubMed]
- Asano F, Shindoh J, Shigemitsu K, et al. Ultrathin bronchoscopic barium marking with virtual bronchoscopic navigation for fluoroscopy-assisted thoracoscopic surgery. Chest 2004;126:1687-93. [Crossref] [PubMed]
- Sato M, Yamada T, Menju T, et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131-9. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188-90. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- Yamanashi K, Sato M, Marumo S, et al. Emphysematous lungs do not affect visibility of virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2016;24:152-7. [Crossref] [PubMed]


