Informed communication with study subjects of radiographically detected osteoporotic vertebral deformity
We read with interests the article of Li et al. (1). This article details the plan of the Prospective Urban Rural Epidemiology (PURE) China Action on Spine and Hip status (CASH) study. It is a prospective large-scale population study with a community-based sampling and recruitment strategy. The aim of PURE CASH study is to determine the prevalence of osteoporosis and spinal fracture, and explore the potential relationship between spinal fracture and bone mineral density (BMD) with QCT measurement. Participants in the PURE CASH study are recruited from 12 centers in seven provinces in China. These data may have great significance for future policy-making and the prevention of osteoporosis and osteoporotic fractures in China (1).
On the aspect of radiological assessment of spine, in the PURE CASH study plan it is noted that ‘the lateral scout view images from the QCT are used to detect vertebral body fractures according to Genant’s semiquantitative (SQ) method. Each vertebral body is classified as normal (grade 0), mild (grade 1, approximately 20–25% depression in height and a reduction in area 10–20%), moderate (grade 2, approximately 25–40% depression in height and a reduction in area 20–40%), or severe (grade 3, more than 40% reduction in height and area) fracture… A subject is considered to have a spinal osteoporotic fracture if any one of the T4–L4 vertebral bodies had a VFA (vertebral fracture assessment) score ≥ grade 1. The vBMD (volumetric bone mineral density) results along with any abnormal CT scan findings will be provided to the participants’ (1). Hereby we like to elaborate our experience using Genant’s semiquantitative (SQ) method (2), as well as the possibility of ‘overcall’ if we describe SQ grade-1 vertebral deformity (VD) as ‘vertebral fracture’ and if investigators communicate to the study subjects as such.
The SQ criteria proposed by Genant et al. (2) are now being widely applied in research setting. Note the initial description of SQ criteria also stressed the importance of qualitative/radiological evaluation. It was noted that ‘aside from morphometric features, most vertebral fractures are readily distinguished by the presence of endplate deformities and buckling of cortices, by the lack of parallelism of end plates, and by the loss of vertical continuity of vertebral morphology… Subtle distinctions between a fractured end plate and the deformity of Schmorl's nodes or the remodeling of the vertebral bodies due to degenerative disk disease and scoliosis can frequently be made qualitatively by an experienced or trained observer’ (2). These points have been emphasized many times later. Genant and Jergas described that ‘in addition to height reductions, the reader pays careful attention to alterations in the shape and configuration of the vertebrae relative to adjacent vertebrae and expected normal appearances. These features add a strong qualitative, sometimes subjective aspect to the interpretation’ (3). Genant also communicated that ‘the first step in the process is to visually determine whether a fracture or a non-fracture deformity exists. The next steps include determining whether endplate deformities (horizontal edges) are present; lack of parallelism of endplates exists, buckling of cortices (on the vertical edges) and, finally, whether there is loss of vertical continuity with adjacent vertebrae’ (4). Appropriate use of Genant’s criteria requires knowledge of developmental deformities [e.g., Scheuermann’s disease (osteochondrosis of vertebral end plates)] and acquired deformities (e.g., osteoarthritis) that do not represent fractures and recognition of features that suggest causes of fractures other than osteoporosis. The common developmental wedge deformities of the mid-thoracic and thoracolumbar regions, the reverse wedging of lower lumbar vertebrae, and the common mild endplate bowing of the lower lumbar vertebrae should be recognized. Nonfractural changes of the vertebrae shape should be evaluated to exclude deformities including developmental short vertebral height, Cupid’s bow deformity, Scheuermann’s disease, and Schmorl’s nodes, and degenerative remodeling (5). An isolated anterior wedging of vertebral body between vertebrae of normal shape may suggest osteoporotic vertebral deformity (oVD), rather than if wedged or biconcave vertebrae are evident throughout much of the thoracic or lumbar spine (6,7). It is understood in the PURE CASH study spine radiograph will be read by experienced radiologists (1), and they will likely read SQ criteria appropriately. However it will be helpful to include Genant et al.’s description of SQ criteria’s qualitative/radiological assessment requirement in the formal study protocol. As the PURE CASH study involves 12 centers in seven provinces in China, without such formal documentation, there could be chances that miscommunication and misinterpretation may occur locally at individual research sites.
Furthermore, we felt it is very difficult to precisely estimate vertebral lateral area reduction. Increasingly, this percentage area reduction requirement has been dropped by users of SQ criteria; instead, only the percentage vertebral height reduction is estimated (4). In addition, according to Genant et al.’s description, oVDs are estimated visually (2). However, without quantitative measurement, we also found it could be difficult to accurately and consistently estimate vertebral height loss. In our experience, the main discord for inter-reader grading disagreement relates to the borderline cases, for example, a perceived reduction in vertebral height of approximately 20% can be categorized as normal by one reader and grade-1 VD by another reader. Similarly, a perceived reduction in vertebral body height of approximately 25% can be categorized as grade-1 or grade-2 VD. Therefore in our practice while we reply on qualitative/radiological approach to define an oVD, to improve consistency we use quantitative measurement for grading. The important component of assessment is that a vertebra should be carefully compared with its neighbors, for their morphology as well as height estimation. The anterior osteophyte and posterior uncinate should be excluded during the measurement. Note where to start to place the cursor for measurement has notable influence for the measured ratio. Again the placing of cursors requires radiological assessment, particularly when multiple neighboring vertebral bodies are involved with deformity. To measure a few times and take the mean may be applied for borderline cases.
Recent work further emphasized the importance of identifying osteoporotic vertebral endplate/cortex fracture (Figures 1,2) [ECF, or ABQ fracture as defined by Jiang et al. (9)]. In addition to vertebral height, particularly attention should be paid to the endplate and vertebral anterior cortex (2,3,8-10). Lentle et al. (11) showed ECF grade-1 vertebral fracture (VF) was associated with higher risk of VFs as well as nonvertebral major osteoporotic fracture, while grade-1 SQ-VD deformity was not associated with higher risk of non-vertebral fracture. We showed subjects with grade-1 SQ-vertebral deformity had a similar BMD compared with subjects without fracture, while subjects with grade-1 ECF VF had lower BMD (12). Our unpublished data shows that within the same mild/moderate VD grades, compared with the subjects without ECF, the subjects with ECF are associated with a higher short term (4-years) future risk of VD progression and new incident VD. Whether scout view images from the QCT are of sufficient image quality to detect subtle changes associated with ECF remains to be validated. It has noted that ‘relative reductions in vertebral height may not be a necessary nor sufficient criterion by which to diagnose a fracture’ (13).
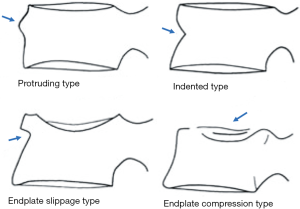
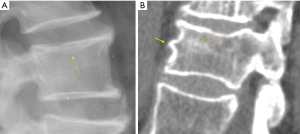
In terms of terminology, as Genant’s criteria do not require a conventional ‘fracture’ sign, we feel radiographical osteoporotic vertebral deformity (roVD) may be the appropriate term for radiographically detected deformity, especially for mild oVD. Different imaging technologies have difference sensitivity and specificity for detecting and classifying vertebral fracture. It is understood that if an endplate/cortex fracture exists, then it will be reasonable to call the involved vertebra as having a ‘fracture’. It has been recently noted that VDs with >34% height loss is usually associated with radiographically identifiable vertebra fracture signs (14), therefore the term vertebral fracture may be appropriate for these roVDs.
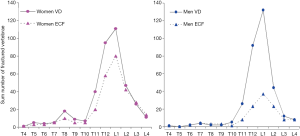
Additionally, oVDs of elderly men and elderly women’s are likely to have distinctly different features. For example, while it is generally accepted that osteoporotic VF is much more common in elderly women than elderly men, the difference in prevalence of roVD between elderly men and elderly women is small (12) (Figure 3). According to some reports, the prevalence of roVD is more common in elderly men than elderly women (15). On the other hand, roVDs in elderly women are more likely to have ECF than elderly men (Figure 3) (12). roVDs with 25–34% height loss can be with or without ECF in women, while roVD with 25–34% height loss usually do not have ECF in men (14). Our unpublished data also shows lower endplate in elderly men is much less likely to fracture than lower endplate in elderly women. These evidences suggest that the Genant’s SQ criteria may not be most suitable for assessing roVD in men. Indeed, Szulc et al. (16) suggested that a cut-off of 30% for wedge deformities from T6 to T9 (thoracic kyphosis site) and that 25% for deformities at other levels may have a high specificity and a moderate sensitivity for identifying VDs related to low BMD in men. A recent Swedish study reported that, if low threshold for oVD (i.e., 10% estimated vertebral height loss in that study) is used, then a clinical relevance of prevalent vertebral fracture in elderly men is low (17).
Despite years’ research, the radiographic criteria for osteoporotic VF and its grading remain debated (6,13,18-20). To communicate with study subjects of mild roVD, appropriate terminology should be used, so to avoid both ‘over-call’ and ‘under-call’. Though group-wise and statistically, even mild oVDs are associated with greater future osteoporotic VF (21), the importance of mild roVD remain unclear at individual subject’s level, and this is particularly the case for males. Indeed, it has been emphasized that Genant’s criteria is developed for epidemiological and large clinical trial usage. It is acknowledged that osteoporosis pharmacotherapy should be strongly considered for patients with an osteoporotic VF of more recent, higher grade, or multiple fractures (22). A grade 1, solitary, asymptomatic, incidentally discovered vertebral fracture is of questionable clinical significance. The clinical prevalence and appropriate management of these silent VDs remain unknown (13,19,20,22).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Li K, Zhang Y, Wang L, Duanmu YY, Tian W, Chen H, Yin L, Bo J, Wang Y, Li W, He L, Zhao WH, Xu SQ, Zhao LF, Zhou J, Wang FZ, Liu Y, Zhu L, Chen YZ, Zhang XL, Hao XG, Shi ZW, Wang JY, Shao JM, Chen ZJ, Lei RS, Ning G, Zhao Q, Jiang YH, Zhi YH, Li BQ, Chen X, Xiang QY, Wang L, Ma YZ, Liu SW, Cheng XG. The protocol for the Prospective Urban Rural Epidemiology China Action on Spine and Hip status study. Quant Imaging Med Surg 2018;8:667-72. [Crossref] [PubMed]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Genant HK, Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporos Int 2003;14 Suppl 3:S43-55. [Crossref] [PubMed]
- Schwartz EN, Steinberg D. Detection of vertebral fractures. Curr Osteoporos Rep 2005;3:126-35. [Crossref] [PubMed]
- Wáng YX, Santiago RF, Deng M, Nogueira-Barbosa MH. Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg 2017;7:555-91. [Crossref] [PubMed]
- Szulc P. Vertebral Fracture: Diagnostic Difficulties of a Major Medical Problem. J Bone Miner Res 2018;33:553-9. [Crossref] [PubMed]
- Wáng YX, Deng M, He LC, Che-Nordin MN, Santiago RF. Osteoporotic vertebral endplate and cortex fractures: A pictorial review. J Orthop Translat 2018. [Crossref]
- Yoshida T, Nanba H, Mimatsu K, Kasai T. Treatment of osteoporotic spinal compression fractures. Conservative therapy and its limitation. Clin Calcium 2000;10:53-8.
- Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 2004;15:887-96. [Crossref] [PubMed]
- Ito Z, Harada A, Matsui Y, Takemura M, Wakao N, Suzuki T, Nihashi T, Kawatsu S, Shimokata H, Ishiguro N. Can you diagnose for vertebral fracture correctly by plain X-ray? Osteoporos Int 2006;17:1584-91. [Crossref] [PubMed]
- Lentle BC, Berger C, Probyn L, et al. for the CaMos Research Group. Comparative Analysis of the radiology of osteoporotic vertebral fractures in women and men: cross-sectional and longitudinal observations from the Canadian Multicentre Osteoporosis Study (CaMos). J Bone Miner Res 2018;33:569-79. [Crossref] [PubMed]
- Deng M, Zeng XJ, He LC, Leung JCS, Kwok AWL, Griffith JF, Kwok T, Leung PC, Wáng YXJ. Osteoporotic Vertebral Fracture Prevalence in Elderly Chinese Men and Women: A Comparison of Endplate/Cortex Fracture-Based and Morphometrical Deformity-Based Methods. J Clin Densitom 2017. Epub ahead of print. [Crossref] [PubMed]
- Lentle B, Trollip J, Lian K. The Radiology of Osteoporotic Vertebral Fractures Redux. J Clin Densitom. 2016;19:40-7. [Crossref] [PubMed]
- Deng M, Kwok TCY, Leung JCS, Leung PC, Wang YX. All osteoporotically deformed vertebrae with >34% height loss have radiographically identifiable endplate/cortex fracture. J Orthop Translat 2018;14:63-6. [Crossref] [PubMed]
- Waterloo S, Søgaard AJ, Ahmed LA, Damsgård E, Morseth B, Emaus N. Vertebral fractures and self-perceived health in elderly women and men in a population-based cross-sectional study: the Tromsø Study 2007-08. BMC Geriatr 2013;13:102. [Crossref] [PubMed]
- Szulc P, Munoz F, Marchand F, Delmas PD. Semiquantitative evaluation of prevalent vertebral deformities in men and their relationship with osteoporosis: the MINOS study. Osteoporos Int 2001;12:302-10. [Crossref] [PubMed]
- Kherad M, Rosengren BE, Hasserius R, Nilsson JA, Redlund-Johnell I, Ohlsson C, Lorentzon M, Mellstrom D, Karlsson MK. Low clinical relevance of a prevalent vertebral fracture in elderly men--the MrOs Sweden study. Spine J 2015;15:281-9. [Crossref] [PubMed]
- Oei L, Koromani F, Rivadeneira F, Zillikens MC, Oei EH. Quantitative imaging methods in osteoporosis. Quant Imaging Med Surg 2016;6:680-98. [Crossref] [PubMed]
- McKiernan FE. Violet Fox: A Clinical View of Vertebral Fractures. J Clin Densitom. 2016;19:35-9. [Crossref] [PubMed]
- Lentle BC, Hg Oei E, Goltzman D, Rivadeneira F, Hammond I, Oei L, Kovacs CS, Hanley DA, Prior JC, Leslie WD, Kaiser SM, Adachi JD, Probyn L, Brown J, Cheung AM, Towheed T. Vertebral Fractures and Morphometric Deformities. J Bone Miner Res. 2018;33:1544-1545. [Crossref] [PubMed]
- Roux C, Fechtenbaum J, Kolta S, Briot K, Girard M. Mild prevalent and incident vertebral fractures are risk factors for new fractures. Osteoporos Int 2007;18:1617-24. [Crossref] [PubMed]
- Kendler DL, Bauer DC, Davison KS, Dian L, Hanley DA, Harris ST, McClung MR, Miller PD, Schousboe JT, Yuen CK, Lewiecki EM. Vertebral Fractures: Clinical Importance and Management. Am J Med 2016;129:221.e1-10. [Crossref] [PubMed]


