Reconstitution of adaptive immunity after umbilical cord blood transplantation: impact on infectious complications
Introduction
Over the last decades, umbilical cord blood (UCB) expanded as an alternative graft source for allogeneic stem cell transplantation (alloHSCT) in patients who lack a suitable HLA-matched related or unrelated donor (1,2). UCB transplantation (UCBT) offers some practical advantages including easy collection, prompt availability and reduced stringency of HLA-matching requirements with the potential of increasing the applicability of alloHSCT to ethnic minorities. However, one of the major concerns after UCBT was the high rate of infection-related morbidity and mortality, particularly in adult UCB recipients (3-5).
Reconstitution of a functional adaptive immune system is an important challenge after alloHSCT. Studies reported that delayed T- and B-cell recovery associated with increased risks of post-transplant opportunistic infections and transplant-related mortality (6-11). Reconstitution of adaptive immunity after alloHSCT is a complex and slow process that can be influenced by several factors, such as recipient age, type of donor (related/unrelated, HLA-matched/HLA-mismatched), type of conditioning (myeloablative/reduced intensity; including or not radiation therapy), ex vivo or in vivo T-cell depletion of the graft, type of graft-versus-host disease (GVHD) prophylaxis, as well as occurrence and treatments of GVHD (12). Some impact of the stem cell source was also reported. For example, differences in the kinetics of T- and B-cell recovery were observed after alloHSCT with mobilized peripheral blood stem cells (PBSC), as compared with bone marrow (BM) (13-15). Since opportunistic infections appeared to be frequent after UCBT, growing interest developed over the last decade about the pattern of immune recovery using this specific graft source.
Several tools have been developed for monitoring the recovery of the adaptive immune system after alloHSCT. Some of them are currently used routinely in clinical laboratories, including measurements of absolute counts and frequencies of main lymphocyte subsets (CD3+CD4+ and CD3+CD8+ T cells, CD20+ or CD19+ B cells) as well as quantification of serum immunoglobulin (Ig) levels. In addition, more complex assays are also available in research laboratories, such as multi-color flow cytometry, functional assays, studies of T- and B-cell repertoire diversity through TCR beta and IgH complementarity determining region 3 (CDR3) size analyses, and detection of T-cell receptor excision circles (TRECs) and kappa-deleting recombination excision circles (KREKs). TRECs and KRECS can be used as markers of thymopoiesis and B-lymphopoiesis, respectively (16,17). These assays have helped us refining our knowledge on recovery of adaptive immunity after alloHSCT, and specifically after UCBT.
In this review, we summarize the current understanding of T- and B-cell reconstitution following UCBT and why this differs from alloHSCT using other stem cell source. We further discussed the links between immune reconstitution and infections after UCBT.
T-cell reconstitution after UCBT
General overview of T-cell reconstitution after alloHSCT
T-cell recovery after alloHSCT proceeds along two different pathways that act in parallel but follow distinct kinetics: (I) homeostatic peripheral expansion of mature T cells (termed the “thymic-independent pathway”); and (II) naive T-cell neo-production from donor hematopoietic progenitor cells through the thymus (termed the “thymic-dependent pathway”) (Figure 1) (12,19).
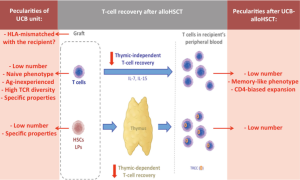
Most circulating T cells during the first months after transplantation arise through the thymic-independent pathway and are the progeny of T cells infused with the graft or of host T cells that survived the pre-transplant conditioning therapy. Peripheral expansion of mature T cells occurs in the peculiar context of lymphopenia and is the consequence of homeostatic mechanisms that control the size of the T-cell pool (20). Briefly, in normal individuals, the mature T-cell pool is highly regulated through T-cell competition for homeostatic cytokines that support their maturation, proliferation and survival [mainly interleukin (IL)-7 and IL-15]. Early after alloHSCT, IL-7 and IL-15 are produced but little consumed because of the lymphopenic state induced by the conditioning regimen. This results in high serum levels of these cytokines (21). Therefore, in such a cytokine climate, the little number of T cells present after transplantation undergo intense expansion, until they reach a number that is in the range of the mature pool in normal subjects. This pathway of immune reconstitution explains several observations such as the high percentage of cells in cycle and the rapid shortening of telomeres in T cells during the first months after alloHSCT (22). Cytokine signals alone are sufficient to induce homeostatic expansion of memory T cells, but naive T cells additionally require TCR engagement with self-HLA for survival and expansion (23). Hence, the magnitude of peripheral expansion is variable among T-cell subsets and is higher for memory than for naive T-cell populations. It also occurs more strongly for CD8+ than for CD4+ T cells. This leads to an inverted CD4/CD8 ratio and to a predominance of memory CD8+ T cells circulating in the peripheral blood during the first months after alloHSCT. Although the peripheral expansion is a polyclonal proliferation, it is not completely antigen-independent since cells that recognize cognate antigens may acquire a proliferative advantage over other T-cell clones. For example, CD8+ T cells recognizing periodically reactivated herpes viruses [i.e., cytomegalovirus (CMV) or Epstein Barr virus (EBV)] may expand rapidly. Therefore, as compared to that of the donor, the T-cell repertoire may narrow after alloHSCT and may be directed toward these viruses, specifically with grafts from adult donors that contain viral antigen-experienced memory T cells and in seropositive recipients (24-26).
Reconstitution of a more diversified T-cell repertoire occurs secondarily, with de novo production of naive T cells through the thymic-dependent pathway (19). It is a long-lasting process that critically depends on lymphoid progenitors arising from donor-derived stem cells, proliferating and seeding the thymus, as well as on optimal thymic microenvironment for T-cell maturation and selection. In the thymus, developing T cells (thymocytes) that bind with appropriate affinity to self (host)-HLA molecules are positively selected (positive selection) and those that recognize self (host)-antigens presented in association with HLA molecules with high affinity are deleted (negative selection) or are deviated into regulatory T cell (Treg) lineage (affinity model of thymocyte selection) (27,28). An essential component of the negative selection process is the display of self-antigens by medullary thymic epithelial cells (mTECs) to developing T cells. This is coordinated by the Autoimmune Regulator (AIRE) gene that initiates the expression of a wide array of tissue-specific self-antigens, creating an “immunological self-shadow” in the thymus (29). Recently, it was suggested that AIREexpressing mTECs could also promote the thymic development of some clones of self-tolerant Treg (29). In young patients, naive T-cell export from the thymus can be observed from day 100 after alloHSCT, but restoration of a diversified naive T-cell pool may require 1 to 2 years.
Several factors can adversely affect the thymic-independent and/or thymic-dependent pathway of T-cell recovery after alloHSCT. The use of ex vivo T-cell depleted graft (30) or in vivo T-cell depleting approaches (i.e., with alemtuzumab, an anti-CD52 monoclonal antibody; or with anti-T cell globulin, ATG) was reported to compromise peripheral T-cell expansion (31-36). Impaired thymopoiesis after ATG-conditioned alloHSCT was also described in some (37) but not all (35) studies, although this may vary depending on the brand of ATG. Acute GVHD was shown to alter metabolism of lymphoid progenitors as well as their homing properties (mobilization from the bone marrow and migration to the thymus) (38,39). Thymopoiesis by itself may be compromised in patients whose thymus is involuted (older patients) or damaged (i.e., because of GVHD) (40). It was also suggested that the degree of HLA mismatch between the donor and the recipient can impact both thymic-independent and -dependent pathways, in part due to higher risk of GVHD but also because of disturbances in the physiologic mechanisms of naive T-cell maintenance in the periphery and of T-cell selection during thymopoiesis, in the setting of HLA-mismatched transplantation (41).
Recovery of the T-cell pool in peripheral blood after (UCB-) transplantation. Peculiarities in the setting of UCBT are highlighted in red. In comparison with PBSC and BM grafts, UCB units are characterized by some quantitative and qualitative differences in their composition. They contain (I) lower total cell dose of hematopoietic stem cells and lymphoid progenitors, (II) lower dose of T cells, (III) almost exclusively naive T cells, (IV) no anti-pathogen specific memory T cells, and (V) a highly diversified TCR repertoire. HLA disparities between the graft and the recipient are also more common in the setting of UCBT. Eventually, UCB-derived HSC, LP and T cells have also specific intrinsic properties [reviewed in (18)], likely due to ontological reasons (fetal origin). All of these factors may participate in delayed T-cell recovery through the thymic-independent and/or -dependent pathway(s) after UCBT. Moreover, some peculiarities have also been described regarding the thymic-independent T-cell recovery after UCBT, such as faster skewing towards memory-like phenotype and a CD4-bias pattern. Ag, antigen; HSCs, hematopoietic stem cells; LPs, lymphoid progenitors; TREC, T-cell receptor excision circle.
Current knowledge about T-cell reconstitution after UCBT
Several studies compared T-cell recovery after UCBT versus BM or PBSC alloHSCT (Table 1) (42-50). Although highly variable in the population they investigated (pediatric versus adult patients, transplantation with either single or double UCB units, HLA-matched or mismatched PBSC- or BM-recipients as control groups, myeloablative or reduced intensity conditioning regimens, and variable GVHD prophylaxes, specifically with the use or not of ATG), the majority of them reported delayed T-cell recovery in UCBT patients (42-45,48-50). Depending on the study, lower counts of circulating T cells in the peripheral blood were observed up to 3–12 months after UCBT, as compared with alloHSCT with PBSC or BM. Delayed CD8+ T-cell recovery was almost consistently described, whilst delayed CD4+ T-cell recovery was reported by some groups (43-45,48,50) but not all (42,49). In a number of these studies, pre-transplant ATG was administered more frequently in UCB-recipients than in patients receiving other graft source (42-44,49,50). In addition, conditioning regimen and GVHD prophylaxis often differed between patients transplanted with UCB and PBSC or BM. These variables have to be taken into consideration when interpreting the results of these studies, as they can be important confounding factors. As an example, prompter CD4+ T-cell reconstitution was indeed observed after UCBT with the omission of ATG (51,52). However, recent studies having monitored immune recovery after “ATG-free” myeloablative double UCBT versus PBSC-alloHSCT in adult patients still reported a transient 3-month delay in T-cell reconstitution in UCB recipients (45,48).
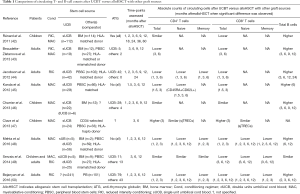
Full table
Whether delayed T-cell recovery after UCBT is related to graft-intrinsic or extrinsic factors (such as specific conditioning and prophylaxis regimens) are not formally known. However, in comparison with PBSC and BM harvests, UCB grafts are characterized by quantitative and qualitative differences in their composition that may predict potential impact on both thymic-independent and thymic-dependent T-cell recovery (Figure 1). This will be discussed in the following paragraphs.
AlloHSCT indicates allogeneic stem cell transplantation; ATG, anti-thymocyte globulin; BM, bone marrow; Cond, conditioning regimen; dUCB, double units umbilical cord blood; MAC, myeloablative conditioning; PBSC, peripheral blood stem cells; RIC, reduced intensity conditioning; sUCB, single unit umbilical cord blood. ?, not specified.
Current knowledge about the thymic-independent pathway of T-cell reconstitution after UCBT
Considering T-cell reconstitution through the thymic-independent pathway, an important limiting factor can be the relatively low dose of T cells contained in one UCB unit (about 1–2 log fewer than BM and PBSC grafts, respectively). This results in lower amount of T cells transferred with the graft that can further undergo peripheral expansion. Moreover, the vast majority of UCB-derived T cells are antigen-inexperienced naive T cells, which are less prone to undergo massive homeostatic proliferation, specifically in an HLA-mismatched transplant setting (that is common in UCBT) (see above). Taken together, these data suggest lower potential for T-cell recovery through the thymic-independent pathway after UCBT. Additionally, since UCB does not contain anti-pathogen experienced memory T cells, there is no possibility for direct transfer of protective T-cell memory immunity from the donor to the host after UCBT.
Faster increase in T cell numbers was observed after double UCBT, in comparison with single UCBT. Although this might simply be related to the higher dose of transferred T cells, frequent HLA mismatches between UCB units in double UCBT might also trigger the expansion of T cells through graft-versus-graft interactions. Hence, it was reported that T cells originating from the predominant UCB unit and recognizing mismatched HLA-alleles expressed by the non-engrafting UCB unit rapidly expanded after double UCBT (53).
Although UCB grafts contain high proportions of naive T cells, low count and low frequency of phenotypically naive (CD45RA+CCR7+) T lymphocytes were reported to circulate in the recipient’s peripheral blood after UCBT, even early after transplantation (44,48,49,54,55). Several authors showed that fetal T cells convert more rapidly than adult T cells into memory-like T cells once proliferating (56), and that the peripheral expansion of naive UCB T cells after alloHSCT associate with a gradual skewing towards memory-like phenotypes (mainly effector memory [CD45RA−CCR7−] and late effector memory [CD45RA+CCR7−] phenotypes) (49,54,55).
Contrarily to the memory CD8+ T-cell biased homeostatic peripheral expansion that occurs after PBSC- and BM-alloHSCT, some authors recently reported a unique CD4+-biased pattern of thymic-dependent T-cell reconstitution after UCBT with the omission of ATG (52). One hypothesis is that fetal naive CD4+ T cells may display higher proliferative response compared to adult T cells, likely due to ontological properties (originating from fetal stem cells rather than adult stem cells) (18,57).
Although rapidly acquiring a memory-like phenotype in the recipient, transferred UCB T cells maintain high TCR diversity after transplantation (58). Fetal peripheral blood is characterized by a complete TCR repertoire at birth (59) and, in comparison with PBSC or BM harvests from adult donors, UCB grafts display larger T-cell polyclonality that reflects the lack of prior antigen stimulation. Using next-generation sequencing assays, van Heijst et al. reported that UCBT (double units, without ATG) was associated with a more diversified TCR repertoire at 6 and 12 months after transplantation, in comparison with conventional and T-cell depleted PBSC-alloHSCT (58). The difference was more pronounced for the CD4+ than for the CD8+ T-cell compartment, in accordance with data having shown that there is a substantially greater TCR diversity in CD4+ compared to CD8+ T cells (40,58,60) and that lymphocyte recovery is CD4+-biased after UCBT (52). To the contrary, the group of Kanda et al. described similar TCR repertoire entropy at 12 months after UCBT and T-cell repleted PBSC-alloHSCT (45). However, the late time-point they analysed might have explained their observation. In fact, in the study of van Heijst et al., TCR diversity in non-UCB recipients improved by 12 months (likely due to appearance of naive T cells coming from de novo thymopoiesis) and approached that of UCB patients at that time point (58).
Current knowledge about the thymic-dependent pathway of T-cell reconstitution after UCBT
Highly variable but generally slow thymic recovery was reported after UCBT, specifically in adult patients (45,46,49,54,55). Thymopoiesis may be affected by several factors after UCBT [nicely reviewed in (41)]. First, although fetal peripheral blood is characterized by higher concentration of hematopoietic stem and progenitor cells than adult peripheral blood (61), each UCB unit contains about a 10–100-fold lower total cell dose compared to BM and PBSC harvests. This can result in a delay in the re-initiation of thymopoiesis. This is specifically true for adult patients transplanted with single UCB unit. By studying immune recovery in a cohort of 32 adult patients treated with single unit UCBT, Komanduri et al. observed a paucity of circulating naive T cells and the near complete absence of TRECs during the first year posttransplant (55). However, in this study, UCB recipients were heavily pre-treated and had impaired thymopoiesis at baseline. In contrast with these results, other groups reported prompt (from 3–6 months after transplantation) detection of TRECs in the peripheral blood of adult recipients of double UCB (7,45). In a study of adult patients undergoing myeloablative transplantation, Kanda et al. described comparable TREC levels among double UCB- and PBSC-recipients by 6 months after transplantation (45). Hence, the use of double UCB can be important for thymic recovery in the adult setting. Although this might simply be related to a cell dose effect, alternative (as yet unidentified) explanations might also be involved since the majority of recipients of double UCB units display single unit chimerism by 3-month post-transplantation.
Independently of the stem cell dose, high level of donor/recipient HLA discordances (that usually characterise UCBT) likely affect intrathymic T-cell selection and adversely impact thymopoiesis. This is supported by the study of Clave et al. who reported comparable low thymic function at 3 months after UCBT and HLA-haploidentical donor alloHSCT in a cohort of pediatric patients, even although HLA-haploidentical recipients had received mega-dose of CD34+-selected stem cells (47).
Clinical factors influencing T-cell recovery after UCBT
Several clinical factors have been described to positively impact T-cell recovery in the specific setting of UCBT. These factors include the use of high dose of total nucleated cells, use of double UCB units, use of UCB from related donor and of HLA well-matched UCB unit, and a positive recipient CMV serology before transplantation (62). Conversely, similarly to what is reported after transplantation with conventional graft source, T-cell depleting approaches (such as with ATG or with alemtuzumab) and occurrence of GVHD significantly hamper T-cell recovery after UCBT (31,62-65). Concerning the impact of ATG, it was recently demonstrated in a large cohort of pediatric patients, that even very minimal exposure to ATG in the setting of UCBT has a significant detrimental effect on early CD4+ T-cell recovery (63).
B-cell reconstitution after UCBT
General overview of B-cell reconstitution after alloHSCT
Immune reconstitution of B cells after alloHSCT is markedly different from that of T cells (12,66). After alloHSCT with PBSC or BM, absolute numbers of circulating B cells classically normalize by 6–12 months (67,68). However, recovery of functional B-cell immunity (i.e. in terms of adequate Ig production) takes several years more, following a pattern that resembles a recapitulation of ontogeny. Specifically, during the 1–2 first months after alloHSCT, the number of circulating B cells remains very low and most of them are transitional B cells (69). These are early marrow emigrant immature B cells that show only partial response to antigen-BCR stimulation. Over the next 3–6 months, total B cell counts gradually rise as well as the proportion of mature naive (CD19+CD27−IgMlowIgDhigh) B cells, the later representing 90% of the circulating B lymphocytes by 9 months. Reconstitution of the memory B-cell (CD19+CD27+) pool may take several (up to 5) years (11,67). It occurs upon antigen exposure but also requires CD40 signals as well as cytokine stimulation from CD4+ T cells (follicular B helper T cells). Hence, delayed T-cell recovery and the reversed CD4/CD8 ratio likely contribute to reduce the numbers of circulating memory B cells after alloHSCT (27).
The production of class-switched antibodies in serum mimics the recovery of class-switched memory B cells. Normal levels of serum IgM are usually measurable from 3–6 months, followed by normalization of serum IgG1/IgG3 and then IgG2/IgG4 between 1–2 years after alloHSCT (66). The last Ig to recover is IgA, which may be undetectable up to 5 years. Because of the radioresistance and the prolonged longevity of plasma cells, antibodies of recipient origin may however be detected during several months or years after alloHSCT.
Among main factors impacting B-cell recovery, both acute and chronic GVHD were associated with delayed quantitative and qualitative B cell reconstitution (11,68). Because they contain antibodies directed against multiple pan-lymphocyte antigens, ATG preparations were thought to exert immunomodulatory effects on B cells (70). However, although some groups observed impaired quantitative B-cell recovery after alloHSCT with ATG (71), others reported higher numbers of circulating B cells in ATG-conditioned patients (35,36). Few studies have assessed the effects of alemtuzumab exposure on circulating B-cell counts after alloHSCT and reported no significant impact (31-33).
Current knowledge about B-cell reconstitution after UCBT
Longitudinal studies of immune reconstitution after UCBT showed that absolute numbers of circulating B cells often surpass the B-cell counts observed in PBSC- or BM-recipients during the first 6–12 months period after transplantation (Table 1) (43-45,48). B-cell numbers generally reach normal levels with a median time of 6 months after UCBT (42-45,48,49). By using KREC assays, Nakatani et al. also recently confirmed enhanced B-cell lymphopoiesis after UCBT compared with PBSC- or BM-alloHSCT (17). Reasons for such faster normalization of total B-cell counts may be multiple. First, this may be related to ontological reasons, with UCB-B cells originating from fetal stem cells rather than adult stem cells (both of which are present in UCB). It can also occur as a “compensatory phenomenon” in response to concomitant profound T-lymphopenia. Indeed, studies in HIV-infected subjects as well as in patients with idiopathic CD4+ T deficiency demonstrated B-cell expansion in the context of T-cell paucity (72,73). Eventually, higher level of B-cell Activating Factor (BAFF), a member of the tumor necrosis factor family that promotes B cell survival, was also reported in UCB recipients in comparison with PBSC recipients (44).
Although numbers of circulating B cells rapidly normalize after UCBT, recovery of full B-cell immunity takes significantly longer. Indeed, during the first year after UCBT, most of circulating B cells are of naive phenotype (46,49). Nevertheless, several groups suggested faster recovery of memory B-cell functions after UCBT as compared with other stem cell sources. By monitoring Ig levels after transplantation, Jacobson et al. observed a quicker recovery of IgG levels after double UCBT than after PBSC transplantation. In their study, IgG levels began to recover between 5 and 6 months after UCBT and reached normal levels by 12 months, whereas they remained below the normal values throughout the first year after PBSC transplantation (44). Another group reported faster discontinuation of Ig replacement therapy in children with immunodeficiency syndromes transplanted with UCB as compared with haploidentical donor (74).
Reconstitution of T- and B-cell immunity after UCBT and clinical implication regarding risk of infections
Infection prevalence and immune response against (viral) pathogens after UCBT
Transplantation with UCB was reported to be associated with a significant risk of infections, specifically during the first 3 months after transplantation (3-5,48,49,51,75-84). Opportunistic infections also account for a major contributing factor of early transplant-related mortality after UCBT. A large retrospective study from the CIBMTR registry revealed a significantly higher incidence of 100-day infection-related mortality (IRM) after UCBT (45%) than after BMT (21–24%) (P=.01) (3). Similarly, a rate of 30–45% of 3-month IRM after UCBT was also reported by other groups (4,5).
Delayed neutrophil engraftment is likely one of the major causes of increased infection risk during the early period after UCBT. This was well demonstrated in a recent study showing that transplantation of ex vivo expanded umbilical cord blood stem cells decreased early infections and hospitalization (85).
Additionally, beyond the pre-engraftment period, UCBT also appears to be associated with increased risk of infections. Whatever is the graft source, the early post-engraftment period (from engraftment until around day 100) is characterized by high prevalence of viral infections, especially due to reactivations of latent viruses. Lack of transfer of memory T cells from donor to recipient as well as lower T-cell counts during the first months after UCBT (see above) may render UCB-recipients more susceptible to viral complications. By performing T-cell functional assays, some groups confirmed impaired T-cell activity against several viruses (including CMV, EBV, BK JC, influenza and respiratory syncytial viruses) in patients transplanted with UCB (55,75). Accordingly, elevated frequencies of infections related to herpes viruses (such as CMV, EBV, varicella zoster and human herpes virus 6) were described (77-82). Focusing on CMV infections, several studies reported the need for increased number of repeated courses of pre-emptive antiviral therapy to control CMV viremia (83) as well as high rate of CMV diseases in seropositive recipients transplanted with UCB (82-84). Some authors suggested that CMV-specific CD8+ T cells could not reliably be detected in UCB recipients until 100 days after transplantation (when thymopoiesis recovers) (55,86). On the contrary, by using more sensitive assays, a recent study showed that a diverse polyclonal CMV-specific T-cell response was detectable as early as day 42 after double UCBT but was unable to expand sufficiently in vivo to efficiently control viral reactivations, likely due to concomitant CD4+ T-cell deficiency and/or to immunosuppressive therapy (87).
Whether or not UCBT also predisposes patients to higher risk of infections in the later post-transplant (>100 days) period is less defined. In the CIBMTR registry analysis of UCB- and BM-recipients, similar rates of infections were observed beyond day 100 in both groups (3). Other studies reported concordant observations (4,49). By analysing adult patients transplanted with double UCB without ATG, Sauter et al. recently showed that serious infections were indeed uncommon after day 120 after UCBT, with 75% of patients free of serious infections after this time (51). This may correlate with progressive recovery of T cells.
Does monitoring circulating T and B cells predict infections after UCBT?
Some studies suggested that monitoring T- and/or B-cell recovery in the peripheral blood might help predicting risks of infections after UCBT. In a large cohort of pediatric patients transplanted with UCB, Admiraal et al. recently reported that posttransplant CD4+ T-cell recovery was a strong predictor of survival (63). In this study, successful early CD4+ T-cell recovery (defined as >50 cells/µL within 100 days after UCBT) significantly increased the chance of overall survival (HR, 0.51; 95% CI, 0.28–0.95; P= 0.035) and lowered the chance of non-relapse mortality (NRM; HR, 0.36; 95% CI, 0.15–0.83; P=0.0017). In patients not reaching CD4+ T-cell recovery, infectious disease was the most common cause of NRM. Similarly, in a longitudinal study of immune reconstitution after HLA-mismatched donor alloHSCT (including UCBT and adult patients), we observed that levels of circulating CD4+ T cells at 3 months and during the 3–12 months period after transplantation inversely associated with the risk of late infections (49). Studies in non-UCB recipients also identified total CD4+ T-cell, naive CD4+ T-cell and TREC levels as major determinants of outcome after alloHSCT, with high values predicting lower incidences of opportunistic infections and mortality (6-10).
B-cell recovery was also reported to predict risk of infections after alloHSCT (11,17). In the specific setting of UCBT, Nakatani et al. observed that earlier recovery of KRECs correlated with fewer infectious episodes (17).
Conclusions and perspectives
Knowledge about T- and B-cell recovery after UCBT steadily increased over the last decade. Overall, current data suggest fast B-lymphopoiesis but delayed (CD8+) T-cell reconstitution in UCB recipients. Depending on the study, lower counts of circulating T cells in the peripheral blood were observed up to 3–12 months after UCBT, as compared with PBSCT or BMT. It remains to be determined whether this is due to graft-intrinsic properties or to extrinsic factors such as specific conditioning and GVHD prophylaxis regimens, or high HLA mismatching between the graft and the recipient. This delayed T-cell recovery likely contributes to the high prevalence of early opportunistic infections observed after UCBT.
Use of double UCB units and the omission or dose reduction of ATG have been shown to improve early immune reconstitution after UCBT, and to reduce the incidence of infections. New strategies for enhancing immune (especially T-cell) recovery after UCBT are currently the matter of intense research. Individualizing ATG exposure could appear as a good one. Dosing would be based on patient characteristics (including absolute lymphocyte count), with the aim of reaching optimal exposure before and after UCBT that would allow optimal immune recovery while protecting against GVHD and graft failure. This approach is currently being investigated in a prospective clinical trial (Dutch trial register NTR4960). Therapeutic drug monitoring (ATG pharmacokinetics measuring) would be an additional tool to target optimal ATG exposure more precisely. However, this would require an operational validated ATG measuring assay. Several other approaches have been assessed to improve both thymo-dependent and thymo-independent pathways of T-cell reconstitution after UCBT. These include, ex vivo expansion of hematopoietic stem cells [reviewed in (88,89)], modulation of thymic niche accessibility by lymphoid progenitors (90), enhancement of thymic function (i.e., through stimulation of the GH pathway or through sex steroid blockade) and treatments with recombinant IL-7 and IL-15 [reviewed in (89,91)]. Posttransplant adoptive transfer of donor-derived T-cell progenitors or virus-specific mature T cells was also used to boost immunity against infections. Nevertheless, these strategies proved much more difficult for UCBT, due to limited cell numbers, the fact that the majority of UCB T cells are antigen-naive and the non-availability of post-transplant donor lymphocyte infusions. Recently, some groups demonstrated that virus-specific T cells could be produced and expanded ex vivo from UCB (92,93) and infusion of such T cells is currently evaluated in ongoing phase I/II clinical studies (www.clinicaltrials.gov#NCT01923766). Although very promising these approaches remains limited by the technical difficulties of the procedure since it necessitates the priming and extensive expansion of naive T cells instead of direct expansion of pre-existing virus-specific memory T cells. A more rapid method would consist of the adoptive transfer of virus-specific T-cells isolated from healthy third party adult donors. This approach has proven effective as treatment of life-threatening infection after UCBT and is also under investigation as a pre-emptive therapy (94,95).
Acknowledgements
The review was in part supported by funds from the FNRS and the Leon Fredericq Foundation from the University of Liège.
Footnote
Conflicts of Interest: S Servais is Postdoctoral Researcher the National Fund for Scientific Research (FNRS) Belgium. The other authors have no conflicts of interest to declare.
References
- Baron F, Labopin M, Ruggeri A, et al. Unrelated cord blood transplantation for adult patients with acute myeloid leukemia: higher incidence of acute graft-versus-host disease and lower survival in male patients transplanted with female unrelated cord blood--a report from Eurocord, the Acute Leukemia Working Party, and the Cord Blood Committee of the Cellular Therapy and Immunobiology Working Party of the European Group for Blood and Marrow Transplantation. J Hematol Oncol 2015;8:107. [Crossref] [PubMed]
- Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013;122:491-8. [Crossref] [PubMed]
- Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004;351:2265-75. [Crossref] [PubMed]
- Parody R, Martino R, Rovira M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 2006;12:734-48. [Crossref] [PubMed]
- Rocha V, Labopin M, Sanz G, et al. Acute leukemia working party of European blood and marrow transplant group,Eurocord-Netcord registry. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004;351:2276-85. [Crossref] [PubMed]
- Storek J, Gooley T, Witherspoon RP, et al. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol 1997;54:131-8. [Crossref] [PubMed]
- Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood 2010;115:4111-9. [Crossref] [PubMed]
- Kim DH, Sohn SK, Won DI, et al. Rapid helper T-cell recovery above 200 x 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant 2006;37:1119-28. [Crossref] [PubMed]
- Bartelink IH, Belitser SV, Knibbe CA, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant 2013;19:305-13. [Crossref] [PubMed]
- Berger M, Figari O, Bruno B, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant 2008;41:55-62. [Crossref] [PubMed]
- Corre E, Carmagnat M, Busson M, et al. Long-term immune deficiency after allogeneic stem cell transplantation: B-cell deficiency is associated with late infections. Haematologica 2010;95:1025-9. [Crossref] [PubMed]
- Ogonek J, Kralj Juric M, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol 2016;7:507. [Crossref] [PubMed]
- Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001;97:3380-9. [Crossref] [PubMed]
- Ottinger HD, Beelen DW, Scheulen B, et al. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood 1996;88:2775-9. [PubMed]
- Abrahamsen IW, Somme S, Heldal D, et al. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica 2005;90:86-93. [PubMed]
- Toubert A, Glauzy S, Douay C, et al. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens 2012;79:83-9. [Crossref] [PubMed]
- Nakatani K, Imai K, Shigeno M, et al. Cord blood transplantation is associated with rapid B-cell neogenesis compared with BM transplantation. Bone Marrow Transplant 2014;49:1155-61. [Crossref] [PubMed]
- Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy 2007;9:111-22. [Crossref] [PubMed]
- Chaudhry MS, Velardi E, Malard F, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation:Time To T Up the Thymus. J Immunol 2017;198:40-6. [Crossref] [PubMed]
- Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007;19:318-30. [Crossref] [PubMed]
- Thiant S, Yakoub-Agha I, Magro L, et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant 2010;45:1546-52. [Crossref] [PubMed]
- Rufer N, Brümmendorf TH, Chapuis B, et al. Accelerated telomere shortening in hematological lineages is limited to the first year following stem cell transplantation. Blood 2001;97:575-7. [Crossref] [PubMed]
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2002;2:547-56. [PubMed]
- Kanakry CG, Coffey DG, Towlerton AM, et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight 2016;1(5).
- Suessmuth Y, Mukherjee R, Watkins B, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRβ repertoire. Blood 2015;125:3835-50. [Crossref] [PubMed]
- Itzykson R, Robin M, Moins-Teisserenc H, et al. Cytomegalovirus shapes long-term immune reconstitution after allogeneic stem cell transplantation. Haematologica 2015;100:114-23. [Crossref] [PubMed]
- Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012;12:157-67. [PubMed]
- Klein L, Kyewski B, Allen PM, et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 2014;14:377-91. [Crossref] [PubMed]
- Anderson MS, Su MA. AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol 2016;16:247-58. [Crossref] [PubMed]
- Martínez C, Urbano-Ispizua A, Rozman C, et al. Immune reconstitution following allogeneic peripheral blood progenitor cell transplantation: comparison of recipients of positive CD34+ selected grafts with recipients of unmanipulated grafts. Exp Hematol 1999;27:561-8. [Crossref] [PubMed]
- Lane JP, Evans PT, Nademi Z, et al. Low-dose serotherapy improves early immune reconstitution after cord blood transplantation for primary immunodeficiencies. Biol Blood Marrow Transplant 2014;20:243-9. [Crossref] [PubMed]
- Marsh RA, Lane A, Mehta PA, et al. Alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery following alemtuzumab, fludarabine, and melphalan RIC HCT. Blood 2016;127:503-12. [Crossref] [PubMed]
- Willemsen L, Jol-van der Zijde CM, Admiraal R, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant 2015;21:473-82. [Crossref] [PubMed]
- Hannon M, Beguin Y, Ehx G, et al. Immune recovery after allogeneic hematopoietic stem cell transplantation following Flu-TBI versus TLI-ATG conditioning. Clin Cancer Res 2015;21:3131-9. [Crossref] [PubMed]
- Servais S, Menten-Dedoyart C, Beguin Y, et al. Impact of Pre-Transplant Anti-T cell globulin (ATG) on immune recovery after myeloablative allogeneic peripheral blood stem cell transplantation. PLoS One 2015;10:e0130026. [Crossref] [PubMed]
- Bosch M, Dhadda M, Hoegh-Petersen M, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012;14:1258-75. [Crossref] [PubMed]
- Na IK, Wittenbecher F, Dziubianau M, et al. Rabbit antithymocyte globulin (thymoglobulin) impairs the thymic output of both conventional and regulatory CD4+ T cells after allogeneic hematopoietic stem cell transplantation in adult patients. Haematologica 2013;98:23-30. [Crossref] [PubMed]
- Glauzy S, André-Schmutz I, Larghero J, et al. CXCR4-related increase of circulating human lymphoid progenitors after allogeneic hematopoietic stem cell transplantation. PLoS One 2014;9:e91492. [Crossref] [PubMed]
- Glauzy S. Alterations of circulating lymphoid committed progenitor cellular metabolism after allogeneic stem cell transplantation in humans. Exp Hematol 2016;44:811-816.e3. [Crossref] [PubMed]
- Castermans E, Hannon M, Dutrieux J, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica 2011;96:298-306. [Crossref] [PubMed]
- Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood 2014;124:3201-11. [Crossref] [PubMed]
- Rénard C, Barlogis V, Mialou V, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol 2011;152:322-30. [Crossref] [PubMed]
- Beaudette-Zlatanova BC, Le PT, Knight KL, et al. A potential role for B cells in suppressed immune responses in cord blood transplant recipients. Bone Marrow Transplant 2013;48:85-93. [Crossref] [PubMed]
- Jacobson CA, Turki AT, Mcdonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012;18:565-74. [Crossref] [PubMed]
- Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:1664-1676.e1. [Crossref] [PubMed]
- Charrier E, Cordeiro P, Brito RM, et al. Reconstitution of maturating and regulatory lymphocyte subsets after cord blood and BMT in children. Bone Marrow Transplant 2013;48:376-82. [Crossref] [PubMed]
- Clave E, Lisini D, Douay C, et al. Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol 2013;4:54. [Crossref] [PubMed]
- Mehta RS, Bejanyan N, Cao Q, et al. Immune reconstitution after umbilical cord blood versus peripheral blood progenitor cell transplantation in adults following myeloablative conditioning. Blood 2016;22:2246.
- Servais S, Lengline E, Porcher R, et al. Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:507-17. [Crossref] [PubMed]
- Bejanyan N, Brunstein CG, Cao Q, et al. Immune reconstitution. after Allogeneic Hematopoietic Cell Transplantation (alloHCT): Comparing Results in Recipients of Unrelated Umbilical Cord Blood (UCB) to Those with an HLA-Matched Sibling Donor Peripheral Blood (MSD PB). Blood 2016;128:4590.
- Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant 2011;17:1460-71. [Crossref] [PubMed]
- Chiesa R, Gilmour K, Qasim W, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naive CD4(+) cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol 2012;156:656-66. [Crossref] [PubMed]
- Lamers CH, Wijers R, Van Bergen CA, et al. CD4(+) T-cell alloreactivity toward mismatched HLA class II alleles early after double umbilical cord blood transplantation. Blood 2016;128:2165-74. [Crossref] [PubMed]
- Ruggeri A, Peffault de Latour R, Carmagnat M, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis 2011;13:456-65. [Crossref] [PubMed]
- Komanduri KV, St John LS, De Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007;110:4543-51. [Crossref] [PubMed]
- Early E, Reen DJ. Rapid conversion of naive to effector T cell function counteracts diminished primary human newborn T cell responses. Clin Exp Immunol 1999;116:527-33. [Crossref] [PubMed]
- Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 2010;330:1695-9. [Crossref] [PubMed]
- van Heijst JW, Ceberio I, Lipuma LB, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med 2013;19:372-7. [Crossref] [PubMed]
- Garderet L, Dulphy N, Douay C, et al. The umbilical cord blood alphabeta T-cell repertoire: characteristics of a polyclonal and naive but completely formed repertoire. Blood 1998;91:340-6. [PubMed]
- Muraro PA, Robins H, Malhotra S, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest 2014;124:1168-72. [Crossref] [PubMed]
- Servais S, Baudoux E, Brichard B, et al. Circadian and circannual variations in cord blood hematopoietic cell composition. Haematologica 2015;100:e32-4. [Crossref] [PubMed]
- Niehues T, Rocha V, Filipovich AH, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children -- a Eurocord analysis. Br J Haematol 2001;114:42-8. [Crossref] [PubMed]
- Admiraal R, Lindemans CA, Van Kesteren C, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 2016;128:2734-41. [Crossref] [PubMed]
- Castillo N, García-Cadenas I, Barba P, et al. Early and Long-Term Impaired T Lymphocyte Immune Reconstitution after Cord Blood Transplantation with Antithymocyte Globulin. Biol Blood Marrow Transplant 2017;23:491-7. [Crossref] [PubMed]
- Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 2014;123:126-32. [Crossref] [PubMed]
- Bemark M, Holmqvist J, Abrahamsson J, et al. Translational Mini-Review series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation-revelation of B cell developmental pathways and lineage phenotypes. Clin Exp Immunol 2012;167:15-25. [Crossref] [PubMed]
- Storek J, Ferrara S, Ku N, et al. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant 1993;12:387-98. [PubMed]
- Storek J, Wells D, Dawson MA, et al. Factors influencing B lymphopoiesis after allogeneic hematopoietic cell transplantation. Blood 2001;98:489-91. [Crossref] [PubMed]
- Marie-Cardine A, Divay F, Dutot I, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol 2008;127:14-25. [Crossref] [PubMed]
- Zand MS. B-cell activity of polyclonal antithymocyte globulins. Transplantation 2006;82:1387-95. [Crossref] [PubMed]
- Roll P, Muhammad K, Stuhler G, et al. Effect of ATG-F on B-cell reconstitution after hematopoietic stem cell transplantation. Eur J Haematol 2015;95:514-23. [Crossref] [PubMed]
- Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood 2007;109:2086-8. [Crossref] [PubMed]
- Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease:correlation with increased IL-7. Proc Natl Acad Sci U S A 2006;103:2262-7. [Crossref] [PubMed]
- Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood 2012;119:2949-55. [Crossref] [PubMed]
- Saliba RM, Rezvani K, Leen A, et al. General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biol Blood Marrow Transplant 2015;21:1284-90. [Crossref] [PubMed]
- Barker JN, Hough RE, van Burik JA, et al. Serious infections after unrelated donor transplantation in 136 children:impact of stem cell source. Biol Blood Marrow Transplant 2005;11:362-70. [Crossref] [PubMed]
- Vandenbosch K, Ovetchkine P, Champagne MA, et al. Varicella-zoster virus disease is more frequent after cord blood than after bone marrow transplantation. Biol Blood Marrow Transplant 2008;14:867-71. [Crossref] [PubMed]
- de Pagter PJ, Schuurman R, Visscher H, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant 2008;14:831-9. [Crossref] [PubMed]
- Dumas PY, Ruggeri A, Robin M, et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Société Française de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant 2013;48:253-6. [Crossref] [PubMed]
- Sashihara J, Tanaka-Taya K, Tanaka S, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood 2002;100:2005-11. [PubMed]
- Scheurer ME, Pritchett JC, Amirian ES, et al. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant 2013;48:574-80. [Crossref] [PubMed]
- Mikulska M, Raiola AM, Bruzzi PA, et al. CMV infection after transplant from cord blood compared to other alternative donors: the importance of Donor-Negative CMV serostatus. Biology of Blood and Marrow Transplantation 2012;18:92-9. [Crossref] [PubMed]
- Tomonari A, Iseki T, Ooi J, et al. Cytomegalovirus infection following unrelated cord blood transplantation for adult patients: a single institute experience in Japan. Br J Haematol 2003;121:304-11. [Crossref] [PubMed]
- Beck JC, Wagner JE, Defor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2010;16:215-22. [Crossref] [PubMed]
- Anand S, Thomas S, Hyslop T, et al. Transplantation of Ex Vivo Expanded Umbilical Cord Blood (NiCord) Decreases Early Infection and Hospitalization. Biol Blood Marrow Transplant 2017. [Epub ahead of print].
- Tormo N, Solano C, Benet I, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8(+)and CD4(+)T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant 2011;46:1437-43. [Crossref] [PubMed]
- McGoldrick SM, Bleakley ME, Guerrero A, et al. Cytomegalovirus-specific T cells are primed early after cord blood transplant but fail to control virus in vivo. Blood 2013;121:2796-803. [Crossref] [PubMed]
- Baron F, Nagler A. Novel strategies for improving hematopoietic Reconstruction after allogeneic hematopoietic stem cell transplantation or intensive chemotherapy. Expert Opin Biol Ther 2017;17:163-74. [Crossref] [PubMed]
- Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol 2014;5:68. [Crossref] [PubMed]
- Baron F, Ruggeri A, Nagler A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev Hematol 2016;9:297-314. [Crossref] [PubMed]
- Lucchini G, Perales MA, Veys P. Immune reconstitution after cord blood transplantation: peculiarities, clinical implications and management strategies. Cytotherapy 2015;17:711-22. [Crossref] [PubMed]
- Park KD, Marti L, Kurtzberg J, et al. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood 2006;108:1770-3. [Crossref] [PubMed]
- Hanley PJ, Lam S, Shpall EJ, et al. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. J Vis Exp 2012.e3627. [PubMed]
- O’Reilly RJ, Prockop S, Hasan AN, et al. Virus-specific T-cell banks for “off the shelf” adoptive therapy of refractory infections. Bone Marrow Transplant 2016;51:1163-72. [Crossref] [PubMed]
- Qian C, Campidelli A, Wang Y, et al. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J Hematol Oncol 2017;10:102. [Crossref] [PubMed]
Cite this article as: Servais S, Hannon M, Peffault de Latour R, Socie G, Beguin Y. Reconstitution of adaptive immunity after umbilical cord blood transplantation: impact on infectious complications. Stem Cell Investig 2017;4:40.


