Three-dimensional speckle-tracking echocardiography detects different patterns of right atrial dysfunction in selected disorders: a short summary from the MAGYAR-Path Study
The right atrium (RA) is the most under-evaluated heart chamber in the clinical practice (1). Its role is to transit deoxygenated blood from the caval veins and coronary sinus to the right ventricle (RV) via the tricuspid valve, but it has effects on heart rhythm development and conductance, and endocrine and baroreceptor regulations as well (2). Similarly, to the left atrium (LA), the RA has a triple function during the cardiac cycle (2,3): (I) it acts as a “reservoir” storing blood arriving from the systemic venous circulation following closure of the tricuspid valve; (II) it acts as a “conduit” with passive filling from the caval veins to the RV after opening of the tricuspid valve; (III) it acts as a “booster pump” contributing to RV filling by atrial contraction in late diastole.
Routine transthoracic echocardiographic RA assessment has a lot of limitations (3). RA dimensions could be directly assessed in apical 4-chamber view (AP4CH), with Doppler and tissue Doppler echocardiography tricuspid inflow and tricuspid annular velocities could be measured, respectively (3). With newer echocardiographic techniques, speckle-tracking echocardiography (STE) allows quantitative assessment of RA deformation, while three-dimensional (3D) echocardiography enables accurate RA volumetric measurements (3,4). 3D-STE merges the benefits of 3D echocardiography and STE: volumetric and strain assessment of heart chambers could be performed at the same time from the same 3D echocardiographic dataset. According to recent practices 3D-STE analysis includes two steps: data acquisition and offline measurement (3,4). Acquisition of 3D echocardiographic dataset is performed with a specific echocardiographic equipment using a matrix phased-array transducer with 3D capability. Using a dedicated software 3D cast of the RA could be created. Several apical and cross-sectional 2D views help this evaluation, on which endpoints of the tricuspid valve and endocardial surface of the RA superior region are set by the examiner (4-8) (Figure 1).
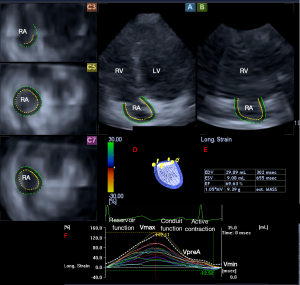
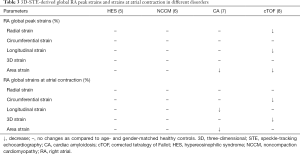
According to the literature, end-systolic largest RA volume (Vmax), early diastolic RA volume before atrial contraction (VpreA) and late-diastolic smallest RA volume (Vmin) are suggested to be measured. From these RA volumes, several volume-based functional properties respecting the cardiac cycle could be calculated which are presented in Table 1 (3). Using the same 3D virtual RA cast, several unidirectional [radial (RS), longitudinal (LS) and circumferential (CS)] and multidirectional [area (AS) and 3D (3DS)] strains could be assessed with a typical twin peaks curve (3). The first peak represents the systolic reservoir function and is called peak RA strain, while the second peak represents late diastolic booster pump phase and is called RA strain at atrial contraction. It is possible to calculate not only global and mean segmental, but segmental strains as well from which different regional strains (basal, midatrial and superior) could be measured for each patient.

Full table
Summarizing these data 3D-STE has an ability of detailed analysis of different phases of RA function as follows (5-8): (I) systolic reservoir phase with total atrial stroke volume (TASV) and emptying fraction (TAEF) together with peak RA strains; (II) early diastolic phase with passive atrial stroke volume (PASV) and emptying fraction (PAEF); (III) late diastolic phase with active atrial stroke volume (AASV) and emptying fraction (AAEF) together with RA strains at atrial contraction.
A particular diagnostic substudy is under way at the Second Department of Medicine and Cardiology Center, University of Szeged, Hungary to assess the above mentioned 3D-STE-derived RA variables in different patient populations using a Toshiba ArtidaTM echocardiography device with a PST-30SBP phased-array transducer (5-8). These studies are parts of Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases (MAGYAR-Path) Study, where “Magyar” means “Hungarian” in Hungarian language.
RA volumes, volume-based functional properties and strains were found to have specific patterns in different disorders as compared to age- and gender-matched healthy subjects as a part of the MAGYAR-Path Study (Tables 2-4) (5-8). Similar ability of 3D-STE for LA has already been confirmed (9).

Full table
Full table

Full table
In these early results increased RA volumes respecting cardiac cycles could be demonstrated in hypereosinophilic syndrome (HES) (5), noncompaction cardiomyopathy with LV involvement (NCCM) (6), cardiac amyloidosis (CA) (7) and corrected tetralogy of Fallot (cTOF) (8). Interestingly, HES and NCCM patients showed similar pattern of mild RA dysfunction affecting systolic reservoir and early diastolic conduit functions (increased TASV and PASV). No changes in RA emptying fractions or strains could be detected. These alterations were present without any clinical signs of right heart involvement in HES and NCCM suggesting subclinical changes (5,6). As expected CA and cTOF were associated with more severe alterations: reductions in RA emptying fractions (TAEF and AAEF) with preserved stroke volumes and impairment of RA strains featuring both systolic reservoir and late-diastolic booster pump functions could be detected (7,8). However, RA conduit function seemed to be intact in CA (7). cTOF showed a different pattern: all RA functions were found to be affected (reduced TAEF, PAEF and strains) (8).
These disease-related alterations could be theoretically explained by RA volume and pressure overloads, filling and emptying features, tissue wall characteristics. Similar alterations of the caval veins, RV and pulmonary artery could also explain the results, but presence and degree of tricuspid regurgitation, LA-RA interactions, conductance and congenital abnormalities, hormonal discrepancies, effects of associated cardiovascular risk factors, lung diseases, different degree left heart failure, autonomic neuropathy, etc. could also have a role. Moreover, differences could be interpreted whether a particular disease affects only left or right heart or both (1-3). Normal values of 3D-STE-derived RA volumetric and functional properties are not defined yet therefore selection of control subjects could also affect comparisons. These factors and their ratio in the development of above mentioned RA morphologic and functional changes theorized to have different effects which could highlight our attention whether pattern of these alterations is specific for certain disorders (or group of diseases), have any differential diagnostic or prognostic value or whether these are age- or gender-related. Therefore, large volume studies with significant number of patients with different disorders are warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tadic M. The right atrium, a forgotten cardiac chamber: an updated review of multimodality imaging. J Clin Ultrasound 2015;43:335-45. [Crossref] [PubMed]
- Mendes L, Cardim N. Right atrial function with speckle-tracking echocardiography: Do we really need it? Rev Port Cardiol 2017;36:901-4. [Crossref] [PubMed]
- Nemes A, Forster T. Echocardiographic evaluation of the right atrium - from M-mode to 3D speckle-tracking imaging. Orv Hetil 2016;157:1698-707. [Crossref] [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography—a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [Crossref] [PubMed]
- Nemes A, Marton I, Domsik P, Kalapos A, Pósfai É, Modok S, Kormányos Á, Ambrus N, Borbényi Z, Forster T. The right atrium in idiopathic hypereosinophilic syndrome: Insights from the 3D speckle tracking echocardiographic MAGYAR-Path Study. Herz 2017. Epub ahead of print. [Crossref] [PubMed]
- Nemes A, Domsik P, Kalapos A, Gavallér H, Oszlánczi M, Forster T. Right atrial deformation analysis in isolated left ventricular noncompaction - insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Rev Port Cardiol 2016;35:515-21. [Crossref] [PubMed]
- Nemes A, Földeák D, Domsik P, Kalapos A, Kormanyos A, Borbényi Z, Forster T. Right atrial volumetric and strain analysis in light-chain (AL) cardiac amyloidosis—a three-dimensional speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging 2017;18:iii182-219.
- Nemes A, Havasi K, Domsik P, Kalapos A, Forster T. Evaluation of right atrial dysfunction in patients with corrected tetralogy of Fallot using 3D speckle-tracking echocardiography. Insights from the CSONGRAD Registry and MAGYAR-Path Study. Herz 2015;40:980-8. [Crossref] [PubMed]
- Nemes A, Domsik P, Kalapos A, Forster T. Is three-dimensional speckle-tracking echocardiography able to identify different patterns of left atrial dysfunction in selected disorders? Short summary of the MAGYAR-Path Study. Int J Cardiol 2016;220:535-7. [Crossref] [PubMed]


