The biomarkers of leukemia stem cells in acute myeloid leukemia
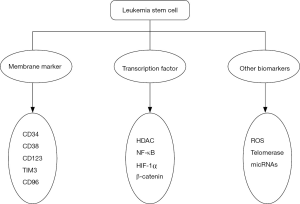
Acute myeloid leukemia (AML), an aggressive malignant disease of hematopoietic system and the most common type of acute leukemia, remains high mortality. AML characterized with the accumulation of granulocytopoiesis in the bone marrow (1). The most commonly used therapy was chemotherapy and stem cell transplantation. However, the majority of the patients died of AML relapse (2,3). DNA alkylators, topoisomerase inhibitors, antibiotics, steroids were approved by FDA for treating AML patients recent years (4). However, AML had a very poor prognosis especially to elder patients (5). More and more evidence has suggested that the small population of leukemia initiating cells or leukemia stem cells (LSCs) is supposed to be the major reason of leukemia initiation, progression, chemotherapeutic drugs resistance and disease relapse (6,7). The mechanisms for LSCs leading to AML relapse were required to be identified (8-10). The targeting of LSCs was considered to be a potential strategy to improve the long-term survival of AML patients (11). Therefore, the biological features for identification of LSCs were important for the drug discovery, targeting therapy and contributed to a better understanding of the molecular mechanism of disease (12,13). Meanwhile, the identification of LSCs in AML is especially significant in disease diagnosis, prognosis, monitoring and drug screening of AML. The identification and targeting of LSCs were depending on the membrane markers, the transcription factor and other specific mechanisms to selectively eliminate LSCs while sparing normal hematopoietic stem cells (HSCs) (Figure 1).
Membrane markers of LSCs in AML
Bonnet and Dick in 1997 reported that the population of cells characterized by the phenotype of CD34+CD38– was able to reconstitute human AML in NOD/SCID mice, which was the first report of LSCs (14,15). This work identified that the subpopulation with the surface antigens CD34+CD38– could be regarded as the specific feature of LSCs in AML. Hematopoietic tissues of AML patients included both LSCs and residual normal HSCs. However, normal HSC shared the same surface markers of CD34+CD38–. Therefore, the identification of LSCs from normal HSCs was important for scientific research and clinical investigation. As reported, the antigen expression level of CD123, interleukin-3 receptor alpha chain, was negatively related to the outcome of chemotherapy and prognosis in AML patients (16). Meanwhile, CD123 has been reported to prominently express on CD34+CD38– cells in leukemia while not normal CD34+CD38– hematopoietic cells (17,18). Therefore, CD123 is an important marker for the identification and targeting of LSCs (19). TIM3 (T-cell Ig mucin3), a negative regulator of Th1-T-cell immunity (20,21), was found to be an important marker used for LSCs and HSCs discrimination (22). Jan reported that TIM3 was highly expressed on the surface of multiple specimens of LSCs, not on normal bone marrow HSCs (23). TIM3 has been identified as a unique AML stem cell surface marker. Saito’ study demonstrated that CD32 and CD25 were highly expressed on the surface of primary human LSCs (24). Meanwhile, elimination of CD32 and CD25 expression on normal human HSCs did not damage the function of normal hematopoietic development. Furthermore, the expression of CD32 and CD25 on human LSCs were sustained after chemotherapy, which suggested that targeting these two surface markers may be effective therapeutic strategies for treatment of AML (24-26). CD96, a trans-membrane glycoprotein, has been reported to express merely on T and NK cells. AML LSCs could be distinguished from normal HSC by the expression of CD96 (27,28). These findings suggested that CD96 was a LSC-specific marker in human AML and excellent candidate target for targeting LSCs (29,30). Targeting IL3R (CD123) with diphtheria toxin (DT)-IL3 fusion proteins was in phase II clinical trial (31). Targeting specific surface markers of LSCs is considered to be a great potential strategy for selectively eliminating LSCs.
The transcription factors of LSCs in AML
Recently, several transcription factors were identified to affect the activity and function of LSCs in AML. As reported, LSCs were greatly associated with refractory AML, multi-drug resistance and relapse. The alternative p53-inactivating is the main mechanism for survival and continued evolution of LSCs during and after chemotherapy (32). Furthermore, the activity of p53 could be regulated by histone deacetylases (HDACs) (33). Therefore, the HDACs protein modulators could be exploited to control the activity of p53 and enhanced the response to chemotherapy and targeting of LSCs. Hypoxia-inducible factor-1α (HIF-1α) is an important regulator for low oxygen level in AML. Meanwhile, HIF-1α played important roles in the self-renew of HSCs and LSCs in AML (34). Bonnet and his colleagues suggested that HIF-1α or HIF-2α was necessary for the survival of LSCs and may be potential therapeutic targets for eradicating LSCs in AML (35).
NF-κB is an important transcription factor in cell survival, proliferation and differentiation. The expression of NF-κB in HSCs is low, while it is significantly over expressed in LSCs (36). Therefore, NF-κB could distinguish HSCs and LSCs, and may serve as a potential therapeutic target for the selective elimination of LSCs sparing HSCs (37,38). Dimethylaminoparthenolide (DMAPT), a NF-κB inhibitor, could selectively eradicate LSCs, which prompted it to be in clinical trials for the treatment of AML, acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL) in the United Kingdom (38,39). DMAMCL (ACT001) was able to eliminate LSCs by inhibiting the activity of NF-κB. ACT001 was in clinical trial in Australia (36,40).
β-catenin is a key molecule of Wnt/β-catenin signaling pathway which is crucial for LSC self-renewal, tumor occurrence, development, recurrence, and drug resistance (41,42). The Wnt/β-catenin was active in human LSCs and in HSCs while β-catenin was unnecessary for the self-renew of adult HSCs (43-45). Meanwhile, pharmacological inhibition of β-catenin impaired LSC function and significantly reduced the growth of human MLL leukemic cells (46,47). Therefore, all these transcription factors might be considered to be potential targets for selectively ablating LSCs.
Other biomarkers and genes
Membrane markers and transcription factors are the main characteristics of LSCs in AML. There are some other types of biomarkers which were reported to be related with the maintenance and survival of LSCs. Intracellular reactive oxygen species (ROS), reactive metabolites containing oxygen, played important roles in stem cell sustaining and function. Meanwhile, ROS had effect on leukemia initiation and progression in a certain degree (48). The level of intracellular ROS in LSCs was relatively lower than that of in HSCs. With this result, agents, inducing overproduction of ROS, can selectively eradicate AML stem cells via modulating ROS production. ROS could be an optimal therapeutic target of LSCs in AML (49). LSCs depended on telomerase to sustain the self-renew and extensive proliferation. Targeting the telomerase might be a perfect therapeutic strategy to eliminate LSCs in AML (50). Moreover, microRNAs like miR-34a, miR-126, miR-21, miR-196b and miR-17-92 played key roles in the regulation of LSCs, which would be developed as novel therapeutic agents against LSCs (51-54).
Conclusions
LSCs in AML were considered to be the root of chemotherapeutic drug resistance and disease relapse. LSCs are the subpopulation cells featured with membrane markers like CD34, CD38, CD123, TIM3, CD25, CD32 and CD96. In addition, the transcription factors were also therapeutic targets in eradicating LSCs, such as HDAC, NF-κB, HIF-1α and β-catenin. Besides membrane markers and transcription factors, intracellular ROS, telomerase and microRNAs were identified to be new targets for ablating LSCs in AML. Identification of the specific features of LSCs will greatly prompt the discovery of potential agents that can selectively eradicate LSCs in AML, which would greatly improve the response to drug resistant and refractory/relapsed AML.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) (81370086 and 81573308).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zeisig BB, Kulasekararaj AG, Mufti GJ, et al. SnapShot: Acute myeloid leukemia. Cancer Cell 2012;22:698-698.e1. [Crossref] [PubMed]
- Sasine JP, Schiller GJ. Acute Myeloid Leukemia: How Do We Measure Success? Curr Hematol Malig Rep 2016;11:528-536. [Crossref] [PubMed]
- Tsai CT, So CW. Epigenetic therapies by targeting aberrant histone methylome in AML: molecular mechanisms, current preclinical and clinical development. Oncogene 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Mussai FJ, Yap C, Mitchell C, et al. Challenges of clinical trial design for targeted agents against pediatric leukemias. Front Oncol 2015;4:374. [Crossref] [PubMed]
- Medinger M, Lengerke C, Passweg J. Novel Prognostic and Therapeutic Mutations in Acute Myeloid Leukemia. Cancer Genomics Proteomics 2016;13:317-29. [PubMed]
- Venton G, Pérez-Alea M, Baier C, et al. Aldehyde dehydrogenases inhibition eradicates leukemia stem cells while sparing normal progenitors. Blood Cancer J 2016;6:e469. [Crossref] [PubMed]
- Crews LA, Balaian L, Delos Santos NP, et al. RNA Splicing Modulation Selectively Impairs Leukemia Stem Cell Maintenance in Secondary Human AML. Cell Stem Cell 2016;19:599-612. [Crossref] [PubMed]
- Mohammadi S, Ghaffari SH, Shaiegan M, et al. Curcumin Veto the Effects of Osteopontin (OPN) Specific Inhibitor on Leukemic Stem Cell Colony Forming Potential via Promotion of OPN Overexpression. Int J Hematol Oncol Stem Cell Res 2016;10:120-9. [PubMed]
- Martinez-Climent JA, Fontan L, Gascoyne RD, et al. Lymphoma stem cells: enough evidence to support their existence? Haematologica 2010;95:293-302. [Crossref] [PubMed]
- Quotti Tubi L, Canovas Nunes S, et al. Protein kinase CK2 regulates AKT, NF-κB and STAT3 activation, stem cell viability and proliferation in acute myeloid leukemia. Leukemia 2017;31:292-300. [Crossref] [PubMed]
- Sands WA, Copland M, Wheadon H. Targeting self-renewal pathways in myeloid malignancies. Cell Commun Signal 2013;11:33. [Crossref] [PubMed]
- Laranjeira AB, Yang SX. Therapeutic target discovery and drug development in cancer stem cells for leukemia and lymphoma: from bench to the clinic. Expert Opin Drug Discov 2016;11:1071-80. [Crossref] [PubMed]
- Pollyea DA, Gutman JA, Gore L, et al. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica 2014;99:1277-84. [Crossref] [PubMed]
- Blair A, Hogge DE, Sutherland HJ. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(-)/HLA-DR-. Blood 1998;92:4325-35. [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Vergez F, Green AS, Tamburini J, et al. High levels of CD34+CD38low/-CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica 2011;96:1792-8. [Crossref] [PubMed]
- Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 2009;5:31-42. [Crossref] [PubMed]
- Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000;14:1777-84. [Crossref] [PubMed]
- Han L, Jorgensen JL, Brooks C, et al. Anti-leukemia efficacy and mechanisms of action of SL-101, a novel anti-CD123 antibody-conjugate, in acute myeloid leukemia. Clin Cancer Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415:536-41. [Crossref] [PubMed]
- Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005;6:1245-52. [Crossref] [PubMed]
- Kikushige Y, Miyamoto T. TIM-3 as a novel therapeutic target for eradicating acute myelogenous leukemia stem cells. Int J Hematol 2013;98:627-33. [Crossref] [PubMed]
- Jan M, Chao MP, Cha AC, et al. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A 2011;108:5009-14. [Crossref] [PubMed]
- Saito Y, Kitamura H, Hijikata A, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med 2010;2:17ra9. [Crossref] [PubMed]
- Terwijn M, Feller N, van Rhenen A, et al. Interleukin-2 receptor alpha-chain (CD25) expression on leukaemic blasts is predictive for outcome and level of residual disease in AML. Eur J Cancer 2009;45:1692-9. [Crossref] [PubMed]
- Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene 2011;30:1009-19. [Crossref] [PubMed]
- Gramatzki M, Ludwig WD, Burger R, et al. Antibodies TC-12 ("unique") and TH-111 (CD96) characterize T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid leukemia. Exp Hematol 1998;26:1209-14. [PubMed]
- Wang PL, O'Farrell S, Clayberger C, et al. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol 1992;148:2600-8. [PubMed]
- Hosen N, Park CY, Tatsumi N, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A 2007;104:11008-13. [Crossref] [PubMed]
- Du W, Hu Y, Lu C, et al. Cluster of differentiation 96 as a leukemia stem cell-specific marker and a factor for prognosis evaluation in leukemia. Mol Clin Oncol 2015;3:833-8. [PubMed]
- Frolova O, Benito J, Brooks C, et al. SL-401 and SL-501, targeted therapeutics directed at the interleukin-3 receptor, inhibit the growth of leukaemic cells and stem cells in advanced phase chronic myeloid leukaemia. Br J Haematol 2014;166:862-74. [Crossref] [PubMed]
- Kuo YH, Qi J, Cook GJ. Regain control of p53: Targeting leukemia stem cells by isoform-specific HDAC inhibition. Exp Hematol 2016;44:315-21. [Crossref] [PubMed]
- Yan M, Qian YM, Yue CF, et al. Inhibition of histone deacetylases induces formation of multipolar spindles and subsequent p53-dependent apoptosis in nasopharyngeal carcinoma cells. Oncotarget 2016;7:44171-84. [PubMed]
- Wang Y, Liu Y, Malek SN, et al. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 2011;8:399-411. [Crossref] [PubMed]
- Rouault-Pierre K, Hamilton A, Bonnet D. Effect of hypoxia-inducible factors in normal and leukemic stem cell regulation and their potential therapeutic impact. Expert Opin Biol Ther 2016;16:463-76. [Crossref] [PubMed]
- Ji Q, Ding YH, Sun Y, et al. Antineoplastic effects and mechanisms of micheliolide in acute myelogenous leukemia stem cells. Oncotarget 2016;7:65012-23. [PubMed]
- Guzman ML, Rossi RM, Karnischky L, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005;105:4163-9. [Crossref] [PubMed]
- Guzman ML, Rossi RM, Neelakantan S, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007;110:4427-35. [Crossref] [PubMed]
- Ghantous A, Sinjab A, Herceg Z, et al. Parthenolide: from plant shoots to cancer roots. Drug Discov Today 2013;18:894-905. [Crossref] [PubMed]
- Zhang Q, Lu Y, Ding Y, et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J Med Chem 2012;55:8757-69. [Crossref] [PubMed]
- Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 2005;5:311-21. [Crossref] [PubMed]
- Dodge ME, Lum L. Drugging the cancer stem cell compartment: lessons learned from the hedgehog and Wnt signal transduction pathways. Annu Rev Pharmacol Toxicol 2011;51:289-310. [Crossref] [PubMed]
- Ysebaert L, Chicanne G, Demur C, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia 2006;20:1211-6. [Crossref] [PubMed]
- Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 2007;12:528-41. [Crossref] [PubMed]
- Jeannet G, Scheller M, Scarpellino L, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood 2008;111:142-9. [Crossref] [PubMed]
- Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 2010;327:1650-3. [Crossref] [PubMed]
- Dietrich PA, Yang C, Leung HH, et al. GPR84 sustains aberrant β-catenin signaling in leukemic stem cells for maintenance of MLL leukemogenesis. Blood 2014;124:3284-94. [Crossref] [PubMed]
- Zhou J, Bi C, Cheong LL, et al. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood 2011;118:2830-9. [Crossref] [PubMed]
- Zhang H, Fang H, Wang K. Reactive oxygen species in eradicating acute myeloid leukemic stem cells. Stem Cell Investig 2014;1:13. [PubMed]
- Kuo YH, Bhatia R. Pushing the limits: defeating leukemia stem cells by depleting telomerase. Cell Stem Cell 2014;15:673-5. [Crossref] [PubMed]
- Wang S, Wang T, Li MZ, et al. Expression of microRNA miR-34a inhibits leukemia stem cells and its metastasis. Eur Rev Med Pharmacol Sci 2016;20:2878-83. [PubMed]
- Raffel S, Trumpp A. miR-126 Drives Quiescence and Self-Renewal in Leukemic Stem Cells. Cancer Cell 2016;29:133-5. [Crossref] [PubMed]
- Velu CS, Chaubey A, Phelan JD, et al. Therapeutic antagonists of microRNAs deplete leukemia-initiating cell activity. J Clin Invest 2014;124:222-36. [Crossref] [PubMed]
- Wong P, Iwasaki M, Somervaille TC, et al. The miR-17-92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res 2010;70:3833-42. [Crossref] [PubMed]
Cite this article as: Ding Y, Gao H, Zhang Q. The biomarkers of leukemia stem cells in acute myeloid leukemia. Stem Cell Investig 2017;4:19.