Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy
Introduction
Antibodies against the cellular surface marker programmed cell death-1 (PD-1) and its associated ligand (PD-L1) allow the endogenous immune response to treat cancer by restoring an effective anti-tumor T-cell response (1). This approach is predicated upon enhancement of the native antitumor response that results from inhibition of the PD-1 signaling pathway. These monoclonal antibodies (mAbs) are available for various tumor types including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, head and neck squamous cell carcinoma, bladder cancer, and Hodgkin lymphoma. As of this writing, the United States Food and Drug Administration (FDA) approved agents for NSCLC include nivolumab, pembrolizumab, and atezolizumab. These agents are recommended by various guidelines including that of the National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) (2,3). Use of these agents, in combination with traditional chemotherapy agents and for various stages of NSCLC, is currently under investigation. Several other antibodies are also currently being evaluated in clinical trials.
Pseudoprogression
Pseudoprogression involves a transient enlargement of the tumor or metastatic sites before regressing in size (4-6). Pseudoprogression occurs in approximately 2 to 6 percent of patients treated with immunotherapy, depending on the criteria used to define the condition. It can occur with any of the immunotherapy agents. Data on the incidence rate in NSCLC for each agent are unavailable. The mean times from baseline to progression and to response are 74 and 169 days, respectively, based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria (6). This pattern of “progression” has been attributed to a temporary inflammatory reaction and may resemble a tumor flare. Because of this, new radiographic assessment protocols have been developed that emphasize specific time intervals before reevaluation. Modified RECIST (RECIST version 1.1) criteria classify tumor responses as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (7). In patients on immunotherapy, because of the possibility of pseudoprogression, follow-up imaging is required after at least 8 weeks on treatment. Hence, the latest iteration of this classification system, termed iRECIST, further stratifies subjects as either unconfirmed or confirmed progressive disease (iUPD vs. iCPD) (7). This differs dramatically from classic anti-tumor response assessment when using traditional chemotherapeutics where radiographic tumor enlargement at any time point is deemed to be a treatment failure. Studies show that in patients with pseudoprogression, immunohistochemical findings on biopsies reveal baseline tumor cells, which may be increased in number, along with an inflammatory response comprised of activated cytotoxic lymphocytes (CD8+ T-cells), TIA-1 (an apoptosis promoting protein), and granzyme B (protein necessary for induction of apoptosis by cytotoxic T-cells) (4,8).
In light of these findings, repeat biopsy may be considered in the setting of enlarging primary tumor or metastatic sites to distinguish pseudoprogression from true progression.
Hyperprogressive disease (HPD)
Some patients on immunotherapy may experience a rapid paradoxical progression of tumor with worsening clinical status, which appears to negatively impact survival (5,9,10). This phenomenon has been termed HPD. Until recently, only anecdotal reports existed describing clinical and radiographic deterioration after initiation of immunotherapy, but the prevalence, natural history, and predictive factors were unknown (11,12). Accelerated disease progression is not specific to anti-PD-1/PD-L1 antibodies and has also been reported with targeted therapy, such as sorafenib and crizotinib, which are both tyrosine kinase inhibitors (13,14). A study of 131 patients with various tumor types from five French hospitals identified 9 percent of patients as having HPD after treatment with anti-PD-1/PD-L1 mAbs (9). Melanoma and NSCLC constituted the two most common histologies. HPD was defined as a ≥2-fold increase in tumor growth rate (TGR) in patients with disease progression between baseline and first assessment by RECIST criteria at 8 weeks. In this study, HPD was not associated with the degree of tumor burden, histologic tumor type, number of metastatic sites, prognostic score, number of previous lines of chemotherapy, or type of prior treatment, whether it was conventional chemotherapy, targeted therapy, or radiotherapy. It was, however, associated with older age. It may also be associated with worsened overall survival (OS). Almost 20 percent of patients older than 65 years of age developed HPD compared to only 5 percent of younger patients (P=0.018). The median OS for patients with HPD was only 4.6 months compared to 7.6 months for non-HPD patients, although the difference was not statistically significant (P=0.19). There was also no difference between the group receiving anti-PD-1 therapy (i.e., nivolumab & pembrolizumab) and the group receiving anti-PD-L1 therapy (i.e., atezolizumab & durvalumab, which is currently undergoing priority review by the FDA). The incidence rate was the same with both PD-1 and PD-L1 antibodies. Interestingly, patients with HPD actually exhibited a lower rate of new lesions than patients without HPD but with progression. As specified in the RECIST 1.1 criteria, TGR was computed solely based on the target lesion; new lesions that developed were not measured.
Emerging data show that in NSCLC the rate of HPD may be even higher. In a multicenter study involving 242 patients, 16 percent were identified as having HPD (5). These patients had significantly lower median progression free survival (1.4 vs. 4.9 months) when compared with patients without HPD. There was no significant difference in terms of tumor burden at baseline, clinical, molecular, or pathological characteristics, PD-L1 expression status, or response rate to treatment before the introduction of immunotherapy. Despite the improved recognition of this clinical entity, the etiology of HPD remains unclear. Potential explanations include oncogenic signaling activation, upregulation of alternative immune checkpoints, or modulation of other protumor immune subsets (15,16). Unfortunately, no biomarkers have been identified that predict an individual’s response to PD-1 inhibitory immunotherapy. Re-biopsy of tumor progression may thus be warranted to confirm that indeed the diagnosis is HPD, as this will result in a change of therapy. In addition, tissue acquisition from these patients may lead to improved understanding of the molecular mechanisms of this phenomenon.
Discussion
While immunotherapy can produce a significant and durable response in patients with NSCLC, physicians caring for patients on these agents must be cognizant of the potential for an aggressive pattern of accelerated progression in a subset of patients. This pattern of rapid progression may lead to diminished progression free and OS (5,9). HPD is an increasingly recognized phenomenon for which positive predictive variables, other than possibly advanced age, have not yet been identified. By the time patients experience progressive symptomatology, airway pathology and pleural space complications may be advanced, as illustrated in Figures 1,2. Interventional pulmonologists or thoracic surgeons should be involved early in the care of patients treated with immunotherapy. Earlier involvement by physicians trained to handle airway and pleural space pathology, may permit earlier detection and hence intervention, in patients with evidence of rapid tumor surge.
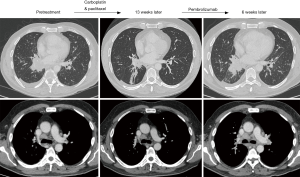
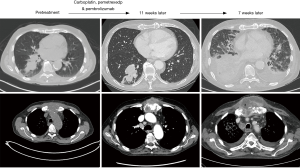
Longitudinal imaging surveillance and repeat biopsy are necessary to differentiate HPD from pseudoprogression. Biopsy in cases of pseudoprogression reveals abundant inflammatory cells, whereas primarily tumor cells are present in HPD (4,8). Further research is required to identify other predictive variables. The time frame of eight weeks has been identified as the threshold for differentiating pseudoprogression from HPD, but in older patients, perhaps imaging should be performed in the interim period in an attempt to identify potential HPD cases at an earlier stage, where a change in therapy or earlier palliative intervention may still be feasible. Future studies should also provide longer term imaging follow-up to assure that HPD and pseudoprogression are indeed separate entities. Tumor response (partial or complete) is expected during long-term follow-up (>10 weeks) when pseudoprogression is encountered, while HDP will demonstrate continual progression of the primary and/or metastatic sites. Routine pre- and post-treatment biopsies would also allow for data collection in an effort to identify molecular or immune markers associated with HPD and pseudoprogression. If these were available, then candidates for immunotherapy could be screened prior to initiation of therapy. Closer follow-up may also be necessary, especially in the older age group, to identify airway and pleural space pathology as early as possible, since these patients may require palliative interventions during these phases of disease.
Conclusions
HPD and pseudoprogression are potentially two distinct response patterns in patients receiving immunotherapy for NSCLC. HPD seems to be associated with worse quality of life and survival. Repeat biopsy is warranted to distinguish these two phenomena. Inability to accurately predict who will develop HPD creates the potential for harm that can only be mitigated by close surveillance and active evaluation by a multidisciplinary team involving medical oncologists, thoracic radiologists, surgeons and interventional pulmonologists, as additional tissue acquisition will change management and eventually elucidate the mechanisms of this clinical entity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Berger KN, Pu JJ. PD-1 pathway and its clinical application: A 20year journey after discovery of the complete human PD-1 gene. Gene 2018;638:20-5. [Crossref] [PubMed]
- Ettinger DS. NCCN Non-Small Cell Lung Cancer Clinical Practice Guidelines, 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541-3. [Crossref] [PubMed]
- Ferrara R, Caramella C, Texier M, et al. Hyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients treated with anti PD1/PD-L1 monoclonal antibodies (IO). Ann Oncol 2017;28:v460-96. [Crossref]
- Kurra V, Sullivan RJ, Gainor JF, et al. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J Clin Oncol 2016;34:abstr 6580.
- Seymour L, Bogaerts J, Perrone A, et al. IRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-ctla-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297-306. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-pd-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Ledford H. Promising cancer drugs may speed tumours in some patients. Nature 2017;544:13-4. [Crossref] [PubMed]
- Lahmar J, Facchinetti F, Koscielny S, et al. Effect of tumor growth rate (TGR) on response patterns of checkpoint inhibitors in non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:abstr 9034.
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-pd-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Kuriyama Y, Kim YH, Nagai H, et al. Disease flare after discontinuation of crizotinib in anaplastic lymphoma kinase-positive lung cancer. Case Rep Oncol 2013;6:430-3. [Crossref] [PubMed]
- Mellema WW, Burgers SA, Smit EF. Tumor flare after start of RAF inhibition in KRAS mutated NSCLC: A case report. Lung Cancer 2015;87:201-3. [Crossref] [PubMed]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]


