Routine jejunostomy tube feeding following esophagectomy
Introduction
It is difficult to adequately feed patients following esophagectomy. During the first postoperative days patients are regularly kept nil-by-mouth, and often complications or gastro-intestinal dysfunction impair oral intake (1-3).These problems may prolong the hospital admission time, result in a re-admission and cause extensive weight loss. Even at a long-term, a decreased caloric intake and persistent weight loss are evident in this patient group (4,5). For that reason a surgically placed jejunostomy is frequently used as route for enteral nutrition directly postoperatively.
However, the overall complication rate of a surgically placed jejunostomy is 13–38%, and serious complications requiring a relaparotomy occur in 0–3% of the patients (6). On the other hand a jejunostomy is considered to be comfortable and effective for long-term nutritional support (2,6). Furthermore routine discharge with home-tube feeding has been advocated recently, which is facilitated by routine creation of a jejunostomy (7). The underlying hypothesis is that routine discharge with tube feeding will decrease hospital stay since previously patients often had to stay admitted due to difficulty in achieving their nutritional goals. Furthermore weight loss and hospital readmissions due to feeding problems may be decreased (7).
In this study the effects of routine jejunostomy tube feeding and routine discharge with home-tube feeding on weight changes, hospital admission time and re-admission rates following esophagectomy were analyzed and set out against the jejunostomy-related complications. Moreover factors predicting the duration of tube feeding were determined to identify subgroups that might especially benefit from selective jejunostomy placement.
Methods
A retrospective cohort study in the University Medical Center Utrecht, a tertiary referral center, was performed. The independent medical ethical committee of the University Medical Center Utrecht approved this study and declared no informed consent had to be obtained due to the retrospective and observational design of the study.
Patients
The patients were retrospectively identified in a prospectively collected electronic database. Consecutive patients undergoing an esophagectomy for cancer with gastric conduit reconstruction and a surgically placed jejunostomy at the University Medical Center Utrecht between January 01, 2009 and January 01, 2014 were included.
Jejunostomy procedure
A jejunostomy was routinely placed in the first jejunal loop distal to the ligament of Treitz. A small caliber tube was introduced into the efferent limb of the jejunal loop and attached to the jejunum with a purse string suture. The jejunal loop was fixed to the anterior abdominal wall with a double purse string suture.
Postoperative feeding
Patients were kept nil-by-mouth during the first 5–7 days following surgery. Nutritional support was initiated from the first day following surgery by protein rich tube feeding (Nutrison Protein Plus®, Nutricia) at a rate of 25 mL/h. The dosage was increased with 25 mL every 6 h until calorie and protein needs were met, calculated with modified Harris-Benedict formula with a surplus of 30–50% for the postoperative period depending on the patients’ condition (8). In case of gastro-intestinal complaints, the amount of tube feeding was reduced until the complaints resolved. An energy dense tube feeding (Nutrison Concentrated®, Nutricia) was temporarily used when there were fluid restrictions and a low-fat tube feeding (Vivonex®, Nutricia) was used in case of chyle leakage.
Oral intake of clear liquids was allowed after postoperative day 5–7 and expanded to liquid and solid food as tolerated, if there was no clinical suspicion for anastomotic leakage. Continuous tube feeding was indicated until possible complications prohibiting oral intake had resolved and the oral intake amounted to more than 50% of the patients’ needs. Supportive overnight tube feeding was given until the oral intake amounted to more than 75% of the patients’ needs. Occasionally, overnight tube feeding was continued when oral intake exceeded 75% of the patients’ needs when patients were severely malnourished preoperatively. The other exception was when patients explicitly wished to either stop or continue tube feeding.
Two regimens were maintained during the studied period. Between January 01, 2009 and June 30, 2011 patients were discharged when oral intake was sufficient without tube feeding, between July 01, 2011 and December 31, 2013 patients were discharged with tube feeding in order to facilitate early discharge.
Data items
Patient characteristics, surgical data, postoperative complications and hospital admission dates were extracted from a prospectively collected electronic database. Outliers and missing data were checked in hospital records. Anastomotic leakage was defined as a leakage requiring any treatment, ranging from opening of the cervical wound to re-operation. Pneumonia was registered when patients were treated with intravenous antibiotics based on a clinically suspected pneumonia and chyle leakage was registered when patients were treated for chyle leakage based on a chylous aspect of thorax drain output. Recurrent nerve injury was registered when vocal cord dysfunction was seen at laryngoscopy.
The following outcome data were collected retrospectively: body weight before and after surgery, start (restart) and stop of full or overnight tube feeding, and jejunostomy-related complications within 180 days postoperatively. Body weight was registered at the moment of diagnosis, at admission for esophagectomy and at 90 and 180 days postoperatively. Jejunostomy-related complications (mortality, dislodgement or occlusion that hampered tube feeding when tube feeding was still indicated, extra-abdominal leakage, insertion site infection requiring treatment, and jejunostomy-related complications necessitating a re-operation) were registered. Dislodgement and occlusion of the jejunostomy tube were only registered when tube feeding was still indicated, thus hampering adequate nutrition. Preliminary termination of tube feeding due to a jejunostomy-related complication was also registered.
Statistical analysis
Statistical analysis was executed with SPSS version 20. Continuous variables were summarized as medians (1st–3rd quartile) and categorical data as frequencies and percentages. In univariate analysis associations were tested with the Pearson’s chi-square, Fishers exact, Spearman correlation, Mann-Whitney-U or Kruskal Wallis test when appropriate. Binary logistic regression was performed to adjust for confounders. Risks were expressed as odds ratios (OR) with corresponding confidence interval (CI). The duration of postoperative tube feeding was included in the multivariate analysis regarding weight loss irrespective of P value because it was a primary variable of interest. All other factors were only included in the multivariate analyses when the P value was <0.1. In the multivariate analyses a P value <0.05 was considered to be statistically significant.
Results
Between January 01, 2009 and January 01, 2014, 236 patients (72% male) underwent an esophagectomy for cancer with a gastric conduit reconstruction and left cervical anastomosis. At time-points that weight was measured, 90 and 180 days postoperatively, 94% and 87% of the patients were alive. Loss to follow-up was 3% and 5% respectively. The follow-up duration was 180 days for all patients.
Baseline characteristics
A total of 179 (76%) patients had one or more comorbidity and 171 (72%) patients received neoadjuvant chemotherapy, chemo-radiation or radiotherapy (Table 1). Most patients, 198 (84%), underwent a minimally invasive esophagectomy, which was a robot-assisted McKeown esophagectomy in 140 (59%) patients, and a laparoscopic transhiatal esophagectomy in 58 (25%) patients. After July 01, 2011 a significant increase in the rate of neoadjuvant chemoradiotherapy (51% vs. 9%, P<0.001) and transthoracic surgery (79% vs. 62%, P<0.001) occurred, with a concomitant increase in anastomotic leakage (18% vs. 30%, P=0.033) and chyle leakage (19% vs. 31%, P=0.039) (Table 2).
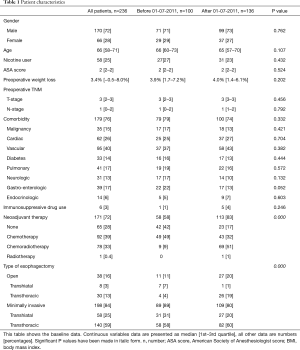
Full table
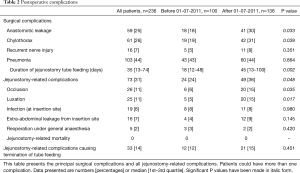
Full table
Jejunostomy-related complications
Jejunostomy-related complications occurred in 73 (31%) patients (Table 2), of which occlusion (n=26, 11%) or luxation (n=25, 11%) were most prevalent. The duration that tube feeding was administered was significantly longer after July 01, 2011, as expected since patients were mostly discharged with home-tube feeding after this date. This was accompanied by a significant increase in the total amount of jejunostomy-related complications (26% vs. 36%, P=0.048), occlusion (6% vs. 15%, P=0.035) and luxation (5% vs. 15%, P=0.017). The median duration of tube feeding was also longer in patients with jejunostomy-related complications; 46 [15–94] versus 24 [12–65] days (P=0.011). Irreversible jejunostomy-related complications that led to the premature termination of jejunostomy tube feeding occurred in 33 (14%) patients. Totally parenteral nutrition or nasojejunal tube feeding had to be started in 16 (7%) of these patients because they could not achieve adequate oral intake or had contra-indications for oral intake such as anastomotic leakage. A reoperation under general anesthesia was required in 5 (2%) patients to treat a jejunostomy-related complication. Two relaparotomies were performed; one for an ischemic proximal small intestine and one patient for intra-abdominal leakage. The other three reoperations were surgical drainage of a large pre-fascia abdominal wall abscesses. Jejunostomy-related mortality did not occur.
Perioperative weights
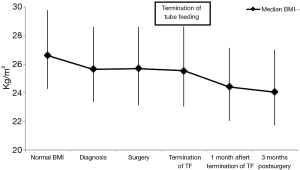
Significant weight loss occurred before cancer diagnosis (Figure 1). Body mass index (BMI) stabilized between diagnosis and surgery, and postoperative weight loss mainly occurred in the first 90 days following surgery. Weight loss primarily occurred after tube feeding was stopped. At the time tube feeding was stopped the median BMI was 25.6 (23.0–28.6) kg/m2, which decreased to 24.4 (22.0–27.1) kg/m2 within 1 month (P<0.001). This was a median of 3.0 (1.0–5.3) kg weight loss, corresponding with a median of 3.9% (1.5–6.3%) weight loss. No significant associations were found between the amount of weight loss at 90 days postoperative and duration of tube feeding or the application of full or supplementary home-tube feeding in univariate and multivariate analysis (Table 3).
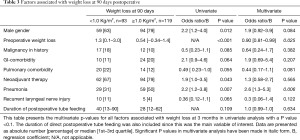
Full table
Duration of postoperative tube feeding
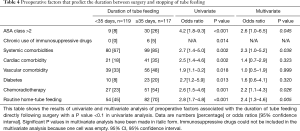
Directly following surgery, tube feeding was given for a median uninterrupted period of 35 [13–74] days (Table 2). Complete tube feeding was given for 12 [10–33] days and over-night supplementary tube feeding for 5 [1–29] days. Tube feeding continued for an uninterrupted period following surgery exceeding 180 days in 10 patients (4%), and until the moment of death in 13 patients (6%). An American Society of Anesthesiologists (ASA) class >2, presence of systemic comorbidities, application of neoadjuvant chemoradiotherapy and discharge with home-tube feeding regimen were independent predictors for a duration of tube feeding of 35 days or longer (Table 4). ASA class >2 was significantly associated with anastomotic leakage (OR 3.2, 95% CI, 1.58–6.64, P=0.001) and pneumonia (OR 2.4, 95% CI, 1.17–4.49, P=0.001) and the application of neoadjuvant therapy was associated with anastomotic leakage (OR 1.7, 95% CI 0.9–3.2, P=0.079) and pneumonia (OR 1.6, 95% CI, 0.9–2.7, P=0.096). When anastomotic leakage and pneumonia were entered into the multivariate model, ASA class and neo-adjuvant chemoradiotherapy were no longer significantly associated with a prolonged duration of postoperative tube feeding.
Full table
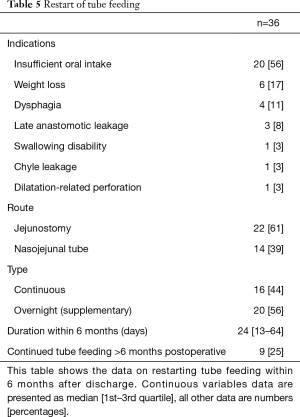
Tube feeding was restarted in 36 (15%) patients during the first 180 days following esophagectomy during the studied period (Table 5). The indication to restart tube feeding was insufficient oral intake in 20 (56%) patients. Tube feeding was restarted via the jejunostomy in 22 (61%) patients. In the remaining 14 (39%) patients a nasojejunal tube had to be placed because the jejunostomy had already been removed. Tube feeding was restarted via the jejunostomy at 34 [26–53] days from surgery, and via the nasojejunal tube at 98 [72–165] days (P>0.0001).
Full table
Discharge with home-tube feeding
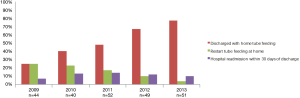
In 2009 a minority of patients (25%) were discharged with home-tube feeding whilst in 2013 the majority of the patients (77%) were discharged with home-tube feeding. Discharge with home-tube feeding was associated with a significant reduction in the number of patients that needed to restart tube feeding after discharge (30% vs. 4%; P<0.001) (Figure 2). Discharge with tube feeding was not correlated with a reduction in the length of hospital stay (median 16 vs. 15 days; P=0.217) or a reduction in hospital readmissions (10% vs. 12%; P=0.669).
Discussion
The present study was performed to evaluate potential benefits and complications of routine jejunostomy tube feeding following esophagectomy. It was shown that weight remained stable during the period of tube feeding following esophagectomy. However, patients were not able to maintain their usual weight without support and lost weight after tube feeding was stopped, independent of the duration of tube feeding. Also a low rate of major jejunostomy-related complications occurred that required reoperation.
Other studies investigating jejunostomy tube feeding report weight loss between 1.0 kg at 1 month after esophagectomy and 7.0 kg at the first postoperative visit (9-12). This is comparable to the weight loss that occurred after the termination of tube feeding in our study. Patients continue to lose weight until a new balance between food-intake and energy requirements is reached (13,14). There are two studies with a follow-up period of 1 and 3 years following esophagectomy, with and without jejunostomy, that show that weight loss occurs during the first 6 months postoperatively (4,15). After that period weight remains relatively stable but at a lower level than the patients pre-operative weight. The current study shows that weight loss cannot be prevented following esophagectomy. The main question is from which moment after surgery weight loss can be accepted.
A theoretical advantage of a jejunostomy is that patients may be discharged with home-tube feeding for a long period. This regime was developed to facilitate the early discharge despite insufficient oral intake and to prevent readmission due to insufficient oral intake. The present study shows that these goals were not achieved. The reason might be that the multivariate analysis was insufficient to correct for the strong influence of the concomitant higher rate of anastomotic leakage, chyle leakage and application of neoadjuvant chemoradiotherapy in the period that discharge with home-tube feeding was routine. However not even a trend to improvement was found.
Though proposed benefits could not be confirmed, jejunostomy tube feeding related complications frequently occurred and were in line with rates reported in literature. This study shows that the risk for tube-related complications is related to the duration that tube feeding is administered. Occlusion, dislocation and insertion site infection of the jejunal feeding tube occurred frequently and were comparable to rates reported in literature (0–7% occlusion, 0–11% dislocation, 1–25% infection) (9,11,12,16-22). Previous reports also showed that severe jejunostomy-related complications requiring reoperation occur in 0–3% of all patients, which was confirmed by the present study (9,11,12,16,18,20,23). Furthermore in a randomized trial it was shown that immediate jejunostomy tube feeding may impair respiratory mechanics and may decrease mobility following esophagectomy (24).
These data question whether jejunostomy tube feeding should be routinely applied following esophagectomy since early enteral feeding can also be applied via a nasojejunal tube. Comparable amounts of nutrients can be applied via this route, and serious complications have not been reported (16). The only drawbacks are frequent dislocation, in 20–35% of all patients (16,25,26) and discomfort for the patient. Thus in case of an indication for long-term tube feeding, such as anastomotic leakage, a jejunostomy tube may be preferable. Another option for direct enteral feeding is oral feeding (27). However, following esophagectomy early initiation of oral intake is believed to increase sequelae of anastomotic leakage and pneumonia rates. Though a safety and feasibility trial has shown encouraging results the results of a randomized controlled trial on this topic have to be awaited (28-30).
It is the question whether a patient tailored approach may be possible, creating a feeding jejunostomy only in those patients that are at high risk for a prolonged need for tube feeding postoperatively. In this study, comorbidities, ASA class 3 or 4, neoadjuvant chemoradiation were independent risk factors for a prolonged duration of tube feeding. The association between ASA class and neoadjuvant therapy with the duration of postoperative tube feeding probably results from the increased risk for anastomotic leakage and pneumonia. This underlines the need for multivariable models that predict which patients will suffer from those complications, since these are most likely to benefit from jejunostomy placement (31).
Conclusions
Several hypothetical benefits of routine jejunostomy tube feeding following esophagectomy over nasojejunal tube feeding were studied. Weight loss was found to be postponed until the moment that jejunostomy tube feeding was stopped. Routine discharge with home-tube feeding did not reduce the length of hospital stay, number of hospital readmissions and weight loss. Meanwhile jejunostomy-related complications, occasionally life-threatening occurred. This study shows that the routine use of jejunostomy tube feeding following esophagectomy can be questioned emphasizing the need for further prospective studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The independent medical ethical committee of the University Medical Center Utrecht approved this study (No. 14-349/c) and declared no informed consent had to be obtained due to the retrospective and observational design of the study.
References
- Akkerman RD, Haverkamp L, van Rossum PS, et al. Long-term quality of life after oesophagectomy with gastric conduit interposition for cancer. Eur J Cancer 2015;51:1538-45. [Crossref] [PubMed]
- Markides GA, Alkhaffaf B, Vickers J. Nutritional access routes following oesophagectomy--a systematic review. Eur J Clin Nutr 2011;65:565-73. [Crossref] [PubMed]
- Akkerman RD, Haverkamp L, van Hillegersberg R, et al. Surgical techniques to prevent delayed gastric emptying after esophagectomy with gastric interposition: a systematic review. Ann Thorac Surg 2014;98:1512-9. [Crossref] [PubMed]
- Martin L, Lagergren P. Long-term weight change after oesophageal cancer surgery. Br J Surg 2009;96:1308-14. [Crossref] [PubMed]
- Carey S, Storey D, Biankin AV, et al. Long term nutritional status and quality of life following major upper gastrointestinal surgery - A cross-sectional study. Clin Nutr 2011;30:774-9. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2015;34:1-6. [Crossref] [PubMed]
- Tomaszek SC, Cassivi SD, Allen MS, et al. An alternative postoperative pathway reduces length of hospitalisation following oesophagectomy. Eur J Cardiothorac Surg 2010;37:807-13. [Crossref] [PubMed]
- Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984;40:168-82. [PubMed]
- Couper G. Jejunostomy after oesophagectomy: a review of evidence and current practice. Proc Nutr Soc 2011;70:316-20. [Crossref] [PubMed]
- Gupta V. Benefits versus risks: a prospective audit. Feeding jejunostomy during esophagectomy. World J Surg 2009;33:1432-8. [Crossref] [PubMed]
- Wani ML, Ahangar AG, Lone GN, et al. Feeding jejunostomy: does the benefit overweight the risk (a retrospective study from a single centre). Int J Surg 2010;8:387-90. [Crossref] [PubMed]
- Ryan AM, Rowley SP, Healy LA, et al. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr 2006;25:386-93. [Crossref] [PubMed]
- Ludwig DJ, Thirlby RC, Low DE. A prospective evaluation of dietary status and symptoms after near-total esophagectomy without gastric emptying procedure. Am J Surg 2001;181:454-8. [Crossref] [PubMed]
- Baker M, Halliday V, Williams RN, et al. A systematic review of the nutritional consequences of esophagectomy. Clin Nutr 2016;35:987-94. [Crossref] [PubMed]
- D'Journo XB, Ouattara M, Loundou A, et al. Prognostic impact of weight loss in 1-year survivors after transthoracic esophagectomy for cancer. Dis Esophagus 2012;25:527-34. [Crossref] [PubMed]
- Han-Geurts IJ, Hop WC, Verhoef C, et al. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. Br J Surg 2007;94:31-5. [Crossref] [PubMed]
- Srinathan SK, Hamin T, Walter S, et al. Jejunostomy tube feeding in patients undergoing esophagectomy. Can J Surg 2013;56:409-14. [Crossref] [PubMed]
- Fenton JR, Bergeron EJ, Coello M, et al. Feeding jejunostomy tubes placed during esophagectomy: are they necessary? Ann Thorac Surg 2011;92:504-11; discussion 511-2. [Crossref] [PubMed]
- Rino Y, Yukawa N, Murakami H, et al. Primary placement technique of jejunostomy using the entristar™ skin-level gastrostomy tube in patients with esophageal cancer. BMC Gastroenterol 2011;11:8. [Crossref] [PubMed]
- Sica GS, Sujendran V, Wheeler J, et al. Needle catheter jejunostomy at esophagectomy for cancer. J Surg Oncol 2005;91:276-9. [Crossref] [PubMed]
- Mercer CD, Mungara A. Enteral feeding in esophageal surgery. Nutrition 1996;12:200-1. [Crossref] [PubMed]
- Gerndt SJ, Orringer MB. Tube jejunostomy as an adjunct to esophagectomy. Surgery 1994;115:164-9. [PubMed]
- Han-Geurts IJ, Verhoef C, Tilanus HW. Relaparotomy following complications of feeding jejunostomy in esophageal surgery. Dig Surg 2004;21:192-6. [Crossref] [PubMed]
- Watters JM, Kirkpatrick SM, Norris SB, et al. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg 1997;226:369-77; discussion 377-80. [Crossref] [PubMed]
- Gabor S, Renner H, Matzi V, et al. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr 2005;93:509-13. [Crossref] [PubMed]
- Page RD, Oo AY, Russell GN, et al. Intravenous hydration versus naso-jejunal enteral feeding after esophagectomy: a randomised study. Eur J Cardiothorac Surg 2002;22:666-72. [Crossref] [PubMed]
- Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247:721-9. [Crossref] [PubMed]
- Weijs TJ, Nieuwenhuijzen GA, Ruurda JP, et al. Study protocol for the nutritional route in oesophageal resection trial: a single-arm feasibility trial (NUTRIENT trial). BMJ Open 2014;4:e004557. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate Postoperative Oral Nutrition Following Esophagectomy: A Multicenter Clinical Trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- Berkelmans GH, Wilts BJ, Kouwenhoven EA, et al. Nutritional route in oesophageal resection trial II (NUTRIENT II): study protocol for a multicentre open-label randomised controlled trial. BMJ Open 2016;6:e011979. [Crossref] [PubMed]
- van Rossum PS, Haverkamp L, Verkooijen HM, et al. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology 2015;274:124-32. [Crossref] [PubMed]


