Mitral valve repair and subvalvular intervention for secondary mitral regurgitation: a systematic review and meta-analysis of randomized controlled and propensity matched studies
Introduction
Secondary mitral regurgitation (MR) develops in up to one-half of patients with an ischemic or non-ischemic dilated cardiomyopathy (1,2). The primary substrate for secondary MR is adverse left ventricular (LV) remodeling and dilatation, which distorts the mitral valve (MV) apparatus geometry. This results in posterolateral and apical displacement of the papillary muscles, an impaired systolic shortening of the interpapillary muscle distance, tethering and restricted systolic closure of the MV leaflets, and subsequently, the development of secondary MR (3-5). Despite guideline-directed medical therapy, revascularization of significant coronary artery disease, and cardiac resynchronization therapy when indicated, secondary MR often persists and is associated with significant morbidity and mortality proportional to its severity (1,2,6).
MV intervention may be considered in these circumstances, which is most often a repair utilizing a ring annuloplasty, or a chordal-sparing MV replacement (7,8). A recent randomized controlled trial comparing MV repair versus chordal-sparing MV replacement for severe secondary MR revealed that recurrent MR occurred in up to 58.8% of patients undergoing repair at 2 years post-operatively (9). However, in those with no MR recurrence, a durable MV repair was associated with greater LV reverse remodeling, as compared with replacement. Given these findings and the potential benefits of MV repair in terms of peri-operative morbidity and avoidance of prosthesis-related complications, there is increasing interest in surgical techniques targeting the subvalvular dysfunction present in secondary MR, with the aim of improving MV repair durability (10-14).
In the present study, a systematic review and meta-analysis was performed to analyze the randomized controlled and propensity-matched studies comparing the safety and efficacy of MV repair utilizing a ring annuloplasty plus subvalvular intervention (Ring + subvalvular) versus a ring annuloplasty (Ring) alone.
Methods
Search strategy
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines (15). A comprehensive literature search was conducted of the PubMed, Embase, Ovid, and Cochrane Library databases, for scientific studies published through January 2017 that reported on the outcomes of MV repair for secondary MR. The Boolean search terms utilized were: [(mitral valve repair OR annuloplasty) AND (mitral regurgitation OR functional mitral regurgitation OR ischemic mitral regurgitation OR secondary mitral regurgitation) AND (subvalvular OR papillary muscle OR chordal)]. Three investigators (Christos G. Mihos, Evin Yucel, Orlando Santana) independently screened the identified articles, and also assessed the respective reference lists of included studies for pertinent publications.
Selection criteria
The studies were considered for inclusion if they met the following criteria: (I) they were randomized controlled or propensity matched investigations, to limit the impact of treatment selection bias and accurately estimate the treatment effects; (II) the population consisted of patients with secondary MR undergoing MV repair; (III) the studies compared a Ring + subvalvular versus Ring MV repair; and (IV) there was reporting of clinical and echocardiographic end-points. The exclusion criteria were: non-English language studies, non-randomized or non-propensity matched studies, investigations of MV repair for primary MR and/or structural abnormalities of the MV apparatus, case series or reports that included only Ring + subvalvular or Ring cohorts, duplicate publications, and review articles. Discrepancies regarding the inclusion of a study were resolved via a group consensus.
Data extraction and appraisal
Two authors (Christos G. Mihos, Evin Yucel) reviewed and extracted the reported data from the studies, which included: the number of patients, baseline demographics, MR severity, LV ejection fraction and size, MV apparatus geometric indices, operative characteristics and type of subvalvular repair, performance of concomitant cardiac procedures, type and size of the ring annuloplasty utilized, operative mortality, peri-operative complications, and length of follow-up. The data extracted from the last available follow-up included: survival, recurrence of moderate or greater MR, and echocardiographic measures of MV apparatus geometry and LV reverse remodeling. The study quality and risk of publication bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias (16).
Assessment of mitral valve apparatus geometry
In order to quantify the extent of alterations in the MV apparatus geometry, and the effects of Ring + subvalvular or Ring MV repair on these indices, three common systolic echocardiographic measurements that were reported in the studies were analyzed. From the parasternal long axis view, the indices of MV leaflet tethering were: (I) MV tenting height (distance from the annular plane to the leaflet coaptation point); and (II) MV tenting area (area enclosed by the annular plane and leaflets) (8). In the parasternal short axis view, the interpapillary muscle distance was measured (distance between the anterolateral and posteromedial papillary muscles) as a marker of papillary muscle displacement (17,18).
Statistical analysis
The analyses were performed utilizing Review Manager 5.3 (RevMan 5.3, Nordic Cochrane Center, Copenhagen, Denmark). The results are presented as mean ± standard deviation, mean and range, median and interquartile range (IQR, 25–75%), or number and percentage, as appropriate. Risk ratio (RR) and the weighted mean difference (MD) with a 95% confidence interval (CI) were calculated utilizing the Mantel-Haenszel and inverse-variance methods, respectively. Forest plots were generated to present the pooled results. The I2 statistic was applied to assess for statistical heterogeneity, which was stratified as none to low (0–49%), moderate (50–74%), and high (75–100%). In the presence of significant study variability (I2 ≥50%), a random effects model was used, while in the absence or presence of low heterogeneity, a fixed effects model was utilized. A P value <0.05 was considered statistically significant.
Results
Study selection, patient demographics, and pre-operative echocardiographic assessment
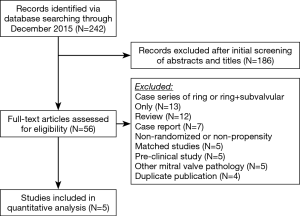
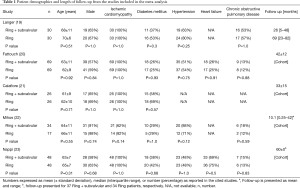
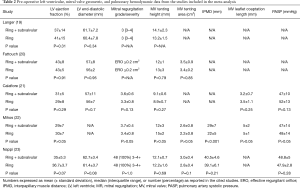
A total of 242 studies were identified utilizing the search criteria, of which 5 met the pre-defined inclusion criteria and comprised the pooled data (Figure 1) (19-23). There was one randomized controlled trial and four propensity matched studies. A total of 207 patients underwent a Ring + subvalvular MV repair, and 190 underwent Ring annuloplasty alone. No difference existed in any of the studies with regards to the patient demographics between the Ring + subvalvular versus Ring groups. The mean age ranged from 61±9 to 70±6 years, and the majority were male (57–91%) patients. The etiology of the cardiomyopathy was ischemic in all patients, except for the study by Mihos et al., in which a non-ischemic cardiomyopathy was present in 38% and 18% of the Ring + subvalvular versus Ring patients (P=0.14), respectively. The mean pre-operative LV ejection fraction ranged from 29±7 to 43±8%, and the LV end-diastolic diameter from 56±2 to 62.7±3.4 mm. All patients had moderate to severe secondary MR, and similar pre-operative MV apparatus geometry, with the exception of a greater mean interpapillary muscle distance in the Ring + subvalvular group in the analysis by Mihos et al., as compared with Ring patients (29±7 vs. 22±5 mm; P<0.001) (Tables 1 and 2).
Full table
Full table
Surgical techniques
In the randomized controlled trial by Nappi et al., the subvalvular procedure consisted of papillary muscle approximation utilizing a 4-mm polytetrafluoroethylene graft, which was placed around the base of each muscle and drawn together (23). The annuloplasty ring implanted in both groups was undersized by two sizes. Mihos et al., reported on the same Ring + subvalvular technique, with the annuloplasty ring sized according to the height and/or surface area of the anterior mitral leaflet (22). In this study, the selection criteria for a Ring + subvalvular repair was an MV tenting height ≥11 mm, MV tenting area ≥2.5 cm2, and/or an end-systolic interpapillary muscle distance >20 mm.
In the study by Fattouch et al., the subvalvular repair performed was a relocation of both papillary muscles via placement of sutures through the respective papillary muscle heads and ipsilateral MV annulus, with traction applied toward the annulus to relieve the leaflet tethering forces (20). Annuloplasty rings in the Ring + subvalvular group were sized according to the height of the anterior leaflet, while undersizing by two sizes was performed in the Ring group. Patients were selected for a Ring + subvalvular repair in the presence of a MV tenting height ≥10 mm. Langer et al., relocated the posterior papillary muscle only with a suture from the muscle head, through the intervalvular fibrosa, and exteriorized through the aortic wall, as a Ring + subvalvular intervention (19). All patients received annuloplasty bands undersized by one or two sizes. Finally, Calafiore et al., combined the surgical cutting of all secondary chordae tendineae with the placement of a 40-mm annuloplasty band as a Ring + subvalvular repair, if the anterior leaflet bending angle was >145° (21). All Ring patients received annuloplasty rings undersized by one or two sizes. No difference between groups was reported in any of the studies regarding the annuloplasty ring size utilized (Table 3).
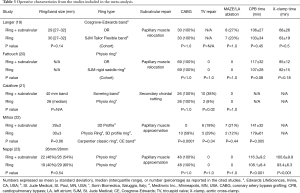
Full table
Operative characteristics
In four studies, concomitant coronary artery bypass grafting was performed in all patients. In the study by Mihos et al., all Ring + subvalvular operations were performed via a right thoracotomy approach, with coronary revascularization having been performed via previous bypass surgery or percutaneous coronary intervention. Accordingly, the Ring group had a higher incidence of bypass surgery (59% versus 0%; P=0.001) and longer operative times, as compared with a Ring + subvalvular repair. Patients undergoing a Ring + subvalvular repair in the study by Calafiore et al., had a higher incidence of concomitant tricuspid valve repair performed, as compared with the Ring group. No other difference was reported in regards to the incidence of concomitant cardiac procedures performed between groups in any of the studies (Table 3).
Post-operative outcomes
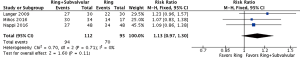
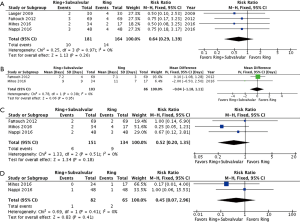
Operative mortality was reported by four studies, with no demonstrable difference between the Ring + subvalvular versus Ring group (RR 0.64, 95% CI, 0.29–1.39; I2 =0%; P=0.26). The mean hospital length of stay was similar between the surgical approaches, and was reported by two studies (MD =−0.04 days, 95% CI, −1.18 to 1.11; I2 =0%; P=0.95). Finally, there was no difference in risk of acute kidney injury (RR 0.52, 95% CI, 0.20–1.35; I2 =0%; P=0.18) or cerebrovascular accident (RR 0.45, 95% CI, 0.07–2.96; I2 =0%; P=0.41) between a Ring + subvalvular versus Ring MV repair, which were reported in three and two studies, respectively (Figure 2).
Follow-up MR recurrence, LV reverse remodeling, MV apparatus geometry, and Survival
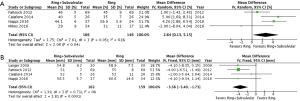
The clinical and echocardiographic follow-up ranged from 10.1 (mean range =0.25–42) to 69 (IQR, 23–82) months. The follow-up mean MR grade was reported by three studies, and was significantly lower for patients who underwent a Ring + subvalvular repair (MD =−0.44, 95% CI, −0.69 to −0.19; I2 =0%; P=0.0005), as was the risk of recurrent moderate or greater MR (RR =0.43, 95% CI, 0.27–0.66; I2 =0%; P=0.0002), which was reported by four studies (Figure 3). Additionally, a Ring + subvalvular repair was associated with greater LV reverse remodeling, as evidenced by a lower mean LV end-diastolic diameter (MD =−3.56 mm, 95% CI, −5.40 to −1.73; I2 =0%; P=0.0001) and a greater LV ejection fraction (MD =2.64%, 95% CI, 0.13–5.15; I2 =61%; P=0.04), reported by four studies for each (Figure 4). The MV apparatus geometry was also significantly improved in the Ring + subvalvular group, as evidenced by a smaller MV tenting height (MD =−2.28 mm, 95% CI, −4.26 to −0.29; I2 =97%; P=0.02) from four studies, MV tenting area (MD =−0.77 cm2, 95% CI, −1.26 to −0.29; I2 =96%; P=0.002) from three studies, and interpapillary muscle distance (MD =−5.97 mm, 95% CI, −7.74 to −4.2; I2 =0%; P<0.00001) from two studies, respectively (Figure 5). Finally, there was no difference in follow-up survival from three reporting studies between the Ring + subvalvular versus Ring group (RR 1.13, 95% CI, 0.97–1.3; I2 =0%; P=0.11) (Figure 6).


Discussion
In the present systematic review and meta-analysis, the study outcomes of a Ring + subvalvular versus Ring MV repair were analyzed to assess the safety and efficacy of adding a subvalvular intervention to MV ring annuloplasty alone for secondary MR. Patient demographics, and baseline MR and LV remodeling severity, were similar between the groups. With the exception of one study, follow-up typically extended to at least 2 years. At last follow-up, when compared with Ring only, a Ring + subvalvular repair was associated with: (I) a smaller MR grade and a 57% reduced risk of moderate or greater MR recurrence; (II) greater LV reverse remodeling and improved LV systolic function; and (III) less MV leaflet tethering and papillary muscle displacement, as evidenced by a smaller MV tenting height, MV tenting area, and interpapillary muscle distance. There was no difference between surgical approaches with regards to peri-operative morbidity and mortality, or survival at follow-up.
The benefits of a Ring + subvalvular MV repair for secondary MR are derived from the application of therapeutic strategies addressing the subvalvular dysfunction that restricts proper valvular mechanics (10-12,14). A concomitant ring annuloplasty is utilized to correct annular dilatation that often co-exists; however, the undersizing strategy often implemented contributes to persistent MV leaflet tethering, placing a greater emphasis on LV support from a subvalvular intervention (24,25). Differences in the technical caveats and effects on the MV apparatus of these interventions should be mentioned, as the choice of subvalvular intervention must be individualized for each patient. With a restrictive circular polytetrafluoroethylene graft to approximate the papillary muscles, the interpapillary muscle distance is reduced, which ameliorates the posterolateral papillary muscle displacement (26,27). This restores a more anatomic subtending position of the papillary muscles, and may be of greater benefit in patients with a prior inferior myocardial infarction and asymmetric MV tethering (28). Papillary muscle repositioning results in movement of the anterolateral papillary muscle closer to the annulus, and reduces the apical restriction of the posteromedial papillary muscle (29,30). These effects appear to be imparted with greatest efficacy when relocating both papillary muscles simultaneously (31). Finally, chordal release (“cutting”) is typically performed on the secondary order (basal) chordae tendineae, which are attached to the ventricular side of the anterior mitral leaflet mid-body (32). It is most efficacious in apically displaced symmetric MV tethering, as seen with anterior myocardial infarction (33). However, controversy does exist regarding possible untoward long-term effects on MV and LV function with chordal release, given the importance of the secondary order chords in maintaining the closing-tethering forth balance of the MV apparatus, and supporting LV geometry (34,35).
The finding of greater LV reverse remodeling with a Ring + subvalvular MV repair is significant. As reverse remodeling occurs, the LV regains a more elliptical shape, which restores a more anatomic configuration of the MV apparatus and decreases the likelihood of recurrent MR (4,25). In a study of 204 patients with secondary MR and ischemic cardiomyopathy undergoing undersized ring annuloplasty, LV reverse remodeling occurred in 41.2%, which was defined as a reduction in the end-systolic volume >15% (36). At a median follow-up of 35 months, patients who experienced LV reverse remodeling had a smaller MV tenting area (1.9±0.3 vs. 2.9±1.0 cm2) and tenting height (8±2 vs. 10±4 mm), as well as interpapillary muscle distance (31±2 vs. 38±7 mm), when compared with no reverse remodeling (P<0.05 for all). Moderate or greater MR recurred in 67.5% of patients without LV reverse remodeling, and in 2.4% of those with reverse remodeling (P<0.001). Similar outcomes have also been reported in patients with secondary MR and non-ischemic dilated cardiomyopathy (37,38).
Currently, no consensus exists in terms of the application of subvalvular interventions in MV repair for secondary MR. However, several pre-operative transthoracic echocardiographic parameters have been identified that predict recurrence of MR after Ring MV repair, which can be used to select candidates who may benefit from a Ring + subvalvular approach. The most commonly used indices include an MV tenting area ≥2.5 cm2, MV tenting height ≥11 mm, end-systolic interpapillary muscle distance >20 mm, and LV end-systolic volume >145 mL (8,17,18). Of these, the end-systolic interpapillary muscle distance has the highest reported sensitivity (96%) and specificity (97%) for recurrent MR (18). A novel parameter, recently reported by the Cardiothoracic Surgical Trials Network, is the LV-MV ring size mismatch, which is the ratio of the LV end-systolic diameter to the implanted MV ring size. Larger ratios indicate a greater disproportion between the ring size needed to restore MV leaflet coaptation and extent of LV remodeling and dilatation. The risk for moderate or greater recurrent MR more than doubles with every 0.5 increase in the mismatch ratio (39,40).
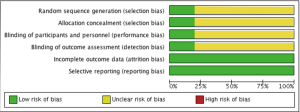
There are several limitations that one should be mindful of when interpreting the present results. Firstly, 4 of the 5 included studies were propensity matched analyses. While the statistical methods employed effectively created Ring + subvalvular and Ring groups with similar characteristics for comparison, this does not eliminate the underlying treatment selection bias present (Figure 7). Secondly, the sample sizes were small, and the studies were underpowered for the detection of differences in mortality between the surgical approaches. Thirdly, the pooled studies included several different and diverse subvalvular interventions, and the impact this may impart on procedural efficacy and clinical outcomes is unknown. Similarly, the type of ring annuloplasty utilized varied widely across the studies. While a complete ring annuloplasty is preferred to a partial annuloplasty band, and saddle-shaped rings may promote a more physiologic MV annular motion and valvular stress distribution, the clinical impact of these factors remains a point of debate (41,42). Fourthly, while uniform definitions were utilized for echocardiographic and clinical outcomes, not all variables were reported in each study, which can affect statistical power and underestimate the treatment effects. Additionally, there was statistical heterogeneity noted in the outcomes of LV ejection fraction, MV tenting height, and MV tenting area. This was mainly due to differences in the magnitude of benefit observed for Ring + subvalvular repair across studies; nevertheless, it stands as an important caveat. Fifthly, only 1 of the 5 studies included patients with non-ischemic dilated cardiomyopathy, so the conclusions drawn here may not fully apply to both ischemic and non-ischemic etiologies. Finally, the length of follow-up varied, and the results must be interpreted within this clinical context, as LV reverse remodeling may continue and MR can recur upwards of three years after MV surgery (2,31,43).
In conclusion, when compared with Ring only, a Ring + subvalvular MV repair is associated with greater LV reverse remodeling and systolic function, less recurrence of moderate or greater MR, and an improved geometry of the MV apparatus, at short-term and mid-term follow-up. A Ring + subvalvular intervention can be performed safely, and may provide a durable repair alternative to MV replacement. Continued research to identify the optimal candidates for this approach, in the form of randomized controlled trials and multicenter registries, and in comparison to conventional MV replacement, is of paramount importance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Magne J, Sénéchal M, Dumesnil JG, et al. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology 2009;112:244-59. [Crossref] [PubMed]
- Kalra K, Wang Q, McIver BV, et al. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 2014;64:1867-79. [Crossref] [PubMed]
- Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr 2011;24:707-19. [Crossref] [PubMed]
- Tibayan FA, Rodriguez F, Zasio MK, et al. Geometric distortions of the mitral valvular-ventricular complex in chronic ischemic mitral regurgitation. Circulation 2003;108 Suppl 1:II116-21. [Crossref] [PubMed]
- Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;103:1759-64. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Mihos CG, Yucel E, Santana O. The role of papillary muscle approximation in mitral valve repair for the treatment of secondary mitral regurgitation. Eur J Cardiothorac Surg. 2017;51:1023-30. [PubMed]
- Wagner CE, Kron IL. Subvalvular techniques to optimize surgical repair of ischemic mitral regurgitation. Curr Opin Cardiol 2014;29:140-44. [Crossref] [PubMed]
- Mihos CG, Larrauri-Reyes M, Santana O. A meta-analysis of ring annuloplasty versus combined ring annuloplasty and subvalvular repair for moderate-to-severe functional mitral regurgitation. J Card Surg 2016;31:31-7. [Crossref] [PubMed]
- Andalib A, Chetrit M, Eberg M, et al. A systematic review and meta-analysis of outcomes following mitral valve surgery in patients with significant functional mitral regurgitation and left ventricular dysfunction. J Heart Valve Dis 2016;25:696-707. [PubMed]
- Santana O, Lamelas J. Surgical options of ischemic mitral regurgitation. Cardiol Rev 2010;18:163-70. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Mihos CG, Pineda AM, Santana O. Targeting the papillary muscles in mitral valve repair for ischemic mitral regurgitation. Rev Cardiovasc Med 2015;16:182-8. [PubMed]
- Roshanali F, Mandegar MH, Yousefnia MA, et al. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg 2007;84:745-9. [Crossref] [PubMed]
- Langer F, Kunihara T, Hell K, et al. RING+STRING: Successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation 2009;120:S85-91. [Crossref] [PubMed]
- Fattouch K, Murana G, Castrovinci S, et al. The role of papillary muscle relocation in ischemic mitral valve regurgitation. Semin Thorac Cardiovasc Surg 2012;24:246-53. [Crossref] [PubMed]
- Calafiore AM, Refaie R, Iacò AL, et al. Chordal cutting in ischemic mitral regurgitation: a propensity-matched study. J Thorac Cardiovasc Surg 2014;148:41-6. [Crossref] [PubMed]
- Mihos CG, Capoulade R, Yucel E, et al. Combined papillary muscle sling and ring annuloplasty for moderate-to-severe secondary mitral regurgitation. J Card Surg 2016;31:664-71. [Crossref] [PubMed]
- Nappi F, Lusini M, Spadaccio C, et al. Papillary muscle approximation versus restrictive annuloplasty alone for severe ischemic mitral regurgitation. J Am Coll Cardiol 2016;67:2334-46. [Crossref] [PubMed]
- Green GR, Dagum P, Glasson JR, et al. Restricted posterior leaflet motion after mitral ring annuloplasty. Ann Thorac Surg 1999;68:2100-6. [Crossref] [PubMed]
- Hung J, Papakostas L, Tahta SA, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation 2004;110:II85-90. [Crossref] [PubMed]
- Hvass U, Tapia M, Baron F, et al. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg 2003;75:809-11. [Crossref] [PubMed]
- Lamelas J, Mihos C, Santana O. Surgical technique: papillary muscle sling for functional mitral regurgitation during minimally invasive valve surgery. Heart Surg Forum 2013;16:E295-7. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Nenna A, et al. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the Papillary Muscle Approximation trial. J Thorac Cardiovasc Surg 2017;153:286-95.e2. [Crossref] [PubMed]
- Tibayan FA, Rodriguez F, Langer F, et al. Annular or subvalvular approach to chronic ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2005;129:1266-75. [Crossref] [PubMed]
- Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg 2002;74:600-1. [Crossref] [PubMed]
- Langer F, Rodriguez F, Ortiz S, et al. Subvalvular repair: the key to repairing ischemic mitral regurgitation? Circulation 2005;112:I383-9. [PubMed]
- Messas E, Guerrero JL, Handschumacher MD, et al. Chordal cutting: a new therapeutic approach for ischemic mitral regurgitation. Circulation 2001;104:1958-63. [Crossref] [PubMed]
- Padala M, Gyoneva LI, Thourani VH, et al. Impact of mitral valve geometry on hemodynamic efficacy of surgical repair in secondary mitral regurgitation. J Heart Valve Dis 2014;23:79-87. [PubMed]
- Rodriguez F, Langer F, Harrington KB, et al. Importance of mitral valve second-order chordae for left ventricular geometry, wall thickening mechanics, and global systolic function. Circulation 2004;110:II115-22. [Crossref] [PubMed]
- Messas E, Bel A, Szymanski C, et al. Relief of mitral leaflet tethering following chronic myocardial infarction by chordal cutting diminishes left ventricular remodeling. Circ Cardiovasc Imaging 2010;3:679-86. [Crossref] [PubMed]
- Gelsomino S, Lorusso R, Capecchi I, et al. Left ventricular reverse remodeling after undersized mitral ring annuloplasty in patients with ischemic regurgitation. Ann Thorac Surg 2008;85:1319-30. [Crossref] [PubMed]
- Takeda K, Sakaguchi T, Miyagawa S, et al. The extent of early left ventricular reverse remodelling is related to midterm outcomes after restrictive mitral annuloplasty in patients with non-ischaemic dilated cardiomyopathy and functional mitral regurgitation. Eur J Cardiothorac Surg 2012;41:506-11. [Crossref] [PubMed]
- De Bonis M, Lapenna E, Verzini A, et al. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg 2008;85:932-9. [Crossref] [PubMed]
- Mihos CG, Yucel E, Santana O. Left ventricle-mitral valve ring size mismatch: understanding the limitations of mitral valve repair for ischemic mitral regurgitation. Ann Transl Med 2017;5:19. [Crossref] [PubMed]
- Capoulade R, Zeng X, Overbey JR, et al. Impact of left ventricular to mitral valve ring mismatch on recurrent ischemic mitral regurgitation after ring annuloplasty. Circulation 2016;134:1247-56. [Crossref] [PubMed]
- Mihos CG, Santana O. Annuloplasty Ring Selection in Ischemic Mitral Regurgitation for Valve Repair During Coronary Artery Bypass Grafting. J Card Surg 2015;30:906. [Crossref] [PubMed]
- Khamooshian A, Buijsrogge MP, de Heer F, et al. Mitral valve annuloplasty rings: review of literature and comparison of functional outcome and ventricular dimensions. Innovations (Phila) 2014;9:399-415. [Crossref] [PubMed]
- Bondarenko O, Beek AM, Twisk JW, et al. Time course of functional recovery after revascularization of hibernating myocardium: a contrast-enhanced cardiovascular magnetic resonance study. Eur Heart J 2008;29:2000-5. [Crossref] [PubMed]