The extent of lymphadenectomy in esophageal resection for cancer should be standardized
Introduction
The incidence of esophageal cancer increases, with approximately 482,000 patients diagnosed with esophageal cancer each year. Lymph node status is an important prognostic parameter in esophageal carcinoma and an independent predictor of survival (1-8). The distribution pattern of lymph node metastases of esophageal cancer is unpredictable. Distribution of lymph node metastases may depend on tumor characteristics such as tumor location, histology, T-stage and the use of neoadjuvant therapy (9-14). The surgical strategy should depend on the distribution pattern of nodal metastases but there is no worldwide consensus on the extent of lymphadenectomy. Lack of knowledge of the distribution pattern of nodal metastases in patients with esophageal carcinoma makes this disease unpredictable, and the optimal extent of the lymphadenectomy is still under debate (15-17).
Distribution of lymph node metastases in squamous cell carcinoma has been mapped by Akiyama in 1994 (3). In different percentages tumors located in the upper, middle and lower esophagus can metastasize to lymph nodes located in cervical, supracarinal, infracarinal and abdominal (among others celiac axis) lymph nodes. In Asia, the incidence of squamous cell cancer is more than 95% of all resected esophageal cancers and the type of lymphadenectomy has been standardized in using the 2- or 3-field lymphadenectomy according with the location of the tumor.
In Europe, esophageal surgery has improved importantly in the last 20 years with 5-year-survival increasing from 15% up to the 45% in the last years. This improvement is due to different factors such as a better diagnosis and staging of patients with esophageal cancer, a better selection of patients for surgery, the implementation of neoadjuvant therapy for stage 2 and 3 diseases and the improvements in anesthesia and Intensive Care.
Moreover, in the last 15 years, there has been an important change of the type of esophageal cancer (from squamous cell cancer to adenocarcinoma) but also its location in the esophagus. Currently, 85% of all tumors in Western Europe are adenocarcinomas mostly located in the distal esophagus and esophagogastric junction, especially in males (1,18). Additionally, combination of obesity and distal adenocarcinoma is very frequent in the western world and 1/3 of patients have a BMI above of 30 (19).
Furthermore, the implementation of neoadjuvant therapy in the Western World for esophageal cancer stages 2 and 3, the MAGIC scheme in the UK and chemoradiotherapy in the continent (CROSS scheme), has been very widely employed (18,20).
There are still controversial issues in esophageal surgery, and one very important and not yet solved is the type of mediastinal and abdominal lymphadenectomy to perform in these patients with distal esophageal and junctional adenocarcinomas. There are many contradictory opinions and ongoing discussions.
An extensive lymphadenectomy may improve survival and is considered the criterion standard according to many clinical guidelines (15). An extensive lymphadenectomy has the disadvantage of greater surgical morbidity (lymphadenectomy alongside both recurrent nerves can cause increasing percentages of recurrence palsy and accompanying pulmonary complications) and it has been suggested that it may not add advantage to the standard treatment (21,22).
The surgical approach will also influence the number and location of resected lymph node stations (23). A transhiatal resection allows for an adequate abdominal lymphadenectomy but a subcarinal, high paraesophageal and paratracheal lymph node dissection is not possible. In Ivor Lewis esophagectomy a 3-field lymphadenectomy will not be performed. However, even in a 3-stage approach some lymph node stations may be left in situ, like splenic hilum nodes or hepatoduodenal ligament nodes, the high paratracheal nodes or the cervical lymph nodes.
Despite the growing incidence in esophageal carcinoma, the extent of the lymphadenectomy is still under discussion. These issues will be addressed by providing an overview of the literature on the extent of lymphadenectomy for esophageal cancer with respect to the supposed lymph node distribution patterns for squamous cell carcinoma and adenocarcinoma, the different lymph node classification systems, the commonly used surgical techniques and outcomes and future perspectives.
Esophageal lymphatic system
The esophagus is an organ crossing three compartments, the neck, the mediastinum and the upper abdomen. Moreover, the esophagus is a muscular tube with mucosal lining and with lymphatic and vascular connections to the great vessels in the mediastinum. But, in the case of malignancy, what is the lymphatic spreading from any location in the esophagus? According to Ji et al. the lymphatic spread of the esophagus has two modes, including penetrating the esophageal wall transversally and shifting longitudinally upwards to the cervical lymph nodes and downwards to the upper abdominal lymph nodes. There are three pathways for lymph node metastasis in esophageal cancer: the longitudinal spreading along the submucosa to regional and non- regional lymph nodes, the second passes transversally through the muscularis propria to regional lymph nodes and the third penetrates perpendiculary through muscularis mucosa to the thoracic duct and the venous system. In this way, it is clear that once the primary tumor infiltrates the submucosa of the esophagus, the change of lymph node metastasis increases (24-26).
Pattern of lymph node metastases
Squamous cell carcinoma
For squamous cell carcinoma, there are a few large retrospective and prospective cohort studies, that describe the distribution pattern of lymph node metastases (3,9,11,13,27). Most patients in these studies have T3 cancers and almost 60% has positive lymph nodes. A wide variation in lymph node metastases is described, with lymph node metastases to the cervical, mediastinal and abdominal regions. The difficulty in comparing the results of these studies is that there is a great variety in the number and localization of the described lymph node stations. One can only conclude that there is great variation and a wide field of spread of lymph node metastases in esophageal squamous cell carcinoma. Therefore, in squamous cell carcinoma a 3 field lymph node dissection should be advised on the basis of literature (9,28,29). The results of a meta-analysis comparing two-field versus three-field lymph node dissection, including two randomized controlled trials and 18 observational studies with over 7,000 patients, show that three-field lymphadenectomy improves overall survival rate but is accompanied by more recurrent nerve palsy and anastomotic leakages (28). The Japanese Esophageal Society (JES) worked out a very detailed classification system with advice about which lymph node stations should be resected in relation to tumor location (see ‘lymph node classification systems’) (30).
Adenocarcinoma
For adenocarcinoma there are few studies that describe the distribution of lymph node metastases (8,14,26,31-35). Also in these studies mainly T3 carcinomas were included and about 70% of patients were node positive. As in squamous cell carcinoma, lymph node metastases are described in the cervical, mediastinal and abdominal regions, although it is unclear in what percentage a cervical lymphadenectomy was performed (31). In these studies, different lymph node stations are described, so also here, these studies can’t be compared in meta-analysis. Studies performed on the junctional esophageal cancer indicate that distal esophageal adenocarcinomas and type I Siewert can metastasize in more than 10% to the supracarinal lymph nodes whereas the esophagogastric junction carcinomas (type II Siewert) only do this in 1–2% (32-34). This means that for all the Siewert I adenocarcinomas and all the adenocarcinomas located in the thoracic esophagus an extended or total lymphadenectomy should be the rule whereas for type II only a mediastinal lymphadenectomy up to subcarinal lymph nodes might be sufficient. However, in other studies even in gastroesophageal junction carcinomas upper mediastinal lymph node metastases are described in a quarter of the patients (14).
Skip metastases
Metastasis to anatomically distant lymph nodes, without metastasis in the direct environment are known as skip metastasis and can develop in the early phase of lymphatic invasion in patients with esophageal cancer (29). Regardless of primary tumor localization, 20% to 40% of patients with esophageal cancer have cervical lymph node metastasis (9,12). It is important to study the prognosis of patients with a solitary lymph node metastasis compared to patients with multiple metastatic nodes meaning systematic disease with poor prognosis (29). These so-called ‘skip metastases’, along with the complex lymphatic system surrounding the esophagus, contribute to the fear of invisible micro metastases distant of the primary tumor site and it prompts surgeons to perform a more extended lymphadenectomy accompanying esophageal resection. In up to 50–60% of esophageal cancer, metastases are found in lymph nodes of the second or third anatomic compartment (upper abdomen, mediastinum, and cervical), skipping lymph nodes of the first compartment (10).
The prognostic impact of lymph node skip metastasis has been unclear. Some reports have shown a favorable prognosis of skip metastasis compared with adjacent or continuous lymph node metastasis and others have not (26,35). Wu et al., compared the prognostic significance of solitary lymph node metastasis in patients with squamous cell carcinoma of the middle thoracic squamous cell carcinoma of the esophagus with a cohort of N0 patients with the same location of the squamous cell carcinoma. They found that solitary lymph node metastasis has a negative impact on survival compared with N0 disease; skip metastasis, however, is comparable to N0 diseases in predicting prognosis (29).
Lymph node classification systems
The lymphatic vessels surrounding the esophagus are complexly aligned and they contribute to a multidirectional spread of lymph node metastasis into the abdomen, the mediastinum and the neck.
Multiple lymph node classification systems are used in the staging of esophageal cancer. The most frequently used classifications are the based on the guidelines of the Japanese Esophageal Society (JES) and the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (30,36).
The guidelines of the JES divide the locations of lymph node metastases into three categories N1, N2 and N3 according to the location of the tumor (cervical, thoracic, abdominal or esophagogastric junction). The Japanese worked out a very detailed classification system that advises which lymph node stations should be resected in relation to the primary tumor: cervical, upper thoracic, middle thoracic, lower thoracic and abdominal. Per tumor location the N1-3 lymph nodes are defined (Tables 1 and 2, Figure 1).

Full table

Full table
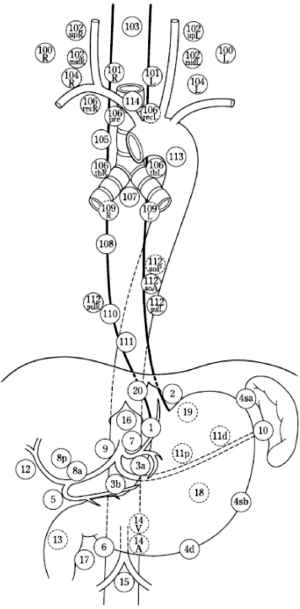
The latest edition of the AJCC Cancer Staging Manual numbers the location of lymph node metastases in the thorax and abdomen (Figure 2). The cervical lymph nodes are not included, there is reference to the head and neck guide. In the 8th edition of the AJCC Cancer Staging Manual, N-status depends on the number of positive lymph nodes, location is not taken into account.
The JES lymph node classification system includes all anatomical locations of the AJCC cancer staging, however the JES classification is more detailed and records more lymph node stations, especially in the cervical and upper mediastinal regions. In addition to different definitions of anatomical locations, the AJCC and JES guidelines have different ways to define the nodal status.
These differences make current literature heterogeneous and studies hard to compare. This advocates the need for a uniform classification system for esophageal cancer regarding definitions of anatomic locations of lymph nodes and the determination of N-status. However, squamous cell cancer continues to be the major type of esophageal cancer in Asia. In contrast, adenocarcinoma predominately affects the Caucasian population in Western countries, these two different diseases might need different staging systems, this however is not studied and remains unknown today (37,38). The location of the primary tumor and histopathologic cell type both influence the location of lymph node metastases, resulting in a different surgical approach and lymphadenectomy (39). Moreover, the Western population is generally more obese, resulting in a different surgical view and anatomy, this may lead to different neoadjuvant therapies, surgical procedures, including lymphadenectomy. These can be explanatory factors why the classification systems differ.
Surgical procedures
Different types of Lymphadenectomy
Different types of a mediastinal lymphadenectomy have been described for squamous cell carcinoma by the ISDE in 1994: the more limited standard, the extended, the total and the 3-field lymphadenectomy (40). The 3-field lymphadenectomy has never been systematically used in Europe for squamous cell carcinoma or adenocarcinoma and only a selected group of academic surgeons have implemented this lymphadenectomy in some studies. In Europe, the standard lymphadenectomy differs between countries and even between centers within countries, as transhiatal and transthoracic operations have been standard procedures existing next to each other, and the optimal surgical approach for the treatment of patients with esophageal adenocarcinoma is still under debate. Transhiatal esophagectomy with limited lymphadenectomy mainly focuses on a decrease of postoperative morbidity and mortality by preventing a formal thoracotomy. The transthoracic esophagectomy with extended 2-field lymphadenectomy attempts to improve the radicality of the resection and thus to increase locoregional tumor control and long-term survival. A transthoracic esophagectomy is, however, associated with increased postoperative morbidity (41).
For esophageal carcinoma at or above the level of the carina transthoracic esophagectomy with a 2- or 3-field lymph node dissection is mandatory, this is accompanied by a cervical anastomosis. For adenocarcinomas located below the level of the carina, either transthoracic esophagectomy with an intrathoracic anastomosis or transhiatal esophagectomy can be performed, depending on surgeon’s preference and patient characteristics. Although others advocate that a transhiatal resections can only be performed for tumors extending not more than 5 cm in the distal esophagus. For adenocarcinomas involving the esophagogastric junction and/or gastric cardia, a survival benefit for transthoracic esophagectomy over transhiatal esophagectomy has never been proven (41).
Sentinel node (SN)
A SN is defined as the first lymph node that receives lymphatic flow directly from the primary tumor, being the first site of metastatic spread. The SN concept states that when pathologic analysis of the detected SN shows no tumor invasion, extensive dissection of the lymph nodes that drain the SN can be omitted (42).
SN surgery in esophageal cancer has first been investigated by Kitagawa in 2000, whereas for other tumors, especially breast cancer and melanoma, its role has already been established (43). The difficulty in esophageal cancer is the multidirectional lymphatic flow and the random and also widespread distribution of lymph node metastases from cervical to abdominal regions (5). This also contributes to the earlier described skip metastases.
Several studies showed that a SN procedure is feasible and associated with a high detection (88–100%) and accuracy rate (78–100%) and a high sensitivity (78–100%). Early esophageal cancer (T1-tumors) is associated with the best results, while advanced cancers are being considered non-suitable because of the destruction of lymph vessels by the tumor and the formation of fibrosis in case of chemoradiation therapy (40,44,45). Early esophageal cancer can be managed with endoscopic mucosal resection or endoscopic submucosal dissection (46). Lymph node metastases are rare (<2%) in case of a low-risk tumor (negative resection margins, tumor confined to mucosa, not poorly differentiated, and absence of vascular or lymphatic invasion) (44). In adenocarcinoma, in case of deep (>500 nanometers) submucosal invasion, a poor differentiation grade, or lymphovascular invasion, the risk of concomitant lymph node metastasis is high, and in these patients, SN surgery could be considered. For squamous cell carcinoma, the risk of lymph node metastases seems to be even higher, with already a 8% risk of metastases in case of m3 disease (47). Currently, a Dutch trial (the SNAP study; NTR5245) is ongoing and recruiting patients for a SN procedure after a radical endoscopic resection of the primary tumor.
Number of lymph nodes to be resected
Overall, via a transthoracic procedure a more extensive lymphadenectomy can be performed, resulting in more harvested lymph nodes. In studies where a comparison is made between the transhiatal and the transthoracic approach, there is a higher lymph node yield after a transthoracic approach. Kutup et al. (2014) obtained by transhiatal approach 17 lymph nodes and through the transthoracic approach 21 nodes (16). This was also the case in the HIVEX trial: the mean number of resected lymph nodes after transhiatal was 16 and after a transthoracic resection 31 lymph nodes (41). In the European study of Anderegg et al., adenocarcinomas of the distal esophagus and GE-junction were operated via transthoracic esophagectomy with an average number of 29 resected lymph nodes (48). They, as many others, found that the presence of lymph node metastases was an independent predictor for survival. Relatively distant lymph node metastases along the celiac axis and/or the proximal field showed to have a negative impact on survival.
Discrepancy exists between staging systems about how many lymph nodes should minimally be resected. For the AJCC this is 10 lymph nodes, in the Dutch guideline 15 lymph nodes, for the German S3 guidelines 20 lymph nodes and for authors like Peyre et al. 23 lymph nodes are the minimum requisite to be resected (36,49). There are authors who claim that the extent of lymphadenectomy should be associated with the stage of the tumor. Rizk et al. recommended for pT1 to resect 10 lymph nodes, for pT2 20 lymph nodes and for pT3–4 at least 30 lymph nodes (17). Others claim the opposite, especially in N0 disease a higher number of resected lymph nodes is associated with better survival, and, moreover, a higher number of negative lymph nodes or a higher lymph node ratio leads to a better survival (50-52).
The value of lymphadenectomy after neoadjuvant chemoradiotherapy
The effect of neoadjuvant therapy on lymph node metastases and the distribution pattern is subject of investigation. Neoadjuvant chemotherapy modifies the number but also the distribution of mediastinal lymph nodes (31). The effect of chemoradiotherapy on lymph nodes may differ between inside and outside radiation field lymph nodes. In addition, neoadjuvant systemic chemotherapy alone may also affect locoregional metastatic lymph nodes. Neoadjuvant chemoradiation is able to treat metastatic lymph nodes. Lymph nodes inside the radiation field are affected by radiotherapy and concurrent chemotherapy, whereas lymph nodes outside the irradiation field are affected by chemotherapy only. Pathology results show fibrosis or sterile nodes in previously affected lymph nodes after successful chemoradiation or systemic chemotherapy. Neoadjuvant therapy and the extent of the radiation field is therefore a new important factor that might influence surgical approach and lymphadenectomy (27). Talsma et al., using the data base of the CROSS study that compared esophagectomy versus chemoradiotherapy followed by resection, came to the conclusion that the number of resected nodes have a prognostic impact on survival in patients after surgery alone. After chemoradiotherapy, the number of resected nodes was not associated with survival. These data question the indication for maximization of lymphadenectomy after nCRT (53).
Relation of lymphadenectomy and survival
Recently, a controversial paper is published by Lagergren et al., studying a cohort of patients who underwent esophagectomy for cancer between 2000–2012. The independent role of the extent of lymphadenectomy in relation to all-cause and disease-specific 5-year mortality was analyzed using Cox proportional hazard regression model. With 83.5% adenocarcinomas and with 47% 5-year survival, they found that the extent of lymphadenectomy was not statistically associated with all-cause or disease-specific mortality. Patients with removed nodes (21–52 nodes) did not demonstrate a statistically significant reduction in all cause 5-year mortality compared with those in the lowest removed lymph nodes (0–10 nodes). They concluded that the extent of lymphadenectomy might not influence 5-year all cause or disease-specific survival. These results challenge current clinical guidelines (54). Others advocate the opposite. A study with 2,303 patients from 9 international centers showed that the number of removed lymph nodes was an independent predictor of survival, and to maximize this benefit a minimum of 23 lymph nodes should be removed (55). Another study with 4,627 patients from leading international esophageal cancer centers showed the same results: improved survival rates following more extensive lymphadenectomy (17).
What do we do in 2017 in the West?
General guidelines state that for squamous cell carcinoma a total 2-field lymphadenectomy should be performed. For these squamous cell carcinomas, in any location in the thorax, after neoadjuvant chemoradiotherapy a 2-field total mediastinal lymphadenectomy should be performed and a cervical lymphadenectomy in selected cases. The type of lymphadenectomy along the recurrent laryngeal nerves is not so complete as it is in Japan. Dissection is not so extended.
For distal located adenocarcinomas (type 1 Siewert), and gastroesophageal junction carcinomas after chemoradiotherapy, a 2-stage esophageal resection is performed followed by intrathoracic anastomosis (Ivor Lewis procedure). Some surgeons perform a limited lymphadenectomy, reserving the supracarinal lymphadenectomy for patients with pre-neoadjuvant treatment suspected lymph nodes in this area. Others perform systematically an extended mediastinal lymphadenectomy in these cases. There is no consensus yet, and evidence is conflicting.
In the Netherlands, the implementation of minimally invasive esophagectomy is around 75% for all cases of esophageal cancer. Minimally invasive esophagectomy is usually performed between 6 and 12 weeks after completion of chemoradiotherapy. In Japan, after neoadjuvant chemoradiotherapy, surgeons prefer not to perform minimally invasive esophagectomy because the changes produced in the mediastinal anatomy and the appearance of mediastinal fibrosis. Surgeons will prefer to perform open mediastinal lymphadenectomy.
Moreover, in Europe 40% of the patients still undergoes a transhiatal resection in which the mediastinal lymphadenectomy is very limited. Indications for transhiatal resection are the fragile patients with a junctional or distal esophageal adenocarcinoma after neoadjuvant chemoradiotherapy.
Future perspectives
There are many questions about this subject: How to standardize the type of lymphadenectomy in esophageal adenocarcinoma and junctional tumors? Which kind of lymphadenectomy to perform with these tumors after neoadjuvant therapy? Are the outcomes for Junctional type 1 and 2 the same after a transhiatal and transthoracic esophageal resection after neoadjuvant therapy? Is it possible to perform a total mediastinal lymphadenectomy and an Ivor Lewis anastomosis without impairment of the vascularization of the proximal esophagus after neoadjuvant chemoradiotherapy? Is it possible to perform en bloc supracarinal lymphadenectomy through minimally invasive esophagectomy from the right side of the trachea? Should the lack of homogeneity concerning classification of lymph node stations should be solved by a common classification system?
Necessity for an extensive study about the type of lymphadenectomy in esophageal cancer
The heterogeneity in the available evidence makes an evidence based choice for surgical strategy impossible. For squamous cell cancer, predominant in Asia, it seems that a standard policy concerning lymphadenectomy is clearly outlined whereas for the increased adenocarcinomas of the distal and junctional esophageal cancer the mediastinal lymphadenectomy policy remains very diverse. An international, observational study will identify lymph node stations that should be resected in relation to tumor characteristics and may clarify if the same surgical strategy is justified in patients with and without neoadjuvant therapy. Furthermore, the prognostic value of different lymph node stations can be analyzed.
Recently, the multinational prospective TIGER-study has been proposed to determine the distribution of lymph node metastases in all patients with resectable esophageal or gastro-esophageal junction carcinoma in whom a transthoracic esophagectomy with at least a 2-field lymphadenectomy is performed in order to develop a uniform worldwide staging system and to establish the optimal surgical strategy for esophageal cancer patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Okholm C, Svendsen LB, Achiam MP. Status and prognosis of lymph node metastasis in patients with cardia cancer - a systematic review. Surg Oncol 2014;23:140-6. [Crossref] [PubMed]
- Zhang M, Li Z, Ma Y, et al. Prognostic predictors of patients with carcinoma of the gastric cardia. Hepatogastroenterology 2012;59:930-3. [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, et al. Three-Field Lymph Node Dissection for Squamous Cell and Adenocarcinoma of the Esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Koenig AM, Prenzel KL, Bogoevski D, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol 2009;16:454-62. [Crossref] [PubMed]
- Lerut T, Coosemans W, De Leyn P, et al. Reflections on three field lymphadenectomy in carcinoma of the esophagus and gastroesophageal junction. Hepatogastroenterology 1999;46:717-25. [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Sharma S, Fujita H, Yamana H, et al. Patterns of lymph node metastasis in 3-field dissection for carcinoma in the thoracic esophagus. Surg Today 1994;24:410-4. [Crossref] [PubMed]
- Chen J, Liu S, Pan J, et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2009;36:480-6. [Crossref] [PubMed]
- Kosugi S, Kawaguchi Y, Kanda T, et al. Cervical Lymph Node Dissection for Clinically Submucosal Carcinoma of the Thoracic Esophagus. Ann Surg Oncol 2013;20:4016-21. [Crossref] [PubMed]
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- van de Ven C, De Leyn P, Coosemans W, et al. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg 1999;15:769-73. [Crossref] [PubMed]
- Allum WH, Blazeby JM, Griffin SM, et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60:1449-72. [Crossref] [PubMed]
- Kutup A, Nentwich MF, Bollschweiler E, et al. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg 2014;260:1016-22. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Dutch Institute for Clinical Auditing. DICA jaarrapportage 2016. Available online: https://www.dica.nl/jaarrapportage-2016/duca
- Cunningham D, Allum W, Stenning S, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesphageal cancer. New Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Darling G. The role of lymphadenectomy in esophageal cancer. J Surg Oncol 2009;99:189-93. [Crossref] [PubMed]
- Markar SR, Noordman BJ, Mackenzie H, et al. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol 2017;28:519-27. [PubMed]
- Talsma AK, Ong CA, Liu X, et al. Location of lymph node involvement in patients with esophageal adenocarcinoma predicts survival. World J Surg 2014;38:106-13. [Crossref] [PubMed]
- Ji X, Cai J, Chen Y, et al. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg 2016;8:90-4. [Crossref] [PubMed]
- Cense HA, van Eijck CH, Tilanus HW. New insights in the lymphatic spread of oesophageal cancer and its implications for the extent of surgical resection. Best Pract Res Clin Gastroenterol 2006;20:893-906. [Crossref] [PubMed]
- Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970-5. [Crossref] [PubMed]
- Cheng J, Kong L, Huang W, et al. Explore the radiotherapeutic clinical target volume delineation for thoracic esophageal squamous cell carcinoma from the pattern of lymphatic metastases. J Thorac Oncol 2013;8:359-65. [Crossref] [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [PubMed]
- Wu J, Chen QX, Zhou XM, et al. Prognostic significance of solitary lymph node metastasis in patients with squamous cell carcinoma of middle thoracic esophagus. World J Surg Oncol 2012;10:210. [Crossref] [PubMed]
- Japanese Society for Esophageal Diseases: Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus. Tokyo: Kanehara & Co, Ltd 9th edition. 2001.
- Castoro C, Scarpa M, Cagol M, et al. Nodal Metastasis From Locally Advanced Esophageal Cancer: How Neoadjuvant Therapy Modifies Their Frequency and Distribution. Ann Surg Oncol 2011;18:3743-54. [Crossref] [PubMed]
- Leers JM, DeMeester SR, Chan N, et al. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinomas of the GE Junction and the distal esophagus. J Thorac Cardiov Surg 2009;138:594-602. [Crossref] [PubMed]
- Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000;232:353-61. [Crossref] [PubMed]
- Parry K, Haverkamp L, Bruijnen RC, et al. Surgical treatment of Adenocarcinomas of the Gastro-esophageal Junction. Ann Surg Oncol 2015;22:597-603. [Crossref] [PubMed]
- Prenzel KL, Bollschweiler E, Schroeder W, et al. Prognostic relevance of skip metastasis in esophageal cancer. Ann Thorac Surg 2010;90:1662-7. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th Ed. New York: Springer; 2017.
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [Crossref] [PubMed]
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer 2012;31:281-6. [Crossref] [PubMed]
- Lerut T, Coosemans W, De Leyn P, et al. Is there a role for radical esophagectomy. Eur J Cardiothorac Surg 1999;16 Suppl 1:S44-7. [Crossref] [PubMed]
- Bumm R, Wang J. More or less surgery for esophageal cancer: extent of lymphadenectomy for squamous cell carcinoma: How much is necessary? Dis Esophagus 1995;8:78.
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Boone J, Hobbelink GG, Schipper EI, et al. Sentinel node biopsy during thoracolaparoscopic esophagectomy for advanced esophageal cancer. World J Surg Oncol 2016;14:117-24. [Crossref] [PubMed]
- Kitagawa Y, Fujii H, Mukai M, et al. The role of the sentinel lymph node in gastro- intestinal cancer. Surg Clin North Am 2000;80:1799-809. [Crossref] [PubMed]
- Takeuchi H, Kawakubo H, Takeda F, et al. Sentinel Node Navigation Surgery in Early Stage Esophageal Cancer. Ann Thorac Cardiovasc Surg 2012;18:306-13. [Crossref] [PubMed]
- Dabbagh Kakhki VR, Bagheri R, Tehranian S, et al. Accuracy of sentinel node biopsy in esophageal carcinoma: a systematic review and meta-analysis of the pertinent literature. Surg Today 2014;44:607-19. [Crossref] [PubMed]
- Ell C, May A, Pech O, Gossner L, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3-10. [Crossref] [PubMed]
- Takubo K, Aida J, Sawabe M, et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology 2007;51:733-42. [Crossref] [PubMed]
- Anderegg MC, Lagarde SM, Jagadesham VP, et al. Prognostic significance of the location of lymph node metastases in patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Ann Surg 2016;264:847-53. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Zhu Z, Chen H, Yu W, et al. Number of Negative Lymph Nodes is Associated with Survival in Thoracic Esophageal Squamous Cell Carcinoma Patients Undergoing Three-Field Lymphadenectomy. Ann Surg Oncol. 2014;21:2857-63. [Crossref] [PubMed]
- Greenstein AJ, Litle VR, Swanson SJ, et al. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer 2008;112:1239-46. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total Number of Resected Lymph Nodes Predicts Survival in Esophageal Cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Koen Talsma A, Shapiro J, Looman CW, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy. Prognostic and therapeutic impact on survival. Ann Surg 2014;260:786-92; discussion 792-3. [Crossref]
- Lagergren J, Mattsson F, Zylstra J, et al. Extent of lymphadenectomy and prognosis after esophageal cancer surgery. JAMA Surg 2016;151:32-9. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]


