Adjuvant radiotherapy for pathological high-risk muscle invasive bladder cancer: time to reconsider?
Introduction
Every year, 2.7 million patients are diagnosed or treated for bladder cancer globally (1,2). Radical cystectomy plus pelvic lymph node dissection (PLND), associated with neo-adjuvant chemotherapy, is a standard of care for the treatment of non-metastatic muscle-invasive bladder cancer (MIBC) (3). Loco-regional recurrence (LRR) after optimal management remains a significant problem in spite of advances in surgical technique and the addition of chemotherapy (3). Pathologic tumor stage is a predictive factor of LRR, which itself is a harbinger of oncological outcomes for MIBC patients (4-6). The aim of this critical literature review is to define the rationale that supports adjuvant radiotherapy, by identifying which patients could benefit from it, examining the appropriate radiation target volumes and considering the suitable radiotherapy techniques.
Materials and methods
In April 2016, we conducted a literature review using the Medline database. The search identified original articles, literature reviews and meta-analyses concerning the radical treatment of MIBC published between 1970 and 2016. Abstracts in any language other than English, editorials, case series or letters to the editor were excluded from the analysis. Key-words included bladder neoplasm, loco-regional relapse, lymph-node dissection, chemotherapy, radiotherapy. Titles and abstracts were analysed and those not meeting the objective of this review were excluded. Additional publications identified in the references of selected articles and those from a free-text internet search were also included.
Surgery for the treatment of MIBC: what are the overall oncologic outcomes after radical cystectomy?
For the past 30 years, surgical series of MIBC patients did not show any improvement in survival. At 5 years, recurrence-free survival (RFS) varied from 53% to 74%, disease-specific survival (DSS) from 66% to 80%, and overall survival (OS) from 39% to 66% (3). Five-year OS results varied considerably depending on the pathological stage, from 60% for pT2 tumors dropping to 10–40% for ≥ pT3 tumors (6). Pathologic pelvic lymph node involvement (pN+) after a pelvic node dissection occurs in 20% of patients regardless of stage, and positive surgical margin occur in 6%. For locally advanced stages, positive surgical margin rates ranged from 0% to 25% (7-10).
LRR: a key event
How commonly does LRR occur?
The Southwest Oncology Group (SWOG)-intergroup 8710 randomized trial comparing radical cystectomy ± neo-adjuvant chemotherapy, included 317 patients and reported 5-year LRR rates of 32%, 29% and 68% for patients with stage ≥ pT3 tumors, pN+ status and positive surgical margins (R1), respectively. LRR was defined as a pelvic mass confirmed by imaging and biopsy (4). In a retrospective study with a median follow-up of 5 years, Pollack et al. reported LRR rates ranging from 29% to 44% depending on the clinical stage cT3b or cT4b (11,12).
Local recurrence appears to occur quite rapidly after radical cystectomy. Local recurrence is most commonly identified within 9 and 18 months after surgery (8,13). In recommendations, LRR rates vary from 4% to 25%; in reality, it appears difficult to characterize LRR incidence independently because metastatic disease is often detected concomitantly and non-isolated. Hence, studies mostly report disease-free survival (DFS) analyses (3). Similarly, studies reporting on predictive factors for relapse are heterogeneous regarding the definition of relapse, the proposed surgical and peri-operative treatment modalities and the follow-up conditions (14).
How does loco-regional control impact on survival?
In their literature review, Skinner et al. demonstrated that loco-regional control was directly associated with OS benefit. At 5 years, patients who had undergone extensive PLND experienced an improved loco-regional control rate compared to those with a limited lymphadenectomy (85% vs. 63%). The OS was also improved, even in the absence of pelvic lymph node involvement (pN0) (15). These data imply a curative role of the PLND. Indeed, the SWOG in the United States has opened a phase III randomized trial (clinical trials: NCT01224665) comparing standard versus extended (including common iliac and pre-sacral nodes) PLND. Many studies seem to correlate pelvic control and metachronous distant metastases (12,16). In their retrospective study of patients who underwent radical cystectomy for MIBC, Ide et al. showed that LRR was an independent predictive factor for the development of distant metastases. In addition, in 63% of pelvic failures, LRR appeared isolated and separated by a free interval of more than 3 months before the appearance of metastases. In parallel, only 5% of LRR were detected 3 months after the occurrence of metastatic relapse (16). Based on these results, it has been hypothesized that metastatic spread may arise from the LRR. Finally, a study highlighted the positive correlation between local control and DSS in the treatment of MIBC (17).
What are the most common sites for LRR?
In Baumann et al.’s series including 442 patients treated by cystectomy and PLND, LRR sites were mapped during radiological standardized follow-up (18). LRR was defined as a soft-tissue lesion located below the aortic bifurcation. A relapse located above the aortic bifurcation, in the inguinal region or in the pelvic bones was considered distant, and therefore a metastasis. As established by Pollack et al. (12), a 3-month period between the LRR and the occurrence of metastatic lesions had to be respected in order to validate the purely loco-regional nature of the relapse. In the series from Baumann et al., 29% of patients had received chemotherapy. In the 5 years following surgery, 80 patients (18%) presented with a LRR (18).
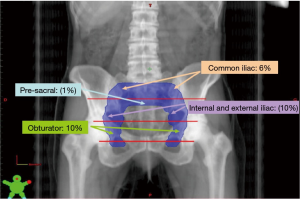
Baumann et al. classified LRR into eight sub-regions of the pelvis: common iliac, internal or external iliac, obturator, pre-sacral and pelvic side-wall nodes, the peri-rectal/rectosigmoid area, the cystectomy bed and ‘other’ (psoas muscle, iliacus muscle, vaginal apex). Approximately, half of failures presented as single lesions. The 5-year overall cumulative incidence was higher for stage ≥ pT3 than for stage ≤ pT2 patients (28% and 8% respectively, P<0.0001). In cases of ≥ pT3 disease, relapses were more frequent in the common iliac (10.1% vs. 3.1%), internal or external iliac (10.8% vs. 4.3%), obturator (10.7% vs. 4.7%), pre-sacral (1.9% vs. 0.0%), and pelvic side-wall (2.4% vs. 0.0%) nodes, as well as in the cystectomy bed (5% vs. 1.3%). Multivariate analysis revealed that pathologic T stage was statistically associated with these sites, but no association was found for relapses in the recto-sigmoid (2% vs. 1.2%) or ‘other’ (1% vs. 0.0%) sub-regions. Stage ≥ pT3 and positive margins were predictive factors for relapse in the cystectomy bed (HR =4.8; P<0.05) as well as in the pre-sacral nodes (HR =7.7; P=0.07). Neither lymph node status (pN0 or pN+) nor the extent of dissection (<10 or >10 resected lymph nodes) were predictive of preferential sites for LRR (18). An example on the LRR pattern of a patient treated by radical cystectomy is shown in Figure 1.
What are the predictive factors of LRR?
A comprehensive analysis of large (more than 150 subjects) surgical series including patients with heterogeneous characteristics revealed that age, surgical technique, volume of the hospital treating the patient, initial tumor stage, pelvic involvement or pathologic stage, extent of lymph node dissection, percentage of nodes resected (lymph node density), as well as surgical margin status could be correlated to LRR. Nevertheless, the predictive nature of these factors often disappears in multivariate analysis and seems to be partially confusing. It is important to note that these series sometimes incorporate peri-operative chemotherapy as part of the treatment plan (4-6,19-26).
In the recent study by Baumann et al., univariate analysis revealed that locally advanced pathological stage of the tumor (≥ pT3), lymph node involvement (pN+), limited node dissection (< ten nodes resected), presence of uretero-hydronephrosis or lymphovascular embolisms, a mixed histology and positive surgical margins are negative predictive factors of 5-year LRR-free survival. On the other hand, age, sex, smoking status, body mass index, chemotherapy administration, extent of node involvement and the type of urinary diversion showed no significant impact. In the multivariate model, only the stage of the disease (≥ pT3) (HR =3.17; P<0.01) and a limited PLND (< ten nodes resected) (HR =2.37; P<0.01) were negative predictive factors. Consequently, three patient subgroups with different LRR risks were defined: low-risk patients (≤ pT2), intermediate-risk patients (≥ pT3 and > ten nodes resected) and high-risk patients (≥ pT3 and < ten nodes resected) with 5-year LRRs of 8%, 23% and 42%, respectively (P<0.01) (5). This stratification was further improved by introducing surgical margin status in the predictive model. High-risk patients were defined as stage ≥ pT3 and < ten nodes resected or positive surgical margins (6). The robustness of this predictive model has been successfully validated in several cohorts, achieving an accuracy higher than 80% (27,28). This nomogram has nevertheless been built in potentially biased surgical cohorts. The same group analyzed the bias inherent to the evolution of surgical techniques over time, to the statistical methods used to estimate LRR risk and to the definition of subgroups with risk of recurrence. The predictive nomogram appears to be valid and could constitute the basis to develop prospective trials to assess the benefit of adjuvant radiotherapy for loco-regional control (14). Table 1 shows the estimated cumulative incidences for 5-year LRR according to the proposed risk stratification in four diverse radical cystectomy cohorts.

Full table
What is the impact of peri-operative chemotherapy on LRR?
The neo-adjuvant chemotherapy approach
Cis-platin based neo-adjuvant poly-chemotherapy is a standard of care (level of evidence 1a) in the treatment of MIBC (3). The aim of this approach is to eliminate micro-metastases, to reduce the size of the tumor in order to facilitate surgery and to prolong patient survival. In the SWOG-Intergroup phase 3 trial, patients were randomly assigned three cycles of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) or no neo-adjuvant chemotherapy. After a median follow-up of 9 years, OS increased (77 vs. 46 months) in the neo-adjuvant chemotherapy arm. However, these results were at the limit of statistical significance (P=0.06) (29). Importantly, the use of neo-adjuvant MVAC did not detectably decrease the risk of LRR (4). The European Organization for Research and Treatment of Cancer (EORTC) also carried out a randomized phase 3 trial assessing the impact of a neo-adjuvant chemotherapy approach (30). Nine hundred and seventy six patients were randomized to receive either no chemotherapy or a triplet regimen in three cycles of cis-platin, methotrexate and vinblastine (CMV) preceding local treatment by cystectomy or radiotherapy. Kaplan-Meier analysis revealed no significant benefit in OS after 3 years of follow-up (P=0.075). A recent update (31), however, has found improvement in OS at 10 years with neo-adjuvant treatment (30% vs. 36%, P=0.037). Similarly, DFS increased 23% (HR =0.77; 95% CI, 0.66–0.90; P=0.001), but no significant benefit was found concerning RFS (P=0.067). Two meta-analyses highlighted that the treatment of MIBC patients with neo-adjuvant poly-chemotherapy based on cis-platin was associated with increased OS (32,33). Nevertheless, a retrospective institutional study from the United States also failed to detect an association between the use of neo-adjuvant chemotherapy and LRR (5).
The adjuvant chemotherapy approach
Results from randomized trials are controversial and prevent the use of adjuvant chemotherapy as standard of care in patients presenting with MIBC (3). Results from Leow et al.’s meta-analysis (34) showed an OS advantage in favor of cis-platin-based adjuvant chemotherapy (HR =0.77; 95% CI, 0.59–0.99; P=0.049). DFS also improved (HR =0.66; 95% CI, 0.45–0.91; P=0.014), especially in patients with lymph node involvement (P=0.01). Nevertheless, the recent 30994 EORTC study did not show any benefit of cis-platin-based immediate adjuvant chemotherapy compared to chemotherapy at the time of recurrence for patients presenting with locally advanced tumor (pT3–T4) and/or lymph node involvement (pN+) at radical cystectomy. A LRR rate of 29% for the immediate chemotherapy arm compared to 43% for the delayed chemotherapy arm was reported, but with no mention of statistical significance (35). Regarding these studies on the use of adjuvant or neo-adjuvant chemotherapy, no significant benefit on loco-regional control is clearly established.
The efficacy and tolerance of perioperative radiotherapy
Perioperative radiotherapy study results
Historically, the retrospective studies of pre-operative radiotherapy [40–50 gray (Gy)] have shown a benefit in LRR-free survival (16–19%) and in OS (12–42%) (36). This benefit appeared specially for stage cT3a-b MIBC patients, but the low tolerance to irradiation seemed to be a limiting factor (36). Six randomized studies have evaluated the contribution of pre-operative radiotherapy (32–54 Gy) and did not find any significant improvement in OS and LRR-free survival. Some of the studies included less common histologies in the epidemiological context of schistosomiasis (37-42). A recent meta-analysis including the latter randomized studies revealed no survival benefit (43). Consequently, pre-operative radiotherapy is no longer indicated in the treatment of patients with MIBC treated by radical surgery.
Post-operative radiotherapy study results
In a notable prospective randomized trial form the National Cancer Institute (NCI) in Egypt, Zaghloul et al. demonstrated the interest of post-operative radiotherapy compared to observation for patients with MIBC (44,45). In the post-operative radiotherapy arm, DFS at 5 years was 44% with conventionally fractionated radiotherapy (CFR) (50 Gy in 25 daily fractions of 2 Gy over 5 weeks) and 49% with hyper-fractionated radiotherapy (HF) (37.5 Gy in 3 daily fractions of 1.25 Gy, with 3-hour inter-fraction intervals over 12 days). This rate dropped to 25% in the observation arm. Loco-regional control at 5 years improved with post-operative radiotherapy: 93% in the CFR arm, 87% in the HF arm and 50% in the cystectomy-only arm (P<0.05). Nevertheless, the population studied by Zaghloul et al. only included 20% of histologies corresponding to urothelial carcinomas more frequently observed in country outside of the Middle East. Another randomized prospective study from the NCI in Egypt compared the use of pre-operative and post-operative radiotherapy, with the same dose scheme in both arms (50 Gy in 25 fractions) (46). OS (53.4% vs. 51.8%, P=0.689) and LRR-free survival (89.3% vs. 80.6%, P=0.410) at 3 years were similar in the pre- and post-operative arms (46). The heterogeneity and small size of the studies above make it difficult to draw definitive conclusions concerning the impact of adjuvant radiotherapy on survival, but a potential trend towards the benefit in terms of LRR is a consistent observation.
Toxicity of peri-operative radiotherapy
Whether adjuvant radiation is tolerable is a matter of controversy. In a series of patients receiving both pre- and post-operative radiotherapy, Reisinger et al. observed a 37% rate of late bowel toxicity of which 22.5% were grade 4 and 7.5% grade 5 (47). On the contrary, in their series of patients treated by adjuvant radiotherapy, Zaghloul et al. (44) reported 18% of late digestive toxicity with CFR and 5% with HF radiotherapy. The study by El-Monim et al. that compared pre- and post-operative radiation (46), reported a 6.5% rate of grade 3 late bowel toxicity. No grade 4 toxicity was observed.
Historically, studies of adjuvant radiation have used 2D radiotherapy and a four-field (opposed anterior-posterior beams and opposed lateral fields) technique. Cranio-caudal limits extended from the L5-S1 intervertebral disc to the lower part of obturators. Lateral limits extended 1–2 cm on both sides of the median basin. At the anteroposterior level, fields extended 2 cm forward of the anterior wall of bladder and up to the anterior edge of the third sacral vertebra. This technique allowed covering the whole outer circumference of the bladder as well as a potential extra-bladder and/or microscopic extension (36). Nevertheless, the doses received by the organs at risk such as the rectum, the femur heads, but particularly the small bowel induced acute and late grade >2 toxicities limited the attractiveness of this approach, especially in western countries. More recently though, investigators have shown in a dosimetric study that modern radiation techniques such as intensity modulated radiation therapy and pencil beam proton therapy can meaningfully reduce dose to GI structures in the post-cystectomy pelvis, likely improving the risk-benefit profile of adjuvant radiation (48).
Why does adjuvant radiotherapy need to be re-evaluated?
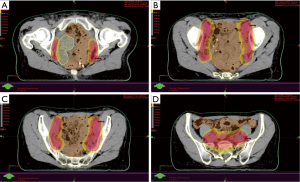
Given the recent data on LRR sites after radical cystectomy and PLND for patients with MIBC, it is now possible to rationalize clinical target volumes (CTVs) in the context of adjuvant radiotherapy (18). The CTVs could be adapted depending on the different at-risk sub-groups previously described and a patient stratification could be applied (6,14). Recently, an international collaboration of radiation oncologists and urologists recommended consensus CTVs that varied depending on surgical margin status of patients (49). For stage ≤ pT2 patients, given the small number of LRR, there is no indication for adjuvant radiotherapy. For stage ≥ pT3 patients and negative margins, the irradiation of common iliac, internal and external iliac, and obturator nodes would allow the inclusion of 76% of potential sites for LRR. Widening the fields at the cystectomy bed as well as the pre-sacral regions increase coverage to 85% and 88% of LRR sites. Finally, for patients with stage ≥ pT3 and positive margins, irradiation of common iliac, internal and external iliac, and obturator regions, of the cystectomy bed and of the pre-sacral region would encompass 79% and 91% of LRR sites (18). The rise of conformal radiation therapy and the progress of tomography have allowed to precisely define CTV and to spare neighboring organs at risk, reducing digestive toxicity. The development of intensity-modulated radiation therapy (IMRT) and of image-guided radiation therapy (IGRT) have notably improved treatment accuracy and allowed to decrease the dose in adjacent tissues. Hence, several studies of pelvic radiotherapy reported a decrease of bowel volume irradiated with current techniques, particularly in cases of post-operatory irradiation, making the post-cystectomy adjuvant approach probably safer (50,51). An example of these technical possibilities is presented in Figure 2.
Conclusions
Radical cystectomy associated to PLND remains a standard of care in the management of localized MIBC. Surgical series report high recurrence rates, especially in patients with locally advanced disease. Early LRR could represent the ground for metastatic spreading and diminish patients’ survival considerably.
Based on this reasoning related to loco-regional control, the role of adjuvant radiotherapy comes to focus. We know to whom, what and how to propose radiotherapy, and French (GETUG-AFU), North-American (NRG), British (NCRI) and Indian (Tata Memorial Hospital) cooperative groups have shown great interest in this approach. We expect future prospective studies to improve the grim prognosis by reducing the risk of relapse and to forge new pathways in the treatment of this disease.
Acknowledgements
We would like to thank Mrs Jone Iriondo-Alberdi for the contribution in the redaction of the English manuscript.
Footnote
Conflicts of Interest: JP Christodouleas discloses employment at Elekta AB; S Shariat owns or co-owns the following patents: methods to determine prognosis after therapy for prostate cancer. Granted 2002-09-06. Methods to determine prognosis after therapy for bladder cancer. Granted 2003-06-19. Prognostic methods for patients with prostatic disease. Granted 2004-08-05. Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010-07-20. He is advisory board member of Astellas, Cepheid, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanofi, Wolff. He is speaker for Astellas, Ipsen, Jansen, Lilly, Olympus, Pfizer, Pierre Fabre, Sanochemia, Sanofi, Wolff. The other authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [Crossref] [PubMed]
- Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004;22:2781-9. [Crossref] [PubMed]
- Baumann BC, Guzzo TJ, He J, et al. A novel risk stratification to predict local-regional failures in urothelial carcinoma of the bladder after radical cystectomy. Int J Radiat Oncol Biol Phys 2013;85:81-8. [Crossref] [PubMed]
- Christodouleas JP, Baumann BC, He J, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer 2014;120:1272-80. [Crossref] [PubMed]
- Yuh B, Wilson T, Bochner B, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol 2015;67:402-22. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [PubMed]
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006;176:2414-22; discussion 2422. [Crossref] [PubMed]
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012;61:1039-47. [Crossref] [PubMed]
- Cole CJ, Pollack A, Zagars GK, et al. Local control of muscle-invasive bladder cancer: preoperative radiotherapy and cystectomy versus cystectomy alone. Int J Radiat Oncol Biol Phys 1995;32:331-40. [Crossref] [PubMed]
- Pollack A, Zagars GK, Cole CJ, et al. The relationship of local control to distant metastasis in muscle invasive bladder cancer. J Urol 1995;154:2059-63; discussion 2063-4. [Crossref] [PubMed]
- Volkmer BG, Kuefer R, Bartsch GC Jr, et al. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? J Urol 2009;181:1587-93; discussion 1593. [Crossref] [PubMed]
- Baumann BC, He J, Hwang WT, et al. Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int J Radiat Oncol Biol Phys 2016;95:703-6. [Crossref] [PubMed]
- Skinner EC, Stein JP, Skinner DG. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol 2007;25:66-71. [Crossref] [PubMed]
- Ide H, Kikuchi E, Miyajima A, et al. The predictors of local recurrence after radical cystectomy in patients with invasive bladder cancer. Jpn J Clin Oncol 2008;38:360-4. [Crossref] [PubMed]
- Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol 2011;185:456-61. [Crossref] [PubMed]
- Baumann BC, Guzzo TJ, He J, et al. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 2013;85:363-9. [Crossref] [PubMed]
- Yossepowitch O, Dalbagni G, Golijanin D, et al. Orthotopic urinary diversion after cystectomy for bladder cancer: implications for cancer control and patterns of disease recurrence. J Urol 2003;169:177-81. [Crossref] [PubMed]
- Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003;169:943-5. [Crossref] [PubMed]
- Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology 2003;61:105-8. [Crossref] [PubMed]
- Cheng L, Weaver AL, Leibovich BC, et al. Predicting the survival of bladder carcinoma patients treated with radical cystectomy. Cancer 2000;88:2326-32. [Crossref] [PubMed]
- Neuzillet Y, Soulie M, Larre S, et al. Positive surgical margins and their locations in specimens are adverse prognosis features after radical cystectomy in non-metastatic carcinoma invading bladder muscle: results from a nationwide case-control study. BJU Int 2013;111:1253-60. [Crossref] [PubMed]
- de Vries RR, Visser O, Nieuwenhuijzen JA, et al. Outcome of treatment of bladder cancer: a comparison between low-volume hospitals and an oncology centre. World J Urol 2010;28:431-7. [Crossref] [PubMed]
- Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227-32. [Crossref] [PubMed]
- Manoharan M, Katkoori D, Kishore TA, et al. Outcome after radical cystectomy in patients with clinical T2 bladder cancer in whom neoadjuvant chemotherapy has failed. BJU Int 2009;104:1646-9. [Crossref] [PubMed]
- Ku JH, Kim M, Jeong CW, et al. Risk prediction models of locoregional failure after radical cystectomy for urothelial carcinoma: external validation in a cohort of korean patients. Int J Radiat Oncol Biol Phys 2014;89:1032-7. [Crossref] [PubMed]
- Novotny V, Froehner M, May M, et al. Risk stratification for locoregional recurrence after radical cystectomy for urothelial carcinoma of the bladder. World J Urol 2015;33:1753-61. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet 1999;354:533-40. [Crossref] [PubMed]
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171-7. [Crossref] [PubMed]
- Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol 2004;171:561-9. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [Crossref] [PubMed]
- Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 2014;66:42-54. [Crossref] [PubMed]
- Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76-86. [Crossref] [PubMed]
- Zaghloul MS. Adjuvant and neoadjuvant radiotherapy for bladder cancer: revisited. Future Oncol 2010;6:1177-91. [Crossref] [PubMed]
- Slack NH, Bross ID, Prout GR Jr. Five-year follow-up results of a collaborative study of therapies for carcinoma of the bladder. J Surg Oncol 1977;9:393-405. [Crossref] [PubMed]
- Anderström C, Johansson S, Nilsson S, et al. A prospective randomized study of preoperative irradiation with cystectomy or cystectomy alone for invasive bladder carcinoma. Eur Urol 1983;9:142-7. [PubMed]
- Blackard CE, Byar DP. Results of a clinical trial of surgery and radiation in stages II and 3 carcinoma of the bladder. J Urol 1972;108:875-8. [PubMed]
- Smith JA Jr, Crawford ED, Paradelo JC, et al. Treatment of advanced bladder cancer with combined preoperative irradiation and radical cystectomy versus radical cystectomy alone: a phase III intergroup study. J Urol 1997;157:805-7; discussion 807-8. [Crossref] [PubMed]
- Ghoneim MA, Ashamallah AK, Awaad HK, et al. Randomized trial of cystectomy with or without preoperative radiotherapy for carcinoma of the bilharzial bladder. J Urol 1985;134:266-8. [PubMed]
- Awwad H, El-Baki HA, El-Bolkainy N, et al. Pre-operative irradiation of T3-carcinoma in bilharzial bladder: a comparison between hyperfractionation and conventional fractionation. Int J Radiat Oncol Biol Phys 1979;5:787-94. [Crossref] [PubMed]
- Huncharek M, Muscat J, Geschwind JF. Planned preoperative radiation therapy in muscle invasive bladder cancer; results of a meta-analysis. Anticancer Res 1998;18:1931-4. [PubMed]
- Zaghloul MS, Awwad HK, Akoush HH, et al. Postoperative radiotherapy of carcinoma in bilharzial bladder: improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys 1992;23:511-7. [Crossref] [PubMed]
- Zaghloul MS, Awwad HK, Soliman O, et al. Postoperative radiotherapy of carcinoma in bilharzial bladder using a three-fractions per day regimen. Radiother Oncol 1986;6:257-65. [Crossref] [PubMed]
- El-Monim HA, El-Baradie MM, Younis A, et al. A prospective randomized trial for postoperative vs. preoperative adjuvant radiotherapy for muscle-invasive bladder cancer. Urol Oncol 2013;31:359-65. [Crossref] [PubMed]
- Reisinger SA, Mohiuddin M, Mulholland SG. Combined pre- and postoperative adjuvant radiation therapy for bladder cancer--a ten year experience. Int J Radiat Oncol Biol Phys 1992;24:463-8. [Crossref] [PubMed]
- Baumann BC, Noa K, Wileyto EP, et al. Adjuvant radiation therapy for bladder cancer: a dosimetric comparison of techniques. Med Dosim 2015;40:372-7. [Crossref] [PubMed]
- Christodouleas JP, Baumann BC, Bosch WR, et al. Development and validation of contouring guidelines for postcystectomy adjuvant radiation of bladder cancer. Int J Radiat Oncol Biol Phys 2015;93:S24-5. [Crossref]
- Wang-Chesebro A, Xia P, Coleman J, et al. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2006;66:654-62. [Crossref] [PubMed]
- Ahamad A, D'Souza W, Salehpour M, et al. Intensity-modulated radiation therapy after hysterectomy: comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys 2005;62:1117-24. [Crossref] [PubMed]


