Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: a systematic review and meta-analysis of matched studies
Introduction
Aortic stenosis (AS) is the most common valvular heart disease and its incidence is increasing with an ageing population (1). Untreated AS carries a high mortality of up to 50% within 2 years of symptom appearance (2-4). Surgical aortic valve replacement remains the gold-standard treatment with proven benefits in survival (5,6). However, approximately a third of patients are unsuitable for operative management secondary to a number of factors including poor left ventricular ejection fraction, advanced age or pre-existing comorbidities (7-9). With advances in technology and technique refinement there is much interest in new treatment alternatives, namely: transcatheter aortic valve implantation (TAVI) and sutureless aortic valve replacement (SUAVR).
There has been an increasing emphasis in the realm of aortic stenosis management on the notion of minimally invasive techniques and interventions. Following the publication of the Cohort A results of the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (10), there has been great debate regarding TAVI for high-risk patients with symptomatic severe aortic valve stenosis. In the PARTNER trial, paravalvular regurgitation was more frequent after TAVI, attributed to the nature of the procedure, which does not involve removal of the native calcified and diseased valve. Paravalvular leak in the PARTNER Cohort A trial was associated with increased late mortality, with significant differences in 2-year mortality reported between patients with mild-severe paravalvular leak versus trace paravalvular leak.
SUAVR was developed as an alternative option for this group of patients, and to help overcome drawbacks of traditional minimally invasive aortic valve replacement including prolonged cardiopulmonary bypass durations, cross-clamp times and operative time (11-15). The use of sutureless bioprosthesis has the potential to facilitate minimally invasive aortic valve replacement by reducing the need for sutures the time required for prosthesis anchoring, thus theoretically curtailing risks associated with prolonged operative durations (13,16). It also allows for minimal paravalvular leak since the native diseased valve is resected. A number of observational studies have reported satisfactory clinical and haemodynamic outcomes with reduced operative time (16-21). In comparison to conventional surgical aortic valve replacement, SUAVR delivers comparable mortality with shorter cross-clamp and cardiopulmonary bypass duration (22). Comparisons between SUAVR and TAVI were pooled in a meta-analysis by Takagi & Umemoto (23), who identified significantly superior short term survival and paravalvular regurgitation rates with SUAVR compared to TAVI. However, small sample size and differences in patient characteristics at baseline may have largely contributed to the findings.
Therefore, the current meta-analysis aims to perform an updated meta-analysis to compare the outcomes of patients undergoing SUAVR and TAVI. To minimise selection bias and underlying differences in baseline characteristics between the two cohorts, we included propensity score matched studies only.
Methods
Literature search strategy
A systematic review of studies comparing SUAVR versus TAVI for the treatment of aortic stenosis was performed according to recommended guidelines (24,25). Five electronic databases including MEDLINE, PubMed, Embase, Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews were searched from their date of inception to September 2016. Appropriate free text and MeSH terms were used to identify all studies: “aortic stenosis”, “AS”, “aortic valve”, “aortic valve replacement”, “AVR”, “sutureless aortic valve replacement”, “transcatheter aortic valve implantation”, “transcatheter aortic valve replacement”, “TAVI”, and “TAVR”. Reference lists of all articles found were searched to further identify potentially relevant studies.
Outcome measures
Baseline parameters collected included age, gender, euroSCORE, diabetes mellitus, hypertension, left ventricular ejection fraction, renal insufficiency, recent myocardial infarction, chronic obstructive pulmonary disease, peripheral vascular disease and pulmonary hypertension. Outcome variables collected included short term mortality, intermediate term mortality (1 and 2 years), length of hospital stay, pacemaker implantation, major bleeding, stroke/transient ischemic attack, acute kidney injury and paravalvular regurgitation.
Eligibility criteria
Studies eligible for this systematic review directly compared SUAVR versus TAVI for patients with aortic stenosis. Only propensity-score matched studies were included in the present systematic review and meta-analysis. Case reports, series with less than ten patients, abstracts, editorials and expert opinions were excluded. If more than one article had been published from the same center with the same dataset, only the article with the most complete dataset published was used. All studies selected were human trials and in English.
Data extraction and critical appraisal
Two reviewers (NW, KP) independently appraised studies from date of inception up to September 2016, using a standard form and extracted data on methodology, quality criteria and outcome measures. All extracted and tabulated data were checked by an additional reviewer (SP). The quality of studies was assessed using assessment criteria recommended by the Centre for Evidence Based Medicine (University of Oxford). Discrepancies between reviewers were resolved by discussion and consensus was reached.
Statistical analysis
The odds ratio (OR) was used as a summary statistic. In the present study, both fixed- and random-effect models were tested. A random-effects model was used as it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 =100% × (Q – df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible because raw data were not available. All P values were 2-sided.
Results
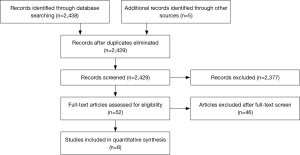
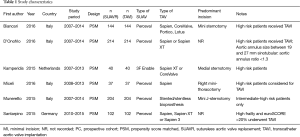
A total of 6 studies (26-31) were identified which compared SUAVR and TAVI via propensity score matching, giving a total of 741 patients in each arm (Figure 1; Table 1). Five of the 6 studies enrolled predominantly high risk patients for TAVI (26-29,31) whilst 1 study compared SUAVR and TAVI in intermediate-high risk patients (30). One study (32) which did not perform propensity score matching, was excluded. 5 of the 6 studies reported follow-up duration of at least 1 year (27-31), whilst one study confined the analysis to hospital outcomes (26). The quality assessment of each study is presented in Table 2.
Full table

Full table
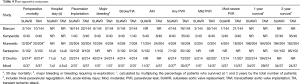
The baseline characteristics of patients undergoing SUAVR compared to TAVI are shown in Table 3. Pooled meta-analysis for SUAVR vs. TAVI showed no significant differences at baseline for age (mean difference −0.16; 95% CI: −0.90–0.57, P=0.66), gender (OR =0.91; 95% CI: 0.74–1.13, P=0.41), diabetes mellitus (OR =1.02, 95% CI: 0.79–1.32, P=0.90), hypertension (OR =1.01; 95% CI: 0.62–1.65, P=0.97), renal insufficiency (OR =0.88; 95% CI: 0.69–1.12, P=0.30), recent myocardial infarction (OR =0.94; 95% CI: 0.48–1.84, P=0.86), chronic obstructive pulmonary disease (OR =0.95, 95% CI: 0.74–1.22, P=0.70), peripheral vascular disease (OR =1.11, 95% CI: 0.80–1.53, P=0.54), and pulmonary hypertension (OR =0.99, 95% CI: 0.72–1.36, P=0.94). However, TAVI patients had a significantly higher log EUROscore than SUAVR by a single point (mean difference 1.00; 95% CI: 0.13–1.87, P=0.02).

Full table
Table 4 shows the postoperative outcomes of the 6 studies comparing SUAVR and TAVI. Pooled meta-analysis of postoperative outcomes comparing SUAVR and TAVI are shown in Figure 2. The perioperative mortality rate showed a non-significant trend favouring improved survival with SUAVR over TAVI (OR =0.55; 95% CI: 0.29–1.06, P=0.07). Patients who underwent SUAVR had a significantly increased length of hospital stay (mean difference 1.84; 95% CI: 0.22–3.47, P=0.03). There was a trend towards major bleeding or bleeding requiring re-exploration in patients who underwent SUAVR compared to TAVI (OR =1.82; 95% CI: 0.90–3.68, P=0.09). There was no significant difference in need for pacemaker implantation (P=0.86), incidence of stroke and transient ischemic attacks (P=0.27) and acute kidney injury (P=0.77).
Full table
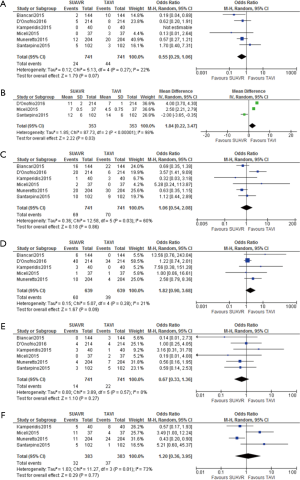
The incidence of paravalvular leak was significantly greater in TAVI patients compared to SUAVR (Figure 3) (OR =0.06; 95% CI: 0.03–0.12, P<0.01). In particular, SUAVR had lower incidences of mild paravalvular regurgitation (OR =0.05; 95% CI: 0.03–0.11, P<0.01) and moderate-severe paravalvular regurgitation (OR =0.12; 95% CI: 0.05–0.27, P<0.01).
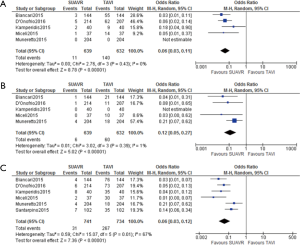
Five of the 6 studies reported intermediate follow-up and were used to determine the survival rate at 1 and 2 years (27-31). 3 studies reported the percentage of patients who survived at 1 year (27,29,30) and 4 studies reported the percentage of patients who survived at 2 years (28-31). TAVI patients had 140% increased odds of dying at 1 year compared to SUAVR (OR =2.40; 95% CI: 1.40–4.11, P<0.01). The risk increased even greater with further follow-up, with 360% increased odds of fatality in TAVI vs. SUAVR at 2-year follow up (OR =4.62; 95% CI: 2.62–8.12, P<0.01).
Discussion
Our findings suggest that SUAVR is a suitable alternative for TAVI in the intermediate to high risk patients with severe aortic stenosis. Although SUAVR is associated with a higher incidence of bleeding and prolonged lengths of hospital stay, there are significantly decreased rates of all grades of paravalvular regurgitation. Takagi and Umemoto (23) performed a meta-analysis which showed acceptable short term outcomes for SUAVR compared to TAVI. Our study updates the analysis with larger patient numbers and longer follow-up. We also attempt to minimize selection bias by including propensity score matched studies only. We found no significant difference in perioperative mortality, however SUAVR was associated with reduced mortality rates at 1- and 2- year follow-up. It is likely that the mortality benefit of SUAVR may be more apparent with longer follow up duration. Furthermore, our study stratifies the incidence of paravalvular leak by severity, and we have shown that SUAVR reduces all grades of regurgitation.
Similar to conventional aortic valve replacement, SUAVR requires valve excision and annular decalcification, but avoids the use of permanent sutures at the decalcified annulus (19,33). Herein lies the potential to reduce operative trauma by minimizing manipulation and facilitating minimally invasive approaches. The faster deployments and reduced procedural time has been associated with a lower rate of transfusion of packed red blood cells, shorter intensive care unit and hospital stay, and reduced hospital costs (34). Furthermore, the idea of minimal invasiveness regarding the surgical incision is complemented by reduced manipulation inside the aortic root, compared with a conventional sutured valve prosthesis implantation. Current guidelines (4) and data from Placement of Aortic Transcatheter Valve (PARTNER) (Cohort B) (2) confirm the benefits of TAVI in inoperable or extreme high-risk surgical patients and the indication for conventional valve replacement in patients at intermediate-risk or low-risk patients.
Minimally invasive SUAVR may be considered an alternative to TAVI in patients with high surgical risk. One of the major limitations of TAVI is the high incidence of paravalvular leak, which is quoted at 70–90% (35-37). This is particularly important as paravalvular regurgitation is associated with increased mortality (38). A reduction in aortic regurgitation after SUAVR is potentially the major advantage of SUAVR over TAVI. Techniques have been developed in an attempt to minimize paravalvular leak in TAVI but with significant complications. Post-dilation has been associated with higher risks of stroke (39) and fatal aortic root injury (40). Regurgitation after TAVI is largely due to a non-uniform compression of the calcified native valve against the aortic wall after deployment of the stent prosthesis. The ridges of calcium hinder adequate stent expansion and subsequent paravalvular spaces are created for possible leaks (37). In contrast, surgery allows for the calcified native valve to be excised and the new prosthesis sutured flush against the aortic annulus, which ameliorates potential paravalvular spaces (37).
Previous studies have shown superior outcomes for SUAVR over TAVI. Biancari showed that SUAVR is associated with lower rates of paravalvular leak and 30-day mortality. However, as the authors suggested, these differences could be attributed to the marked differences in baseline characteristics of patients undergoing SUAVR and TAVI. This is largely due to the indications for TAVI, which are high risk patients who would not be otherwise fit for conventional surgery (2,4). Therefore, studies have employed propensity score matching in an attempt to ameliorate the selection bias when comparing SUAVR and TAVI. Miceli et al. (29) found 1 year survival rates of 91.6% vs. 78.6% at 1 year and 91.6% vs. 66.2% at 2-years after propensity score matching, with no significant differences at baseline between the two groups. Similarly, D’Onofrio et al. (27) also found a 1 year survival rates favouring SUAVR (94.2% vs. 91.6%). However, both studies had non-significant findings, largely due to inadequate sample sizes and high drop out rates. Our results suggest that SUAVR is associated with better 1- and 2- year survival rates. Although this result can be partially explained by the slightly higher euroSCORE in the patients who underwent TAVI, the improved survival is likely a true finding. Similarly, Muneretto (30) found SUAVR to have significantly superior survival outcomes at 2 years (94.9% vs. 79.5%). The authors also concluded that TAVI was an independent predictor for all-cause mortality.
TAVI was initially designed as an alternative for high risk patients and was shown to be non-inferior to conventional surgical aortic valve replacement in this specific subset of patients (2,38). Such encouraging results led to the inclusion of the transcatheter approach in the most up-to-date guidelines and consensus statements on valvular heart disease as an additional therapeutic option for high-risk or inoperable patients (4,41). However, the benefits of TAVI in intermediate risk patients has not been as well elucidated, with CESANA IDEM trials currently being conducted to address this question. Furthermore, of the 4 studies (27-29,31) with intermediate follow-up that recruited high risk patients for TAVI, there were no significant difference in 1- and 2- year mortality. However, when considering intermediate-high risk patients, Muneretto et al. found significantly better survival rates for SUAVR at 1 and 2 years (30). Overall it is difficult to conclude which method has superior intermediate survival rates because differences in patient population likely play an important factor. However, it is likely that SUAVR may be more appropriate for intermediate risk patients. This difference may be explained by 2 key factors: firstly, propensity score matching may not account for all differences at baseline as these studies were non-randomised and in our pooled analysis, the log EUROscore was higher in TAVI by a single point; secondly the dramatically reduced incidences of paravalvular regurgitation may also be responsible for the reduced morality rates.
Limitations
This meta-analysis has several key limitations and must be interpreted with care. Firstly, we were not able to control for heterogeneity between studies. Although we only included studies with propensity score matching, the lack of randomization can subject the studies to selection bias, which were uncontrolled for. Secondly, there has been limited number of studies examining long term outcomes of SUAVR. Currently our results are limited to short term outcomes and estimated intermediate mortality rates. The role of TAVI versus SUAVR or minimally invasive AVR in the setting of reoperation remains unclear, although a recent review of the literature suggests that both methods are feasible (42). Long-term outcomes of SUAVR will be in part addressed by a multi-center international registry (43). Future directions include a prospective multi-center randomized controlled trial comparing TAVI and SUAVR in intermediate-risk and higher-risk patients with aortic stenosis.
Conclusions
Our findings suggest that SUAVR remains an alternative to TAVI in the intermediate-high risk patients with severe aortic stenosis. The main advantage for SUAVR, likely lies in the lower rates of paravalvular regurgitation when compared to TAVI. Future studies are needed to compare long term outcomes in randomized trials of intermediate-high risk patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-142. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Turina J, Hess O, Sepulcri F, et al. Spontaneous course of aortic valve disease. Eur Heart J 1987;8:471-83. [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167-214. [PubMed]
- Kvidal P, Bergström R, Hörte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000;35:747-56. [Crossref] [PubMed]
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982;66:1105-10. [Crossref] [PubMed]
- Varadarajan P, Kapoor N, Bansal RC, et al. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006;82:2111-5. [Crossref] [PubMed]
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [Crossref] [PubMed]
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-9. [Crossref] [PubMed]
- Elmariah S, Palacios IF, McAndrew T, et al. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv 2013;6:604-14. [Crossref] [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [Crossref] [PubMed]
- Phan K, Croce B, Yan TD. Minimally invasive aortic valve replacement. Ann Cardiothorac Surg 2015;4:218. [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11. [PubMed]
- Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25. [PubMed]
- Phan K, Xie A, Tsai YC, et al. Ministernotomy or minithoracotomy for minimally invasive aortic valve replacement: a Bayesian network meta-analysis. Ann Cardiothorac Surg 2015;4:3-14. [PubMed]
- Wendt D, Thielmann M, Pizanis N, et al. Sutureless aortic valves over the last 45 years. Minim Invasive Ther Allied Technol 2009;18:122-30. [Crossref] [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Fischlein T. Sutureless valve implantation in a patient with bicuspid aortic valve. Int J Cardiol 2012;157:e21-2. [Crossref] [PubMed]
- Shrestha M, Folliguet T, Meuris B, et al. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis 2009;18:698-702. [PubMed]
- Eichstaedt HC, Easo J, Härle T, et al. Early single-center experience in sutureless aortic valve implantation in 120 patients. J Thorac Cardiovasc Surg 2014;147:370-5. [Crossref] [PubMed]
- Flameng W, Herregods MC, Hermans H, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011;142:1453-7. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Concistrè G, et al. REDO aortic valve replacement: the sutureless approach. J Heart Valve Dis 2013;22:615-20. [PubMed]
- Takagi H, Umemoto T. ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Sutureless aortic valve replacement may improve early mortality compared with transcatheter aortic valve implantation: A meta-analysis of comparative studies. J Cardiol 2016;67:504-12. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [PubMed]
- Biancari F, Barbanti M, Santarpino G, et al. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016;31:427-33. [Crossref] [PubMed]
- D'Onofrio A, Salizzoni S, Rubino AS, et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;152:99-109.e2. [Crossref] [PubMed]
- Kamperidis V, van Rosendael PJ, de Weger A, et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score-matched high-risk populations with severe aortic stenosis. JACC Cardiovasc Interv 2015;8:670-7. [Crossref] [PubMed]
- Miceli A, Gilmanov D, Murzi M, et al. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardiothorac Surg 2016;49:960-5. [Crossref] [PubMed]
- Muneretto C, Alfieri O, Cesana BM, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in "real-world" patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg 2015;150:1570-7; discussion 1577-9. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Clinical Outcome and Cost Analysis of Sutureless Versus Transcatheter Aortic Valve Implantation With Propensity Score Matching Analysis. Am J Cardiol 2015;116:1737-43. [Crossref] [PubMed]
- Doss M, Buhr E, Moritz A, et al. Sutureless aortic valve replacement: catheter-based transapical versus direct transaortic implantation. J Heart Valve Dis 2012;21:758-63. [PubMed]
- Santarpino G, Pfeiffer S, Schmidt J, et al. Sutureless aortic valve replacement: first-year single-center experience. Ann Thorac Surg 2012;94:504-8; discussion 508-9. [Crossref] [PubMed]
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43. [Crossref] [PubMed]
- Détaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv 2009;2:821-7. [Crossref] [PubMed]
- Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011;97:899-906. [Crossref] [PubMed]
- Rajani R, Kakad M, Khawaja MZ, et al. Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2010;75:868-72. [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Nombela-Franco L, Rodés-Cabau J, DeLarochellière R, et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv 2012;5:499-512. [Crossref] [PubMed]
- Barbanti M, Yang TH, Rodès Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244-53. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- Phan K, Zhao DF, Wang N, et al. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis 2016;8:E83-93. [PubMed]
- Di Eusanio M, Phan K, Bouchard D, et al. Sutureless Aortic Valve Replacement International Registry (SU-AVR-IR): design and rationale from the International Valvular Surgery Study Group (IVSSG). Ann Cardiothorac Surg 2015;4:131-9. [PubMed]