Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and meta-analysis
Introduction
Fluorescent imaging using indocyanine green (ICG) is an emerging method during cancer surgery that aids the surgeon with intraoperative decision making. The technology is gaining clinical acceptance in many surgical fields (1-4), including upper gastrointestinal cancer surgery. One of the most common types of cancer in the upper gastrointestinal tract is esophageal cancer, with the incidence still increasing (5,6). Treatment of esophageal cancer is a multidisciplinary effort with (neo-)adjuvant chemotherapy or chemoradiotherapy and surgery, of which surgical resection with lymphadenectomy remains the cornerstone of treatment for curative intent. Although esophagectomy improves overall survival, it comes at the cost of surgical morbidity and mortality.
Anastomotic leakage and graft necrosis are feared complications occurring in 5–20% after esophagectomy with continuity restoration and are associated with a high mortality (7). Among the risk factors that influence anastomotic integrity, poor perfusion is a surgically modifiable factor. Intraoperative real-time fluorescence angiography using ICG can assess perfusion, thereby enabling precise delineation of the ideal site for anastomosis and assessment of final anastomotic vitality. However, no quantitative threshold of the fluorescence signal is known for adequate perfusion.
Another complication after esophagectomy is chylothorax, and occurs in up to 4% of the patients (8,9). If the chyle leak cannot be managed conservatively, surgical exploration with ligation of the thoracic duct is necessary in rare cases. However, despite preoperative imaging, identifying the thoracic duct and chyle fistula during reoperation is often difficult due to post-operative changes.
In this systematic review we aim to provide an overview of the use of fluorescence imaging with ICG during esophagectomy and to investigate how this technology could prevent surgical morbidity. Our main interest is in the following surgical applications: anastomotic perfusion assessment and detection of chyle fistula. Furthermore, we aim to report what studies have been performed on the evaluation of anastomotic fluorescence angiography in quantitative values.
Methods
Article inclusion
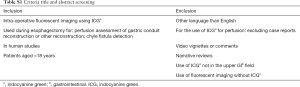
The PRISMA standard for systematic reviews was used. A PubMed and Embase database search was conducted in November 2018 to identify all relevant articles on ICG fluorescent imaging during upper gastrointestinal cancer surgery (Table 1). Title and abstract were screened by two authors (MD Slooter and WJ Eshuis) to meet predetermined criteria (Table S1). Conflicts were discussed in order to reach consensus. The reference lists of included articles were scanned to potentially obtain additional articles.

Full table
Full table
Subsequently two authors (MD Slooter and WJ Eshuis) performed full-text screening for eligibility. To meet the eligibility criteria, manuscripts had to be full-text, presenting original data on at least one of the surgical applications of interest: anastomotic perfusion assessment or detection of chyle fistula. For articles concerning perfusion assessment, case-reports, and safety and/or feasibility studies were excluded. Conflicts on the eventual in- or exclusion of an article were discussed in order to reach consensus.
Outcomes
Studies on perfusion assessment had to record the correlation of fluorescence angiography to clinical outcome (particularly anastomotic leakage and/or graft necrosis), intraoperative change in management due to fluorescence angiography results and/or evaluation of fluorescence in quantitative values. Outcomes of interest for chyle fistula detection were: visualisation of the thoracic duct or chyle fistula.
Quality assessment
Quality assessment was performed for all included articles to detect potential bias using the Newcastle-Ottawa scale for cohort studies. Quality assessment was executed by two authors (MD Slooter and WJ Eshuis), and conflicts were discussed in order to reach consensus.
Statistics
Incidence rates were pooled when more than three studies (with a total N>10 patients) recorded similar outcomes. Pooled incidence rates were calculated using an inverse variance method in RStudio version 1.1.453 (R Foundation for Statistical Computing, Vienna, Austria) with corresponding 95% confidence interval (CI). A meta-analysis using the Mantel-Haenszel method, to compare ICG to non-ICG, was conducted in Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK). The outcome was displayed as an odds ratio (OR) with corresponding 95% CI. Heterogeneity was assessed with the Cochran’s Q test (significance level at P<0.05) and quantification of heterogeneity was achieved by the I2 test (low: 0–25%, moderate: 25–50%, large: 50–75%, and very large: >75%). A random effects model was used when Cochran’s Q test was not significant or I2 test was moderate to very large.
Results
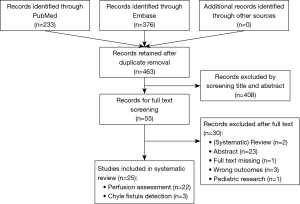
Some 463 articles were screened of which 408 were excluded after title and abstract screening and another 30 articles after subsequent eligibility screening. A total of 25 articles were included in this review: 22 on perfusion assessment, and three on the detection of chyle fistula. A PRISMA flow diagram is shown in Figure 1.
Perfusion assessment
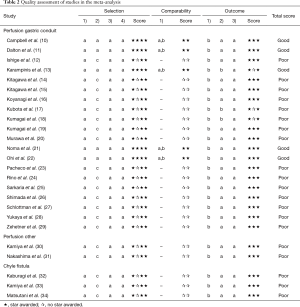
Twenty-two observational studies were identified, including 11 prospective, nine retrospective studies and five studies that included a non-exposed cohort. The articles were of poor to good quality (Table 2).
Full table
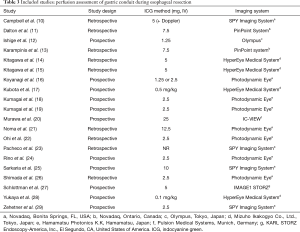
Twenty studies assessed perfusion of the gastric conduit before anastomotic reconstruction (10-29) (Table 3). To assess perfusion, ICG (1.25–25 mg) was injected intravenously during the surgical procedure and the fluorescence signal was detected within a minute after injection. Seven different near infrared camera systems were used for fluorescent detection. No adverse events due to ICG were reported in any of the studies.
Full table
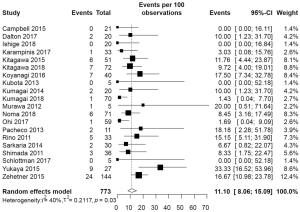
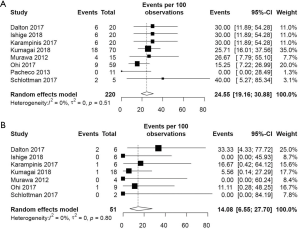
The pooled incidence of anastomotic leakage and graft necrosis in the ICG group was 11.10% (95% CI: 8.06–15.09%) (Figure 2). Eight studies reported change in management due to fluorescence angiography using ICG and the pooled change in management rate was 24.55% (95% CI: 19.16–30.88%) (Figure 3A). Change in management included resection of the poorly perfused part of the gastric conduit and change in anastomotic site. After change in management, the pooled incidence of anastomotic leakage and graft necrosis was 14.08% (95% CI: 6.55–27.70%) (Figure 3B).
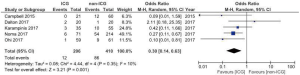
We included five studies with a non-exposed cohort in a meta-analysis to compare ICG to non-ICG on anastomotic leakage and graft necrosis (Figure 4). Campbell et al. studied the effect of the combination of ICG fluorescence angiography and Doppler examination in 30 patients (10), of which nine with Doppler only. In the meta-analysis, the patients assessed by Doppler only were excluded. The meta-analysis analysis showed that less anastomotic leakages and graft necrosis occur in the ICG group (OR 0.30, 95% CI: 0.14–0.63).
Kitagawa et al. (2015 and 2018) used fluorescence angiography to determine the shape of the gastric conduit (14,15). They correlated outcomes of fluorescence angiography to anastomotic leakage and compared the results in two groups: ICG before (N=46) and after (N=26) gastric conduit reconstruction (15). The results showed a tendency towards lower leak rates, although it did not reach statistical significance (6.5% vs. 15.4%; P=0.244).
Furthermore, we identified two articles that used fluorescence angiography for different purposes than the assessment of gastric conduit perfusion: one article studied the perfusion of a free jejunal graft (30) and one the perfusion of a pedicled omental flap (31). The first study by Kamiya et al. will be discussed below (see ‘Quantification’) (30). Nakashima et al. reported fluorescence angiography of the pedicled omental flap on cervical anastomosis after esophagectomy, and poorly perfused omental tissue was excised on the demarcation line (31). Anastomotic leakage occurred in one out of 38 patients (2.6%) in that series.
Quantification
We identified five out of 22 articles that evaluated fluorescence angiography in quantitative values (12,16,19,28,30).
Kumagai et al. proposed a 90-second rule: all anastomoses were reconstructed in the area that was enhanced within 90 seconds after initial enhancement at the distal end of the gastric conduit (19). The tip was excised in 50% (35/70), and in 18 of those 35 cases there was change in anastomotic site (initial enhancement after median 95.5 seconds to 41.0 seconds after excision). In none of the patients the anastomosis was performed at a site with enhancement after more than 90 seconds. Anastomotic leakage occurred in one out of 70 cases (1.4%) at an anastomotic site that was enhanced after 77 seconds.
Three articles evaluated the fluorescence signal using time-fluorescence intensity curves (12,28,30). Ishige et al. showed ‘normal’ curves (characterized by a sharp high peak followed by rapid decline to plateau level) for gastric tissue before disconnection of perigastric vessels (12). After gastric conduit reconstruction, the curves at the anastomotic site showed a ‘normal’ pattern in 30% (6/20) and a ‘gradual’ pattern in the other cases. However, no leakages occurred. Yukaya et al. identified three types of curves: a normal, inflow delayed and outflow delayed type (28). Anastomotic leakage occurred in 23.1% (3/13) in the normal type, 44.4% (4/9) in the inflow delayed type and 40% (2/5) in the outflow delayed type, with no significant difference among the types. Kamiya et al. assessed ICG flow of free jejunal grafts and identified delayed increase of ICG fluorescence in five out of 26 patients (30). In those patients the anastomosis was revised and no leakage occurred. In two out of 21 cases with ‘good’ perfusion, graft necrosis developed.
Koyanagi et al. classified two groups according to ICG flow speed (cm/s): a simultaneous group with similar speed in gastric conduit wall and that of the greater curvature vessels, and a delayed group where the ICG fluorescence was obviously slower in the gastric conduit wall compared to the greater curvature vessels (P<0.001) (16). Anastomotic leakage developed in 0% (0/25) in the simultaneous group and in 46.7% (7/15) in the delayed group (P<0.001).
Chyle fistula detection
Three articles were identified regarding ICG lymphography to detect the thoracic duct and chyle fistula. All articles were case-reports and ICG lymphography was applied in the setting of a reoperation in all reported cases (32-34). ICG lymphography was reported in a total of three patients (aged 62–70 years) who developed a chylothorax after esophagectomy for cancer. Patients underwent reoperation at day 10 (N=1) or day 28 (N=2) after the initial procedure. During all procedures the chyle fistula could not be identified by conventional assessment. To perform fluorescence lymphography, ICG was either injected subcutaneously at the bilateral inguinal region (1.5 mL, 0.5%) (33,34) or into the mesentery of the small bowel (2 mL, 0.5%) (32). The thoracic duct was detected 10–14 minutes after subcutaneous injection at bilateral inguinal region (33,34). The thoracic duct was detected in all cases and the chyle fistula in one case.
Discussion
In this systematic review we identified literature on fluorescence imaging using ICG during esophagectomy to prevent surgical morbidity. The use of ICG for perfusion assessment of the gastric conduit before anastomosis is safe and leads to a reduction in anastomotic leakage and graft necrosis (OR 0.30, 95% CI: 0.14–0.63). The three case-reports we identified regarding chyle fistula detection during reoperation for chylothorax, reported a successful detection of the thoracic duct in all cases and of the chyle fistula in one case.
The pooled results show that fluorescence angiography has an added value, as change in management occurs in 25%, and less anastomotic leakages are found when fluorescence angiography is used as compared to when it is not used. However, anastomotic leakages still occur in the group of patients who underwent a change in management due to fluorescence angiography (14%). This relatively high percentage of leakage can be explained by anastomotic tension and selection bias in a patient group with relatively worse vascularization to begin with. Achieving an anastomosis in the perfused area might come at the cost of an anastomosis under tension. In Figure 5A, a case of change in management due to fluorescence angiography in our center is shown. Additional resection of the poorly perfused gastric conduit (between red and green line) may cause more tension on the anastomosis, although perfusion is better at the chosen site. The gastric conduits in the ‘change in management’ group are more at risk. In this group, perfusion of the gastric conduit is deemed insufficient in such an extent that the surgeon considered change in management. Even if fluorescence angiography shows delineation of good versus poor perfusion, continuity restoration is one of the main goals during esophagectomy that should be pursued whenever possible.
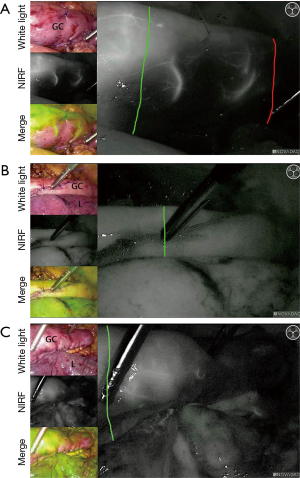
There is no consensus on the dose of ICG. The dose varied from 1.25 to 25 mg per bolus. The lowest dose already allows a clear and reliable measurement, but is too low in some cases (experience in our center). A high dose might interfere with a second measurement, as the background signal remains high. Our recommendation would be a dose of 0.05 mg/kg/bolus in case of gastric conduit perfusion evaluation. If the signal is not clear, an extra bolus of 2.5 mg of ICG can be given. After 15 minutes a new measurement is possible. In this way anastomotic perfusion can be assessed before and after anastomotic construction. Figure 5 shows examples of gastric conduit perfusion assessment with ICG in our center: Figure 5B,C demonstrate well-perfused planned anastomotic sites, and 5C shows a case with change in management.
Although fluorescence angiography using ICG allows a feasible real-time assessment of perfusion, the lack of objectivity and a threshold for adequate perfusion is a major limitation. Quantification of the fluorescent signal may overcome this limitation and is important to strengthen the added value of the technology in the future. Time to fluorescent enhancement is an easy quantitative parameter (19), and can be already applied without additional software. Time-fluorescence intensity curves are promising quantitative values, and might be useful in the future when correlated to anastomotic leakage and graft necrosis.
The use of a pedicled omental flap for wrapping the anastomosis is well-established, as a meta-analysis showed significant reduction in the anastomotic leakage rate (3% vs. 12%; P<0.01) (35). In these procedures, fluorescence angiography might further improve patient outcomes in the future by reducing the risk of necrotic omental tissue.
Evidence on thoracic duct and chyle fistula detection is limited. However, the thoracic duct was detected after ICG injection in all described cases that were reviewed. When a chyle leak is suspected during the initial procedure or when it is difficult to locate the leak during reoperation, surgeons could consider this method.
Limitations of this review are the limited number and the quality of articles. Poor quality was mainly due to absence of a non-exposed cohort. Furthermore, a meta-analysis by Ladak et al. was recently published on fluorescence angiography to assess the perfusion of the gastric conduit (36). In this review we identified two extra articles, not including a non-exposed cohort (12,19). Ladak et al. address anastomotic leakage only, while we included graft necrosis in the outcomes, as ICG fluorescence angiography should prevent this complication from occurring. In the present review we excluded Kitagawa et al. from the meta-analysis. Kitagawa et al. (2015 and 2018) performed fluorescence angiography to guide gastric conduit reconstruction and compared two groups before and after gastric conduit reconstruction (14,15). We interpreted that both groups received perfusion assessment before anastomotic reconstruction and therefore included all patients in the ICG group. We also excluded patients that received perfusion assessment with Doppler only from the analysis (10). However, we found similar outcomes: Ladak et al. found a pooled incidence of 10% and an OR of 0.31 (95% CI: 0.15–0.63) compared to 11% and an OR of 0.30 (95% CI: 0.14–0.63) found in our review.
Conclusions
Fluorescence imaging using ICG is a promising and safe technology to reduce surgical morbidity after esophagectomy with continuity restoration. Future studies are needed to confirm risk reduction in anastomotic leakage and graft necrosis by ICG fluorescence angiography and to demonstrate the feasibility of ICG lymphography for thoracic duct and chyle fistula detection.
Acknowledgements
The authors thank FS van Etten-Jamaludin, clinical librarian, for assistance with the literature search.
Footnote
Conflicts of Interest: MI van Berge Henegouwen—Educational grant Stryker, Research grant Olympus, Consultant for Medtronic; SS Gisbertz—Research grant Olympus, Consultant for Medtronicb. The other authors have no conflicts of interest to declare.
References
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [PubMed]
- van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013;119:3411-8. [Crossref] [PubMed]
- Togami S, Kawamura T, Fukuda M, et al. Prospective study of sentinel lymph node mapping for endometrial cancer. Int J Gynaecol Obstet 2018;143:313-8. [Crossref] [PubMed]
- Degett TH, Andersen HS, Gogenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 2016;401:767-75. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Rao DV, Chava SP, Sahni P, et al. Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus 2004;17:141-5. [Crossref] [PubMed]
- Crucitti P, Mangiameli G, Petitti T, et al. Does prophylactic ligation of the thoracic duct reduce chylothorax rates in patients undergoing oesophagectomy? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:1019-24. [Crossref] [PubMed]
- Campbell C, Reames MK, Robinson M, et al. Conduit Vascular Evaluation is Associated with Reduction in Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:806-12. [Crossref] [PubMed]
- Dalton BGA, Ali AA, Crandall M, et al. Near infrared perfusion assessment of gastric conduit during minimally invasive Ivor Lewis esophagectomy. Am J Surg 2018;216:524-7. [Crossref] [PubMed]
- Ishige F, Nabeya Y, Hoshino I, et al. Quantitative Assessment of the Blood Perfusion of the Gastric Conduit by Indocyanine Green Imaging. J Surg Res 2019;234:303-10. [Crossref] [PubMed]
- Karampinis I, Ronellenfitsch U, Mertens C, et al. Indocyanine green tissue angiography affects anastomotic leakage after esophagectomy. A retrospective, case-control study. Int J Surg 2017;48:210-4. [Crossref] [PubMed]
- Kitagawa H, Namikawa T, Munekage M, et al. Visualization of the Stomach's Arterial Networks During Esophageal Surgery Using the HyperEye Medical System. Anticancer Res 2015;35:6201-5. [PubMed]
- Kitagawa H, Namikawa T, Iwabu J, et al. Assessment of the blood supply using the indocyanine green fluorescence method and postoperative endoscopic evaluation of anastomosis of the gastric tube during esophagectomy. Surg Endosc 2018;32:1749-54. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Oguma J, et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine 2016;95:e4386. [Crossref] [PubMed]
- Kubota K, Yoshida M, Kuroda J, et al. Application of the HyperEye Medical System for esophageal cancer surgery: a preliminary report. Surg Today 2013;43:215-20. [Crossref] [PubMed]
- Kumagai Y, Ishiguro T, Haga N, et al. Hemodynamics of the reconstructed gastric tube during esophagectomy: assessment of outcomes with indocyanine green fluorescence. World J Surg 2014;38:138-43. [Crossref] [PubMed]
- Kumagai Y, Hatano S, Sobajima J, et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus 2018.31. [PubMed]
- Murawa D, Hunerbein M, Spychala A, et al. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chirurgica Belgica 2012;112:275-80. [Crossref] [PubMed]
- Noma K, Shirakawa Y, Kanaya N, et al. Visualized Evaluation of Blood Flow to the Gastric Conduit and Complications in Esophageal Reconstruction. J Am Coll Surg 2018;226:241-51. [Crossref] [PubMed]
- Ohi M, Toiyama Y, Mohri Y, et al. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus 2017;14:351-9. [Crossref] [PubMed]
- Pacheco PE, Hill SM, Henriques SM, et al. The novel use of intraoperative laser-induced fluorescence of indocyanine green tissue angiography for evaluation of the gastric conduit in esophageal reconstructive surgery. Am J Surg 2013;205:349-52; discussion 352-3. [Crossref] [PubMed]
- Rino Y, Yukawa N, Sato T, et al. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med Imaging 2014;14:18. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Philadelphia, Pa) 2014;9:391-3. [PubMed]
- Shimada Y, Okumura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 2011;8:259-66. [Crossref] [PubMed]
- Schlottmann F, Patti MG. Evaluation of Gastric Conduit Perfusion During Esophagectomy with Indocyanine Green Fluorescence Imaging. J Laparoendosc Adv Surg Tech A 2017;27:1305-8. [Crossref] [PubMed]
- Yukaya T, Saeki H, Kasagi Y, et al. Indocyanine Green Fluorescence Angiography for Quantitative Evaluation of Gastric Tube Perfusion in Patients Undergoing Esophagectomy. J Am Coll Surg 2015;221:e37-42. [Crossref] [PubMed]
- Zehetner J, DeMeester SR, Alicuben ET, et al. Intraoperative Assessment of Perfusion of the Gastric Graft and Correlation With Anastomotic Leaks After Esophagectomy. Ann Surg 2015;262:74-8. [Crossref] [PubMed]
- Kamiya K, Unno N, Miyazaki S, et al. Quantitative assessment of the free jejunal graft perfusion. J Surg Res 2015;194:394-9. [Crossref] [PubMed]
- Nakashima Y, Saeki H, Yukaya T, et al. Blood Flow Assessment with Indocyanine Green Fluorescence Angiography for Pedicled Omental Flap on Cervical Esophagogastric Anastomosis after Esophagectomy. J Am Coll Surg 2016;222:e67-9. [Crossref] [PubMed]
- Kaburagi T, Takeuchi H, Oyama T, et al. Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: report of a case. Surg Today 2013;43:206-10. [Crossref] [PubMed]
- Kamiya K, Unno N, Konno H. Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: report of a case. Surg Today 2009;39:421-4. [Crossref] [PubMed]
- Matsutani T, Hirakata A, Nomura T, et al. Transabdominal approach for chylorrhea after esophagectomy by using fluorescence navigation with indocyanine green. Case Rep Surg 2014;2014:464017. [Crossref] [PubMed]
- Wiggins T, Markar SR, Arya S, et al. Anastomotic reinforcement with omentoplasty following gastrointestinal anastomosis: A systematic review and meta-analysis. Surg Oncol 2015;24:181-6. [Crossref] [PubMed]
- Ladak F, Dang JT, Switzer N, et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019;33:384-94. [Crossref] [PubMed]