Navigation bronchoscopy for diagnosis and small nodule location
Introduction
Lung cancer is the most common cause of cancer death among men and women in the United States (1). Unfortunately, the diagnosis is usually made at advanced stages with reported 5-year survival rates of approximately 15% (2). An early diagnosis and timely surgical resection is essential to improve outcomes and decrease mortality (3,4). Survival rates as high as 70% can be achieved in patients who undergo surgical resection at the earliest stage of disease (5,6).
Lung cancer screening programs with low-dose CT-scan have allowed detection of small lung nodules (7). Nevertheless, an accurate diagnosis from these small, peripheral lung lesions can still be challenging with the use of conventional procedures. Options available to diagnose newly identified lung lesions include: flexible fiberoptic bronchoscopy (FFB), CT-guided transthoracic biopsy, bronchoscopy with endobronchial ultrasound (EBUS) and thoracoscopic or open thoracic surgery.
FFB is useful for visible endobronchial and central lesions, with an overall sensitivity of roughly 88% (8,9). However, its performance as a diagnostic tool for small peripheral lesions is limited and has been reported to be between 19% and 62% (10,11). In nodules measuring less than 20 mm the diagnostic yield can be as low as 14% (12).
On the other hand, CT-guided transthoracic procedures display a higher diagnostic yield. CT-guided fine-needle aspiration has an overall sensitivity of 82% and an accuracy of 88%, but its performance might vary depending on lesion size and location (13). A sensitivity of over 90% has been reported for CT-guided transthoracic core needle biopsies, but the rate of complications is not negligible, with hemorrhage and pneumothorax occurring in as many as 30% of the cases (14-16).
The rate of accurate diagnosis for solitary pulmonary nodules using bronchoscopy with radial ultrasound probe is around 70–77% (17). This technique is operator dependent and involves blind navigation through the bronchial tree. Consequently, difficulty localizing the lesion is present in about 20% of cases (18,19). Although Kurimoto et al. (20) found no difference in the diagnostic rates amongst different lesion sizes, Eberhardt et al. reported a decrease in diagnostic yield, in lesions smaller than 20 mm (21).
The highest diagnostic yield (close to 100%) is achieved with thoracoscopic and open surgery (22). However, these approaches are more invasive and may be limited by poor pulmonary reserve in some patients (23). Electromagnetic navigational bronchoscopy (ENB) is a novel technique that offers a less invasive procedure for the diagnosis of small, peripheral lung nodules. It may also be a better option in certain circumstances (severe emphysema) where CT guided biopsy would be associated with a greater risk for complications.
ENB combines virtual and conventional bronchoscopy for the localization of lung nodules and allows the guidance of diagnostic and/or dye marking instruments (10,24). The most widely used and reported system is the SuperDimension system (Medtronic, Minneapolis, MN, USA) The planning phase is performed using the patient’s CT scan that is loaded onto a computer prior to the procedure. The target lesion is identified and the most appropriate bronchial pathway is carefully chosen for guidance during navigation. The system has an extended working channel (EWC), that is passed through the working channel of the bronchoscope and a locatable guide (LG) that is placed within the EWC. The LG is trackable on the navigation system providing the link between the real-time and virtual bronchoscopy. At the start of the procedure a standard bronchoscopy is performed and landmarks from this bronchoscopy registered on the software of the system. The standard bronchoscopy and virtual bronchoscopy created from the initial CT will then be linked. The surgeon or pulmonologist will then navigate towards the target lesion using both the standard and virtual images. At some point the bronchoscope will wedge within a segmental bronchus. The surgeon or pulmonologist will then advance the EWC and LG towards the target lesion using the virtual image only. Once the target lesion is reached, the LG is removed allowing placement of biopsy forceps, cytology brushes and aspiration needles through the EWC. It is also possible to obtain washings, place fiducials to guide stereotactic body radiation therapy and inject dye to mark small lesions for minimally invasive resections.
Two other navigation systems are commercially available, namely The LungPoint Virtual Bronchoscopic Navigation System (Bronchus Technologies, Inc., Mountain View, CA, USA) and the SPIN Drive System (Veran Medical Technologies, St. Louis, MO, USA). A potential advantage of the Veran system is that this uses “trackable” instruments that may improve accuracy as biopsies are performed. Currently data with these other systems are limited and no comparative information is available (25,26).
ENB for the diagnosis of small lung nodules
ENB has been shown to aid in the diagnosis of lung lesions with a lower rate of complications compared with more invasive techniques. However, the diagnostic yield for small, peripheral lung nodules is variable and clear selection criteria for patients that may benefit from this intervention have not been well defined.
The diagnostic yield of ENB has been reported between 59% and 94% (24,27-37) (Table 1). This large variability across several studies highlights the need to define systematic selection criteria and standardized protocols for the use of ENB.
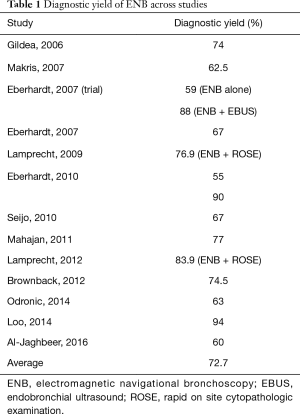
Full table
Several variables that affect the performance of ENB have been evaluated. The presence of a bronchus sign on CT-scan (38), the use of rapid on site cytopathologic examination (ROSE) (30,34), combining ENB with endobronchial radial ultrasound (28,31), PET scan (30), and the use of general anesthesia and fluoroscopy could improve the diagnostic yield of ENB. Moreover, the experience of the operator given by the number of procedures performed is a good predictor of success. The diagnostic yield of ENB has been shown to increase from 63% during the first year of work to 90% after two years of experience (33,34).
One of the first studies conducted to assess the utility of ENB was performed by Gildea et al. (27). The overall diagnostic yield was 74%. However, when analyzed by lesion size, the diagnostic yields ranged between 66.6% and 72%. Although all the nodules were considered peripheral, the distance from the pleural surface to each nodule was not reported, probably confounding the results since higher diagnostic yields could be expected for centrally located lesions.
On the other hand, Eberhardt et al. carried out a randomized controlled trial, finding a diagnostic yield of 59% that did not change with nodule size or location (28). This trial compared the use of EBUS with that of ENB alone or EBUS plus ENB for the diagnosis of peripheral lung lesions. The diagnostic yield with the combination of both tests was higher (88%) than either one alone, proving the adjunctive effect of endobronchial ultrasound. A more recent study by the same group showed different diagnostic rates depending on the biopsy technique. The overall diagnostic yield was 75.5%. Samples acquired using suction catheter had higher yields compared to forceps, where the diagnostic rates dropped to 55% (31). Again, this study found that the combination of ENB and EBUS achieved a higher diagnostic yield (93%) compared to 48% when the lesion was not visible under ultrasound (31).
In another study conducted by Eberhardt et al. there was a nonsignificant trend towards an increase in the diagnosis of lesions located within the right middle lobe compared with other locations (24). Similarly, Chen et al. showed that lesions located within the lower lobes may be more challenging to reach via ENB due to greater movement during respiration compared with upper lobe nodules (39).
Other diagnostic tools used in combination with ENB have shown favorable results. ROSE has been used immediately after ENB tissue sampling, although this technique is not readily available at all centers.
Loo et al. showed an overall diagnostic yield of 94% with the use of ENB and fine needle aspiration, and a diagnostic yield of 87% for lung lesions <2 cm, which was attributed to the use of ROSE (36). Similarly, Lamprecht et al. (34) showed diagnostic rates of over 80% with the combination of ENB, PET-scan and ROSE. The diagnostic yields varied depending on size. A correct diagnosis was achieved in 76% of lesions smaller than 20 mm and 90% of those larger than 20 mm. However, these differences were not statistically significant and both studies showed false-negative and false-positive results, affecting the sensitivity and specificity of ROSE.
As mentioned above, the biopsy technique could also affect the probability of obtaining a definitive diagnosis after ENB. Diagnostic yields of 55% to 77% have been reported with the use of biopsy forceps and 54% for bronchial brushing (29,31-33,35,38). Combining ENB with fine needle aspiration biopsy (FNA) has allowed surgeons to achieve diagnostic yields as high as 87% for lesions <20 mm and 100% for those >20 mm (36). The concurrent use of bronchial brushing and transbronchial biopsies did not improve these outcomes (36). Furthermore, Odronic et al. also found that the sensitivity of ENB-FNA was higher when compared to the use of biopsy forceps and bronchial brushings (35). However, they suggest that that the combination of these techniques could improve sensitivity. Our own preference is to use a number of techniques including biopsy forceps, bronchial brushing and washings and needle aspiration.
CT-scan findings have also been shown to influence the diagnostic performance of ENB. Seijo et al. (38) found that the diagnostic yield of ENB improved significantly from 31% when a bronchus sign on CT scan was absent to 79% when such sign was present. Size of lesion was also a variable that significantly affected the rates of definitive diagnosis in that study (38). In contrast, Brownback et al. found a 13.9% absolute increase in the diagnostic yield when a bronchus sign was identified on CT scan, but this finding was not statistically significant. Lesion size did not affect the diagnostic rates significantly either in this study (33).
In our own analysis of 100 ENB’s performed in 95 patients (data not published), the diagnostic yield of this technique was associated with lesion size, location and the presence of a bronchus sign on CT-scan. Lesion size ≥2 cm, location within the central and intermediate regions, location within the upper and middle lobes and the presence of a bronchus sign were all factors associated with an increased probability of obtaining a definitive diagnosis.
The large range in diagnostic yield reported among studies could be explained by differences in study design, lesion size, location, biopsy techniques employed, the inclusion of adjunctive resources such as radial EBUS and ROSE, and differences in learning curve/operator experience. This variability has led to the conflicting findings reported. As further studies and experiences are reported, it is hoped that factors that impact the performance of ENB will be better defined, improving selection criteria for ENB rather than an alternative diagnostic modality. It is also important to note that all studies consistently demonstrate lower complication rates with ENB compared to that reported by CT guided techniques.
Additional uses of ENB
Fiducial placement for SBRT
Besides the diagnostic potential of ENB for small peripheral lung nodules, this technique is also useful for the placement of fiducials and dye marking for subsequent stereotactic body radiation therapy (SBRT) and minimally invasive surgical resection.
It has been shown that the use of ENB for the placement of fiducial markers is a safe and feasible procedure for subsequent SBRT (40). The deployment of these fiducials showed lower rates of complications compared to transthoracic placement, and higher retention rates in close proximity within the tumor or the location of initial placement, allowing successful completion of radio surgical treatments (40,41).
ENB-guided dye marking for minimally invasive resection
The increased number of small lung lesions detected by CT scan has also increased the number of cases referred for surgical resection. The surgical approach of these lesions may be challenging since visualization and palpation of small nodules is limited during minimally invasive resection.
ENB-guided dye marking has shown to be a safe and feasible procedure for the identification of lung nodules during video-assisted thoracoscopic (VATS) and robotic-assisted thoracoscopic resection (RATS) (42,43). This potentially has great utility for thoracic surgeons performing these procedures.
Krimsky et al. used ENB-guided dye marking for the localization of 21 lung nodules with a median size of 13.4 mm (range, 7–29 mm) (43). Indigo carmine and methylene blue were used. In 81% of the cases the dye was identified close to the lesions, in one case the dye marking extravasated into the pleural space, and in 3 cases the dye was not identified. No complications related with ENB were reported.
Recent studies have also shown a good performance of ENB-guided dye marking with significantly less rates of complications when compared to other percutaneous marking procedures (44-46). Marino et al. reported a success rate of 97% for the localization of 70 lung lesions with a median lesion size of 8 mm (range, 4–17 mm) and a median distance from the pleural surface of 6 mm (range, 1–19 mm). The failure rate was 2.9%, which is significantly lower than that of transthoracic methylene blue marking and hook-wire localization (46). Similarly, Awais et al. (45) found a success rate of 100% for the localization of 33 lung nodules with a median size of 10 mm (range, 4–27 mm) and a median distance from the pleural surface to the center of the lesion of 13 mm (range, 3–44 mm). Complications were reported in two patients, which seemed to be independent of the ENB procedure.
Conclusions
Electromagnetic Navigational Bronchoscopy is a novel technique that has proven to be useful for the diagnosis of small lung nodules. This technique increases the likelihood of obtaining tissue samples from lesions that were unreachable with the use of standard bronchoscopy. It is also of great utility for the localization of small, non-palpable lung nodules for subsequent minimally invasive resection, as well as for the placement of fiducial markers for therapeutic purposes in patients with advanced stage disease.
There is variability in diagnostic yields from ENB with an average of 72% (Table 1) reported. Although not statistically significant, the majority of the studies have shown differences in the diagnostic rates related to lesion size (24,28,29,32,34-36) suggesting that bigger lesions are more likely to be diagnosed with ENB. Other variables that have been suggested to improve the performance of ENB include presence of bronchus sign on CT scan and location in the middle and upper lobes.
Future studies should focus on establishing well-defined selection criteria for ENB that will help guide the selection of optimal diagnostic approach when evaluating a new lung nodule. Additionally, methods to improve the performance of ENB, such as the biopsy technique (e.g., brush, fine-needle, core or cup biopsy) and adjunctive approaches (such as the use of radial EBUS) will need to be established.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016.
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators , Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Kanarek NF, Hooker CM, Mathieu L, et al. Survival after community diagnosis of early-stage non-small cell lung cancer. Am J Med 2014;127:443-9. [Crossref] [PubMed]
- Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest 1992;101:1013-8. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S-28S. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Weiser TS, Hyman K, Yun J, et al. Electromagnetic navigational bronchoscopy: a surgeon's perspective. Ann Thorac Surg 2008;85:S797-801. [Crossref] [PubMed]
- Wallace JM, Deutsch AL. Flexible fiberoptic bronchoscopy and percutaneous needle lung aspiration for evaluating the solitary pulmonary nodule. Chest 1982;81:665-71. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [Crossref] [PubMed]
- Sachdeva M, Ronaghi R, Mills PK, et al. Complications and Yield of Computed Tomography-Guided Transthoracic Core Needle Biopsy of Lung Nodules at a High-Volume Academic Center in an Endemic Coccidioidomycosis Area. Lung 2016;194:379-85. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809-14. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest 2006;129:147-50. [Crossref] [PubMed]
- Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004;24:533-7. [Crossref] [PubMed]
- Asahina H, Yamazaki K, Onodera Y, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest 2005;128:1761-5. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Eberhardt R, Ernst A, Herth FJ. Ultrasound-guided transbronchial biopsy of solitary pulmonary nodules less than 20 mm. Eur Respir J 2009;34:1284-7. [Crossref] [PubMed]
- DeCamp MM Jr, Jaklitsch MT, Mentzer SJ, et al. The safety and versatility of video-thoracoscopy: a prospective analysis of 895 consecutive cases. J Am Coll Surg 1995;181:113-20. [PubMed]
- Shafiek H, Valera JL, Togores B, et al. Risk of postoperative complications in chronic obstructive lung diseases patients considered fit for lung cancer surgery: beyond oxygen consumption. Eur J Cardiothorac Surg 2016;50:772-9. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Eberhardt R, Kahn N, Gompelmann D, et al. LungPoint--a new approach to peripheral lesions. J Thorac Oncol 2010;5:1559-63. [Crossref] [PubMed]
- Santos RS, Gupta A, Ebright MI, et al. Electromagnetic navigation to aid radiofrequency ablation and biopsy of lung tumors. Ann Thorac Surg 2010;89:265-8. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. [Crossref] [PubMed]
- Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung 2009;187:55-9. [Crossref] [PubMed]
- Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration 2010;79:54-60. [Crossref] [PubMed]
- Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol 2011;18:133-7. [Crossref] [PubMed]
- Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchology Interv Pulmonol 2012;19:91-7. [Crossref] [PubMed]
- Lamprecht B, Porsch P, Wegleitner B, et al. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respir Med 2012;106:710-5. [Crossref] [PubMed]
- Odronic SI, Gildea TR, Chute DJ. Electromagnetic navigation bronchoscopy-guided fine needle aspiration for the diagnosis of lung lesions. Diagn Cytopathol 2014;42:1045-50. [Crossref] [PubMed]
- Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol 2014;122:191-9. [Crossref] [PubMed]
- Al-Jaghbeer M, Marcus M, Durkin M, et al. Diagnostic yield of electromagnetic navigational bronchoscopy. Ther Adv Respir Dis 2016;10:295-9. [Crossref] [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest 2015;147:1275-81. [Crossref] [PubMed]
- Nabavizadeh N, Zhang J, Elliott DA, et al. Electromagnetic navigational bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy: analysis of safety, feasibility, and interfraction stability. J Bronchology Interv Pulmonol 2014;21:123-30. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014.4. [PubMed]
- Tay JH, Wallbridge PD, Larobina M, et al. Electromagnetic Navigation Bronchoscopy-directed Pleural Tattoo to Aid Surgical Resection of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol 2016;23:245-50. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]


