Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios
Introduction
Male factors are responsible for approximately half of all infertility cases (1). The initial assessment of the male patient involves a conventional semen analysis (SA), which may fail to provide a complete understanding of fertility potential. Due to variations in sperm quantity and quality (2), it is difficult to use SA to make management decisions. Sperm function tests can help assess whether they are likely to complete complex actions such as sperm transport through the female reproductive tract, acrosome reaction and penetration of the zona pellucida (3). However, with the advent of intracytoplasmic sperm injection (ICSI), sperm function tests have fallen out of favor in most andrology laboratories.
Within the last decade, infertility researchers have turned their attention to sperm molecular architecture for good reason—mammalian fertilization and subsequent embryo development depend in part on the inherent integrity of sperm DNA (4). Sperm DNA is bound to protamine and is naturally present in a compact state, protecting it from damage during transport (5). Some damage can occur, which can be repaired in the cytoplasm of the oocyte. When the damage exceeds the cytoplasm’s repair threshold, however, infertility can ensue (6).
Both in vitro and in vivo studies have shown that sperm DNA integrity is negatively associated with fertility (7-10). Elevated Sperm DNA fragmentation (SDF) may affect fertility by hindering fertilization, early embryo development, implantation, and pregnancy (11).
The etiology of SDF is multifactorial. A number of cellular events contribute to impaired fertility and sperm DNA damage including abnormal chromatin packaging and/or remodeling during spermatogenesis (12,13), excessive reactive oxygen species (ROS) production (14,15) and/or decreased seminal antioxidants (16), and apoptotic events during sperm maturation within the epididymis (17). Exposure to environmental toxins and pollutants, drugs, chemo-radiation, cigarette smoking, febrile illness, varicocele and advanced age have also been proposed as factors that can increase SDF (18-20).
While SDF is increasingly being available in the urologists’ armamentarium for the evaluation of infertile men, its accurate clinical implication remains poorly understood. Currently, there seems to be insufficient evidence to support the routine use of SDF in male factor evaluation (21) nevertheless the importance of DNA fragmentation in spermatozoa has been acknowledged in the latest American Urological Association (AUA) and European Association of Urology (EAU) guidelines on male infertility (21,22). Although a precise understanding of the specific utility of such test in different clinical scenarios is still lacking, studies defining specific indications for DNA testing are now emerging (23-25).
This review will help explain the current indications of sperm DNA testing as well as the management of increased SDF. Using clinical scenarios, it is intended to be a useful reference for assisting practicing urologists and reproductive specialists outside the expertise of genetics in identifying the circumstances in which SDF testing should be of greatest clinical value.
Evidence acquisition
A comprehensive search was performed through PubMed up until June 2016. Original and review articles investigating the significance of SDF testing were included. A panel comprised of five urologists (Ahmad Majzoub, Sandro C. Esteves, Edmund Ko, Ranjith Ramasamy, Armand Zini) and one andrologist (Ashok Agarwal) with expertise in male infertility were selected to provide evidence-based recommendations. These colleagues have been considered opinion leaders according to the following criteria: clinical experience with the use of SDF testing in male infertility scenarios and/or assisted reproductive technology, demonstrated by peer-reviewed publications and presentation at major international meetings.
For the first part, the group of experts prepared an illustrative review about the tests clinically available for SDF testing. In the second part, clinical scenarios commonly found in the urologic office of participants were described, followed by an evidence-based analysis of the clinical utility of SDF under that particular case and recommendations by consensus.
Evidence synthesis
SDF tests
There are two types of assays that have been developed to measure SDF: those that can directly measure the extent of DNA fragmentation through the use of probes and dyes and those that measure the susceptibility of DNA to denaturation, which occurs more commonly in fragmented DNA. The eight described methods to assess SDF are briefly presented below and summarized in Table 1. The most commonly used tests are terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), the sperm chromatin dispersion test (SCD), and the sperm chromatin structure assay (SCSA) (26).
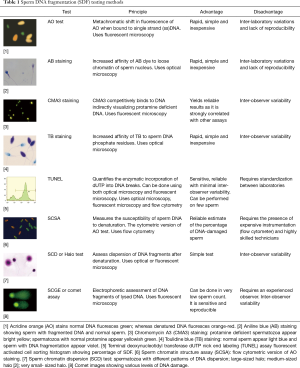
Full table
Acridine orange (AO) test
AO is a nucleic acid-selective cationic fluorescent dye that interacts with double strand (ds)DNA or single strand (ss)DNA by intercalation or electrostatic attraction, respectively. When bound to dsDNA, it mimics fluorescein, having an excitation maximum at 502 nm and an emission maximum at 525 nm (green). However, when it associates with ssDNA, the excitation maximum shifts to 460 nm (blue) and the emission maximum shifts to 650 nm (red). It is this metachromatic shift that allows the extent of sperm DNA damage to be determined.
Following mild acid denaturation of sperm DNA, AO binds to ds (i.e., non-denatured) DNA producing green fluorescence or to ss (i.e., denatured) DNA producing red fluorescence. The number of cells with red fluorescence can be measured, which approximates the quantity of sperm with DNA damage in the sample.
There are a number of advantages to this technique—it is fast, simple and inexpensive. Nonetheless, the presence of heterogeneous slide staining with multiple intermediate colors and the considerable inter-laboratory variations and lack of test reproducibility make AO a less reliable test of SDF (27,28).
Aniline blue (AB) staining
AB staining is another technique that depends on the use of dyes. AB is an acidic dye that has a great affinity for lysine-rich histones in the nucleus of immature sperm, which stain blue (29). On the other hand, the protamine-rich nuclei of mature spermatozoa with abundant arginine and cysteine react negatively and remain unstained. Increased AB staining of sperm indicates loose chromatin packing.
This is a simple and inexpensive technique requiring a simple bright field microscope for analysis. While the results of AB staining correlate well with those of the AO test (30), heterogenous slide staining remains a prominent drawback of this technique.
Toluidine blue
Toluidine blue is a basic thiazine metachromatic dye with a high affinity for sperm DNA phosphate residues. It becomes heavily incorporated in damaged chromatin where it produces a violet-blue intense coloration. The sample can be analyzed using an ordinary microscope. However, intermediate coloration increases the inter-observer variability. Toludine blue staining generally correlates well with other methods of sperm DNA testing (31).
Chromomycin A3 (CMA3) staining
CMA3 is a guanine-cytosine-specific fluorochrome that competes with protamines for the same binding sites in the DNA. Therefore, when the test is highly positive, it reflects a low DNA protamination state associated with poorly packaged sperm chromatin (32). When compared with AB staining, the CMA3 assay provided equivalent results during sperm chromatin evaluation (32,33).
SCSA (6)
The SCSA measures the susceptibility of sperm DNA to denaturation when it is exposed to heat or acids. It is a flow cytometry-based assay that can evaluate large numbers of cells (10,000 cells) rapidly and robustly (11). It is the flow cytometric version of AO staining in which the extent of DNA denaturation is determined by measuring the metachromatic shift from green fluorescence to red fluorescence (34). An advantage of SCSA is that it has a standardized protocol for all users, minimizing inter-laboratory variation. The clinical threshold is an SDF index of 30% meaning that samples can contain up to 30% of DNA damaged cells and still be considered normal. Its disadvantage is that it requires a flow cytometer, making it less attractive to clinical andrology laboratories due to equipment costs. Without this equipment, specimens must be sent to a central laboratory, which lengthens the turnaround time.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
As its name suggests, this assay detects “nicks” or free ends of DNA by utilizing fluorescent nucleotides (35). The samples are evaluated with flow cytometry or a standard fluorescence microscope; the assay quantifies the incorporation of dUTP into ss- or dsDNA breaks through an enzymatic reaction creating a signal, which increases with the number of DNA breaks. While TUNEL is believed by many to be the gold standard for SDF testing (36), it lacks strict standardization, which makes comparison between laboratories more difficult and explains why many clinical thresholds exist (37).
Mitchell and colleagues recently modified the TUNEL assay to reduce inter-laboratory discrepancies. They attempted to relax the entire chromatin structure with dithiothreitol (DTT) before fixation to allow access to all “nicks” (38). Recently, a modified TUNEL protocol using bench top flow cytometer was shown to measure SDF accurately and in a large number of samples simultaneously (39).
Single cell gel electrophoresis assay (comet)
The comet assay (40) quantifies the amount of DNA damage per spermatozoon. The name of the assay comes from the mass of DNA fragments that stream out of the sperm head, resembling a ‘comet’ tail. The staining intensity and length of the comet tail represents the amount of migrated DNA, indicating different degrees of SDF (40). One major advantage of this assay is that it can be used in patients with severe oligozoospermia as only about 5,000 sperm are required (11). In addition to its ability to detect single and double strand breaks, the comet assay can identify altered bases. While the comet assay is informative because it is possible to analyze the different types of DNA damage in a single cell using neutral and alkaline electrophoresis, the method is not suited for rapid diagnosis and requires highly specialized personnel to analyze the results.
SCD
The SCD, also known as the Halo test (41), is based on the concept that sperm with fragmented DNA do not produce the characteristic halo of dispersed DNA loops that are observed in sperm with non-fragmented DNA following acid denaturation and removal of nuclear proteins.
Agarose-embedded sperm are subjected to a denaturing solution to remove nuclear proteins and expose the damaged DNA (ssDNA, fragmented DNA). After lysis, spermatozoa with intact DNA exhibit characteristic loops around the sperm nucleus (creating a halo effect) (42) whereas spermatozoa with DNA damage do not.
Halos can be observed via bright field microscope if the staining is done with an eosin and azure B solution. If DNA-directed fluorochromes are used, the analysis requires a fluorescence microscope. The technique is simple and does not require complex instrumentation. There may be some inter-observer subjectivity, however, when categorizing the halos, which otherwise is a competent assay for SDF quantification.
Generally, SDF measurement provides a more accurate representation of a male’s fertility status principally because it has a lower biologic variability than conventional semen studies (43-45). Despite that, a few obstacles still hinder its wide availability among andrology laboratories or prevent it from being routinely used for male fertility evaluation. We still do not understand the true nature of sperm DNA damage and exactly what it is that each test measures. The prognostic accuracy of SDF tests depends on the precision of their technique of implementation. Considerable inter-laboratory variability exists, influencing the reliability of test results. Furthermore, thresholds (or cutoffs) for many of these tests have not been clearly described. Finally, SDF results can be greatly affected by laboratory or clinical conditions such as the degree of sperm nuclear decondensation or the ejaculation abstinence period (46,47).
Indications for SDF testing
Clinical varicocele
Clinical scenario #1: a 28-year-old man presents with primary infertility of 2 years’ duration. SA shows mild oligozoospermia [based on the 2010 WHO reference ranges (48)]. His wife is 28 years old with a normal fertility evaluation. During physical examination, a small varicocele is detected during Valsalva maneuver [grade 1 based on Dubin & Amelar classification (49)]. He is otherwise healthy with no significant reproductive or medical/surgical history.
Varicocele is a clinical condition associated with considerable debate. While its detrimental effects on semen parameters and overall pregnancy rate are well documented (50), and it is prevalent in up to 20% of the adult male population (51), a substantial number of affected men are able to conceive without difficulties.
Because surgery is the mainstay treatment for varicocele, efforts were made to search for adjunct laboratory tests that would help improve patient selection and identify those who would benefit most after surgery. Interest in SDF testing began after a significantly positive association with varicocele was detected in early reports (7).
The occurrence of SDF can be explained by a brief understanding of varicocele pathophysiology. While several theories have been proposed to explain the deleterious effects of varicocele on testicular function, testicular hyperthermia is perhaps the most commonly accepted one. It has long been observed that minor changes in testicular temperature can affect spermatogenesis (52) as many of the enzymes responsible for DNA synthesis in the testis are temperature dependent (53), mainly favoring temperatures lower than 98.2±0.72 °F (normal body temperature). The anatomic position of the testis in the scrotal sac together with the countercurrent cooling mechanism provided by the pampiniform plexus of veins are responsible regulating testicular temperature (54). The blood stasis that occurs with a varicocele disrupts the countercurrent cooling effect, causing the testicular temperature to rise, which subsequently results in abnormal DNA synthesis and defective spermatogenesis (55).
Intratesticular blood stasis is another theory that may help explain the occurrence of DNA damage in men with varicocele. The abnormal dilatation of the pampiniform plexus of veins reduces testicular blood inflow resulting in hypoxia and oxidative stress. It is generally accepted that oxidative stress is the most important intermediary state in the development of testicular dysfunction (56,57). A small amount of oxidative stress is required for normal sperm functions including sperm capacitation, hyperactivation, and sperm-oocyte fusion along with other critical cellular processes. However, negative consequences occur when levels exceed antioxidant capacity (58). Spermatozoa are extremely sensitive to oxidative stress as they lack the necessary enzyme repair systems (59). As a result, free radicals negatively affect spermatozoa in three main ways: membrane lipid peroxidation, DNA damage, and induction of apoptosis (60,61). DNA damage occurs because free radicals directly attack the purine and pyrimidine bases destabilizing the DNA molecule and causing anomalies such as point mutations, polymorphisms, deletions, translocations, and double-stranded breaks (62).
SDF levels in varicocele patients
Many studies have explored the prevalence of SDF in varicocele patients. In their literature review, Zini and Dohle (63) identified 16 case-control studies that investigated the association between varicocele and SDF. In nine studies, infertile men with varicocele were compared to infertile men without varicocele. A significant association between infertile men with varicocele and sperm DNA damage was demonstrated in 4 of the 9 studies. In the remaining 7 studies involving fertile men, there was also an association between varicocele and SDF (63). In a recent multicenter study involving 593 men, Esteves et al. (23) evaluated SDF in various etiologic conditions, including 98 men with varicocele and 80 fertile controls. The highest SDF rates were observed in the men with varicocele (35.7%±18.3%) and in those with leukocytospermia (41.7%±17.6%). Rates of SDF in testicular cancer and repeated in vitro fertilization (IVF)/ICSI failure (P<0.05) were high in these two groups as well.
Interestingly, a specific subpopulation with massive nuclear SDF, so-called degraded sperm, was distinguished from the whole population of fragmented sperm. This class was not exclusive of varicocele patients but was overrepresented in this group (P<0.001). Using receiver operating characteristics (ROC) analysis, DDSi—defined as the proportion of degraded sperm in the whole population of spermatozoa with fragmented DNA—identified patients with varicocele with 94% accuracy (23).
Influence of varicocele surgery on SDF
The association between varicocele and increased SDF was further validated by several investigators who examined the effect of varicocelectomy on sperm DNA damage. Zini and Dohle reviewed 511 patients belonging to 9 prospective and 3 retrospective studies (63) comparing men with clinical varicocele with a control group. A reduction of SDF (measured with 8-hydroxy-2-deoxyguanosine, COMET, TUNEL, SCSA and AB staining) was reported by all studies after varicocelectomy (63).
More recent studies have reported similar results but further assessed the impact of this reduction on pregnancy rates. Smit et al. examined 49 patients who had a 1-year history of infertility and underwent varicocelectomy (64). Postoperatively, SDF assessed by SCSA significantly decreased from 35.2% to 30.2% (P=0.019). Natural pregnancy was reported by 37% of patients who had a significantly lower SDF than patients who did not conceive naturally or who conceived with assisted reproduction (64).
Ni et al. compared 42 subfertile patients with left clinical varicocele with 10 normozoospermic healthy donors with proven fertility (65). Patients were evaluated with polymerase chain reaction and SCSA to analyze the sperm protamine-1/2 mRNA ratio and DNA fragmentation index before and after surgery (65). The female partners of 10 of the patients naturally conceived 6 months after surgery (23.81%), and these men had a statistically significant reduction in their protamine-1/2 mRNA ratio and DNA fragmentation index after surgery (65). It has also been shown in a meta-analysis of seven studies that varicocelectomy decreases SDF with a mean difference of −3.37% (95% CI, −4.09 to −2.65; P<0.00001) compared to no treatment (66).
Low grade varicocele and SDF
The patient in this clinical scenario had a low-grade varicocele, which further complicates the case. Little is known about the impact of varicocele grade on SDF. Almost all studies exploring the association between DNA damage and varicocele failed to examine this association among different grades. Sadek et al. reported similar preoperative measurements in clinical grades 2 and 3 while evaluating the influence of varicocelectomy on SDF measured with AB staining (67). In the aforementioned study, only grade 3 varicocele patients had a statistically significant reduction in SDF after surgery (67). Ni et al. reported a significant reduction in the protamine-1/2 mRNA ratio in grade 3 varicocele and a significant reduction in DNA fragmentation in grades 2 and 3 disease after surgery (65).
Clearly, there is insufficient evidence to highlight the clinical utility of DNA fragmentation testing in low-grade varicocele. As such, clinical decisions are based on the available literature, which has consistently shown a significant association between low-grade varicocele and subfertility. A recent study evaluated 482 infertile patients with varicocele who underwent surgical ligation (68). There was a statistically significant improvement in semen parameters after surgery in all three grades of varicocele. More importantly, lower grade varicocele patients had postoperative natural pregnancies that were similar to those of grade 3 varicocele patients (68). The authors recommend SDF testing for patients with low-grade varicocele and borderline SA because it would aid in surgical decision making. Furthermore, surgery recommendations based solely on conventional SA results, especially in this subset of patients, could miss those with already compromised sperm function and otherwise “normal” conventional parameters.
Recommendation
While further studies are required, current evidence suggests that DNA fragmentation testing may allow clinicians to better select varicocelectomy candidates among those men with clinical varicocele and borderline to normal semen parameters (Table 2). SDF is recommended in patients with grade 2/3 varicocele with normal conventional semen parameters and in patients with grade 1 varicocele with borderline/abnormal conventional semen parameter results (Table 3, grade C recommendation).
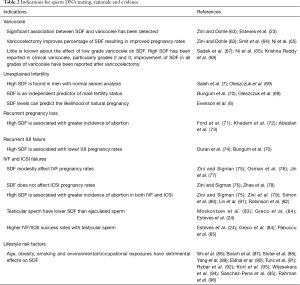
Full table

Full table
Unexplained infertility/recurrent pregnancy loss/intrauterine insemination (IUI) failure
Clinical scenario #2: a 29-year-old man presents with secondary infertility of 3 years’ duration. He was evaluated with several conventional semen analyses, the results of which were within reference limits (48). His wife is 24 years old with a normal fertility evaluation. The couple has a history of three miscarriages, all of which occurred before the 12th week of gestation. Subsequently, they underwent 2 IUI cycles (total motile sperm inseminated >5 million) with no clinical pregnancy. The man is otherwise healthy with no significant reproductive or medical/surgical history.
Unexplained infertility is a term given when the results of a fertility evaluation are normal. These include SA, ovulation assessment and a hysterosalpingogram. While estimates of the prevalence rate of unexplained infertility vary, it is thought to occur in about 10–30% of couples seeking evaluation (97,98) and is perhaps an obvious demonstration of the limitations of conventional semen testing. Furthermore, it has changed our understanding of the pathophysiology of infertility and initiated the search for new diagnostic tools that can further expand our knowledge (99).
SDF in unexplained infertility
Over the last few years, several studies have investigated the correlation between SDF and conventional sperm parameters (100). Some studies reported an inverse association between DNA fragmentation rates and sperm quality overall, as assessed by sperm concentration, motility and morphology (101-104). Several other studies failed to find such a significant association (105,106). Thus, men with unexplained infertility may indeed have a high SDF index, suggesting that impairment of sperm DNA integrity can arise in men with otherwise normal semen parameters (70). Oleszczuk et al. compared 119 men with unexplained infertility to 95 men with proven fertility; the SDF index was above 30% in 17.7% of men with unexplained infertility and in 10.5% of the proven fertile men (P=0.005) (69). In another study, Saleh et al. observed that the SDF index, assessed by the SCSA assay, was higher in infertile men with normal SA (23%; interquartile range, 15–32%) than in fertile controls (15%; interquartile range, 11–20%) (8).
SDF and natural pregnancy
SDF has been found to be a valuable prognostic tool in assessing the chances of natural pregnancy in couples. The chances of natural pregnancy are reduced when the SDF index, measured by SCSA, is between 20–30% and is virtually nonexistent when the SDF index is higher than 30% (6). A meta-analysis involving three studies and 616 couples demonstrated that a high SDF, determined by the SCSA test, was associated with failure to achieve natural pregnancy with an odds ratio (OR) of 7.01 (95% CI, 3.68– 13.36) (79).
Furthermore, a few studies have linked SDF to recurrent miscarriage, defined as three consecutive pregnancy losses prior to 20 weeks’ gestation (71). Using SCD, Khadem et al. compared 30 couples with RSA to another 30 control couples (72). The SDF was higher in the RSA group than in the control group (43.3% vs. 16.7%, P=0.024) (72). Another study using the SCD test similarly demonstrated a significantly higher SDF in the RSA group compared to the control group (P≤0.05) (73).
SDF and IUI success rates
High levels of SDF may be associated with lower IUI pregnancy rates. Duran et al. evaluated semen samples from 154 IUI cycles. SDF was measured using TUNEL or AO testing. The SDF level was significantly higher among the failed cycles, where no woman inseminated with a sample having >12% of sperm with fragmented DNA, by TUNEL, achieved a pregnancy (74). Another study by Bungum et al. (70) measured SDF using SCSA in 387 IUI cycles. They reported significantly lower biochemical pregnancy (3% vs. 24%), clinical pregnancy (3% vs. 23.7%) and delivery rates (1% vs. 19%) in patients with an SDF index >30% vs. ≤30%, respectively (70).
Recommendation
A high DNA fragmentation index in clinical scenario #2 patient would provide a possible explanation for RSA and IUI failure. Therefore, it is reasonable to offer SDF testing to infertile couples with RSA or prior to initiating IUI (Table 2) as these couples may be better served by IVF or ICSI sooner rather than later (Table 3, grade C recommendation).
IVF and/or ICSI failure
Clinical scenario # 3: a 33-year-old man presents with secondary infertility. A previous SA revealed oligoasthenoteratozoospermia, and the couple was counseled to undergo IVF. After an unsuccessful IVF cycle, they were subjected to an ICSI cycle that resulted in a clinical pregnancy. However, 10 weeks after pregnancy initiation, his wife had a miscarriage.
SDF effect on IVF success rate
The relationship between SDF and the outcomes of conventional IVF has been extensively investigated. Major controversies exist in this particular topic and are principally related to the heterogeneous nature of the conducted studies. Multiple factors may affect the outcome measures such as the assays used to measure DNA fragmentation, female age and fertility status and the source of the utilized sperm. Two systematic reviews have reported a modest relationship between sperm DNA damage and pregnancy rates with IVF (75,107). Zini and Sigman evaluated 9 IVF studies (6 using TUNEL and 3 SCSA) and reported lower pregnancy rates in patients with a high SDF with a combined OR of 1.57 (95% CI, 1.18–2.07; P<0.05). Likewise, another review involving 553 patients who underwent conventional IVF showed a statistically significant association between SDF (measured by TUNEL, SCSA and COMET) and pregnancy rate with an OR of 1.27 (95% CI, 1.05–1.52; P=0.01) (76). However, delivery rates were not analyzed, and subgroup analyses indicated that the SDF measurement method influenced the magnitude of effect size.
Aiming to understand female factor contribution, Jin et al. assessed the influence of SDF on the clinical outcomes of assisted reproductive technology (ART) in women with normal ovarian reserve versus reduced ovarian reserve (77). SDF, measured with SCD, significantly affected IVF outcome only in the patients with reduced ovarian reserve. The authors concluded that oocyte quality may be the pivotal determinant for the negative effect of SDF (77).
SDF effect on ICSI success rate
The impact of SDF on ICSI has also been studied. While controversy remains, compelling evidence suggests SDF has a negligible effect on ICSI outcome measures. The systemic review by Zini and Sigman failed to find a significant association between SDF and ICSI pregnancy rates (combined OR, 1.14; 95% CI, 0.86–1.54) (75). Another meta-analysis of 2,756 couples revealed that a lower pregnancy rate in the context of high SDF was noted only in the patients undergoing conventional IVF but not ICSI (78). This difference in outcome measures between conventional IVF and ICSI cycles may be explained by technical differences between the two methods of ART. In IVF, the gametes are subjected to prolonged culture, which may augment the effect of SDF. Indeed, culture media can significantly influence IVF outcomes as reported by Dumoulin et al. (108) who demonstrated that the birth weight of IVF babies can be markedly influenced by minor differences in culture conditions. Conversely, ICSI sperm are injected directly into the optimal environment of the oocyte soon after ejaculation, which may protect them from culture media or laboratory-induced damage. Furthermore, during IVF, the oocytes are exposed to marked oxidative stress (resulting from ROS accumulation in vitro and the absence of endogenous defense mechanisms) that is thought to be the principle cause for SDF in the first place (109). Meanwhile in ICSI, the oocyte is protected from this attack and uses its energy to repair the damage in the sperm immediately following fertilization (110).
SDF and risk of pregnancy loss after ART
Several studies have reported a relationship between SDF and pregnancy loss after both conventional IVF and ICSI (78-82). The systematic review by Zini and Sigman showed that SDF was associated with a significant increase in the rate of pregnancy loss after IVF and ICSI with a combined OR of 2.48 (95% CI, 1.52–4.04; P<0.0001) (75). Another review of 16 cohorts utilizing AO, TUNEL and COMET for DNA fragmentation measurement confirmed a similar result where a significant increase in pregnancy loss was noticed in patients with high DNA damage compared with those with low DNA damage [risk ratio (RR) =2.16 (95% CI, 1.54–3.03; P<0.001)] (82).
Several strategies have been proposed to minimize the influence of abnormal chromatin integrity on ART outcomes. They include: intake of oral antioxidants (111), varicocele ligation (66), frequent ejaculation (112) and sperm selection methods such as magnetic cell sorting (113) or intracytoplasmic morphologically selected sperm injection (114). While varying success rates for these strategies have been reported to reduce SDF, their effect on ART outcomes is still unknown, thus questioning the clinical value of routine application in cases of high SDF before ART (115). Another more promising maneuver is the utilization of sperm harvested from the testes instead of ejaculated sperm. It is believed that most DNA damage occurs during the epididymal transit of sperm (116,117). This is mainly because the sperm is more vulnerable to DNA damage before disulphide cross-linking of its chromatin occurs in the epididymis (118). A few reports have confirmed this phenomenon by finding significantly higher levels of SDF in ejaculated sperm compared with testicular sperm (24,83,84).
Greco et al. observed that SDF levels, as assessed by TUNEL, were lower in testicular sperm (4.8%±3.6%) than in ejaculated sperm (23.6%±5.1%; P<0.001), and they reported higher pregnancy rates by ICSI using testicular sperm (44.4% vs. 6%; P<0.05) (84). In a recent prospective observational study, Esteves et al. compared 81 testicular sperm cycles to 91 ejaculated sperm cycles in couples whose male partners had oligozoospermia and high SDF (24). Despite reporting a significantly higher fertilization rate with ejaculated sperm, the live birth rate was significantly higher and the miscarriage rate significantly lower in the testicular sperm cycles (24). The authors also found that SDF assessed by the SCD method was 5-fold lower in testicular sperm than in ejaculated sperm (40.7%±9.9% vs. 8.3%±5.3%; P<0.001). In another study of similar design but involving normozoospermic men with high SDF, the fertilization rate and miscarriage rate did not differ; however, a significantly higher pregnancy rate was reported in testicular sperm cycles (85).
Recommendation
While further research in this area is still warranted, DNA fragmentation testing in patients with recurrent ART failure is indicated as it can provide useful prognostic information on subsequent ART cycles (Table 2). Several studies have shown some benefit in using testicular sperm rather than ejaculated sperm in men with oligozoospermia, high SDF and recurrent IVF failure (Table 3, grade B–C recommendation).
Borderline abnormal (or normal) SA with risk factors
Clinical case #4: a 48-year-old man working at a pesticide factory presents with primary infertility of 6 years’ duration. He has smoked 1 pack of cigarettes per day for the past 25 years. His body mass index is 46 kg/m2 while the rest of his physical examination is otherwise unremarkable. His SA reveals mild oligoasthenoteratozoospermia.
This scenario highlights the influence different modifiable lifestyle factors have on male fertility. SDF is influenced by a number of factors that disrupt the balance between oxidants and reductants. As stated previously, oxidative stress is the key to the pathophysiology of male infertility. Like any other cell in the body, spermatozoa produce small amounts of ROS during mitochondrial energy production (119). These are generally counterbalanced by antioxidants in the mitochondria and in seminal fluid (120). An imbalance between ROS and antioxidants triggers a state of oxidative stress, which may damage sperm DNA.
Lifestyle influence on SDF
A number of lifestyle factors have been linked with oxidative stress-induced SDF. Age is a non-modifiable example (18,121). Advancing male age has been associated with increased frequency of sperm DNA defects (86-88). The most commonly accepted hypothesis is that it occurs secondary to age-associated increases in oxidative stress (18).
Smoking is the primary cause of preventable illness—it affects nearly every organ of the body. Cigarette smoke contains more than 7,000 chemicals (122) that can adversely affect fertility in a number of ways. Studies have confirmed that smoking has a detrimental effect on conventional semen parameters (123), sperm fertilizing capacity (124) and risk of infertility (89). SDF is also shown to be consistently higher in smokers than in nonsmokers (35). In one study, patients with idiopathic infertility were categorized into three groups: fertile non-smokers (n=16), infertile non-smokers (n=36), and infertile smokers (n=34). All patients underwent SA and SDF testing by SCSA. The percentage of DNA fragmentation was significantly higher in the infertile smokers than in the infertile nonsmokers. Moreover, significant negative correlations were noticed between the degree of DNA damage and worsening of semen parameters (90). Chemicals such as nicotine (125), cadmium (126), lead (127) and benzopyrene (128) were specifically investigated and found to cause sperm DNA damage.
Obesity is another factor associated with male infertility and abnormal semen parameters secondary to endocrine dysregulation, increased scrotal temperatures, fatty accumulation of toxins and/or altered sexual health (129). Its influence on SDF has been investigated in several studies with conflicting results. While a few reports failed to detect a significant association (91,92), larger studies did confirm its presence (93,130,131). Kort et al. evaluated 520 male partners of infertile couples using SCSA (93) and found a positive correlation between body mass index and SDF, with the mean SDF rising from 19.9% in men with a normal body mass index (20–24 kg/m2) to 27.0% in obese men (>30 kg/m2) (93). Similar results were also reported in studies utilizing COMET (131) or TUNEL (130) for DNA fragmentation measurement.
Occupational exposure is yet another subject that is considerably linked to male infertility. Many toxins and/or pollutants have been described, which cannot possibly be completely covered in this publication. Wijesekara et al. (94) interviewed 300 men who were undergoing infertility evaluation looking for the duration of and physical distance from exposure to environmental and occupational chemicals. They reported lower sperm parameters in the exposed group when compared to the non-exposed group. Lead and cadmium were detected in 38.3% and 23% of exposed men, respectively, and their levels were inversely related to the distance from the source of environmental or occupational exposure (94).
Exposure to organochlorine pollutants such as polychlorinated biphenyls and metabolites of dichlorodiphenyltrichloroethane has been associated with DNA fragmentation in spermatozoa. Using SCSA, Sánchez-Peña et al. reported a significant influence from exposure to organophosphorus pesticides by which 75% of exposed workers had a SDF >30% whereas unexposed controls had mean SDF of 9.9% (95).
Bisphenol A is another compound widely utilized in plastic containers used in food and drink industries. Rahman et al. recently revealed that high concentrations of BPA can alter sperm function, fertilization, and embryonic development via regulation and/or phosphorylation of fertility-related proteins in spermatozoa (96). Bisphenol A was also found to alter sperm DNA integrity. Wu et al. incubated semen samples in 1 and 10 µM of bisphenol A and reported a significant direct correlation between SDF and bisphenol A concentrations in vitro (P<0.001) (132).
Recommendation
Infertile men with evidence of exposure to pollutants or those found to have a modifiable lifestyle risk factor during evaluation should be offered SDF testing (Table 2). The sperm DNA test can help reinforce the importance of lifestyle modification (e.g., cessation of cigarette smoking, antioxidant therapy), predict fertility and monitor the patient’s response to intervention (Table 3, grade C recommendation).
Conclusions
Sperm DNA is an integral element in the success of human reproduction. There is fair evidence indicating that SDF testing is a useful diagnostic tool in male fertility evaluation. While it has been extensively researched over the past two decades, newer studies help us clarify the role of SDF testing and its indications. SDF should be included in the evaluation of male factor fertility along with SA.
Acknowledgements
A Agarwal acknowledges the support for this work by the American Center for Reproductive Medicine, Cleveland, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Esteves SC, Sharma RK, Gosálvez J, et al. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol 2014;46:1037-52. [Crossref] [PubMed]
- Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003;9:331-45. [Crossref] [PubMed]
- Erenpreiss J, Spano M, Erenpreisa J, et al. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl 2006;8:11-29. [Crossref] [PubMed]
- Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-49. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril 2003;80:1431-6. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Nada EA, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 2003;79 Suppl 3:1597-605. [Crossref] [PubMed]
- Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online 2006;12:466-72. [Crossref] [PubMed]
- Spanò M, Bonde JP, Hjøllund HI, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000;73:43-50. [PubMed]
- Lewis SE, John Aitken R, Conner SJ, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online 2013;27:325-37. [Crossref] [PubMed]
- Sakkas D, Moffatt O, Manicardi GC, et al. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod 2002;66:1061-7. [Crossref] [PubMed]
- Shamsi MB, Kumar R, Dada R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J Med Res 2008;127:115-23. [PubMed]
- Moustafa MH, Sharma RK, Thornton J, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod 2004;19:129-38. [Crossref] [PubMed]
- Venkatesh S, Riyaz AM, Shamsi MB, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia 2009;41:251-6. [Crossref] [PubMed]
- Shamsi MB, Venkatesh S, Kumar R, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys 2010;47:38-43. [PubMed]
- Gosálvez J, López-Fernández C, Fernández JL, et al. Unpacking the mysteries of sperm DNA fragmentation: Ten frequently asked questions. Journal of Reproductive Biotechnology and Fertility 2015;4:1-16. [Crossref]
- Sharma R, Biedenharn KR, Fedor JM, et al. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol 2013;11:66. [Crossref] [PubMed]
- Sharma R, Harlev A, Agarwal A, et al. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol 2016;70:635-45. [Crossref] [PubMed]
- Rubes J, Selevan SG, Evenson DP, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod 2005;20:2776-83. [Crossref] [PubMed]
- Jarow J, Sigman M, Kolettis PN, et al. The optimal evaluation of the infertile male: best practice statement reviewed and validity confirmed 2011. Available online: https://www.auanet.org/education/guidelines/male-infertility-d.cfm
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on male infertility. Available online: https://uroweb.org/guideline/male-infertility/
- Esteves SC, Gosálvez J, López-Fernández C, et al. Diagnostic accuracy of sperm DNA degradation index (DDSi) as a potential noninvasive biomarker to identify men with varicocele-associated infertility. Int Urol Nephrol 2015;47:1471-7. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Majzoub A, Esteves SC, Gosálvez J, et al. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl 2016;18:205-12. [Crossref] [PubMed]
- Shamsi MB, Imam SN, Dada R. Sperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of infertility. J Assist Reprod Genet 2011;28:1073-85. [Crossref] [PubMed]
- Chohan KR, Griffin JT, Lafromboise M, et al. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl 2006;27:53-9. [Crossref] [PubMed]
- Auger J, Mesbah M, Huber C, et al. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl 1990;13:452-62. [Crossref] [PubMed]
- Erenpreiss J, Bars J, Lipatnikova V, et al. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 2001;22:45-53. [PubMed]
- Hammadeh ME, Stieber M, Haidl G, et al. Association between sperm cell chromatin condensation, morphology based on strict criteria, and fertilization, cleavage and pregnancy rates in an IVF program. Andrologia 1998;30:29-35. [Crossref] [PubMed]
- Manicardi GC, Bianchi PG, Pantano S, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod 1995;52:864-7. [Crossref] [PubMed]
- Franken DR, Franken CJ, de la Guerre H, et al. Normal sperm morphology and chromatin packaging: comparison between aniline blue and chromomycin A3 staining. Andrologia 1999;31:361-6. [Crossref] [PubMed]
- Darzynkiewicz Z, Traganos F, Sharpless T, et al. Thermal denaturation of DNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res 1975;90:411-28. [Crossref] [PubMed]
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl 2011;34:2-13. [Crossref] [PubMed]
- Sharma R, Ahmad G, Esteves SC, et al. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet 2016;33:291-300. [Crossref] [PubMed]
- Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184-91. [Crossref] [PubMed]
- Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003;24:59-66. [PubMed]
- Ankem MK, Mayer E, Ward WS, et al. Novel assay for determining DNA organization in human spermatozoa: implications for male factor infertility. Urology 2002;59:575-8. [Crossref] [PubMed]
- Erenpreiss J, Bungum M, Spano M, et al. Intra-individual variation in sperm chromatin structure assay parameters in men from infertile couples: clinical implications. Hum Reprod 2006;21:2061-4. [Crossref] [PubMed]
- Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25. [Crossref] [PubMed]
- Oleszczuk K, Giwercman A, Bungum M. Intra-individual variation of the sperm chromatin structure assay DNA fragmentation index in men from infertile couples. Hum Reprod 2011;26:3244-8. [Crossref] [PubMed]
- Agarwal A, Gupta S, Du Plessis S, et al. Abstinence Time and Its Impact on Basic and Advanced Semen Parameters. Urology 2016;94:102-10. [Crossref] [PubMed]
- Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28:164-71. [Crossref] [PubMed]
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril 1970;21:606-9. [Crossref] [PubMed]
- Jarow JP. Effects of varicocele on male fertility. Hum Reprod Update 2001;7:59-64. [Crossref] [PubMed]
- World Health Organization. Laboratory manual of the WHO for the examination of human semen and sperm-cervical mucus interaction. Ann Ist Super Sanita 2001;37:I-XII, 1-123. [PubMed]
- Henning H, Masal C, Herr A, et al. Effect of short-term scrotal hyperthermia on spermatological parameters, testicular blood flow and gonadal tissue in dogs. Reprod Domest Anim 2014;49:145-57. [Crossref] [PubMed]
- Fujisawa M, Yoshida S, Matsumoto O, et al. Decrease of topoisomerase I activity in the testes of infertile men with varicocele. Arch Androl 1988;21:45-50. [Crossref] [PubMed]
- Zorgniotti AW. Testis temperature, infertility, and the varicocele paradox. Urology 1980;16:7-10. [Crossref] [PubMed]
- Hienz HA, Voggenthaler J, Weissbach L. Histological findings in testes with varicocele during childhood and their therapeutic consequences. Eur J Pediatr 1980;133:139-46. [Crossref] [PubMed]
- Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online 2006;12:630-3. [Crossref] [PubMed]
- Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res 2009;129:357-67. [PubMed]
- Guthrie HD, Welch GR. Effects of reactive oxygen species on sperm function. Theriogenology 2012;78:1700-8. [Crossref] [PubMed]
- Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002;23:737-52. [PubMed]
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003;79:829-43. [Crossref] [PubMed]
- Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol 2013;10:26-37. [Crossref] [PubMed]
- Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol 2012;9:678-90. [Crossref] [PubMed]
- Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril 2011;96:1283-7. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2013;189:S146-50. [Crossref] [PubMed]
- Ni K, Steger K, Yang H, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol 2014;192:170-6. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Sadek A, Almohamdy AS, Zaki A, et al. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril 2011;95:1705-8. [Crossref] [PubMed]
- Krishna Reddy SV, Shaik AB, Sailaja S, et al. Outcome of Varicocelectomy with Different Degrees of Clinical Varicocele in Infertile Male. Advances in Andrology 2015. Available online: http://dx.doi.org/ [Crossref]
- Oleszczuk K, Augustinsson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. [Crossref] [PubMed]
- Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9. [Crossref] [PubMed]
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76-83. [PubMed]
- Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia 2014;46:126-30. [Crossref] [PubMed]
- Absalan F, Ghannadi A, Kazerooni M, et al. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet 2012;29:11-4. [Crossref] [PubMed]
- Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 2009;30:219-29. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015;103:910-6. [Crossref] [PubMed]
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. [Crossref] [PubMed]
- Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 2011;57:78-85. [Crossref] [PubMed]
- Simon L, Brunborg G, Stevenson M, et al. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 2010;25:1594-608. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang Lee R, Li SH, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod 2012;27:2908-17. [Crossref] [PubMed]
- Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Pabuccu EG, Caglar GS, Tangal S, et al. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Shi Q, Martin RH. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet 2000;90:219-26. [Crossref] [PubMed]
- Bosch M, Rajmil O, Egozcue J, et al. Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age. Eur J Hum Genet 2003;11:754-9. [Crossref] [PubMed]
- Sloter E, Nath J, Eskenazi B, et al. Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil Steril 2004;81:925-43. [Crossref] [PubMed]
- Yang F, Li L, Chen JP, et al. Couple's infertility in relation to male smoking in a Chinese rural area. Asian J Androl 2016. [Epub ahead of print]. [PubMed]
- Elshal MF, El-Sayed IH, Elsaied MA, et al. Sperm head defects and disturbances in spermatozoal chromatin and DNA integrities in idiopathic infertile subjects: association with cigarette smoking. Clin Biochem 2009;42:589-94. [Crossref] [PubMed]
- Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia 2011;43:121-8. [Crossref] [PubMed]
- Rybar R, Kopecka V, Prinosilova P, et al. Male obesity and age in relationship to semen parameters and sperm chromatin integrity. Andrologia 2011;43:286-91. [Crossref] [PubMed]
- Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl 2006;27:450-2. [Crossref] [PubMed]
- Wijesekara GU, Fernando DM, Wijerathna S, et al. Environmental and occupational exposures as a cause of male infertility. Ceylon Med J 2015;60:52-6. [Crossref] [PubMed]
- Sánchez-Peña LC, Reyes BE, López-Carrillo L. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol 2004;196:108-13. [Crossref] [PubMed]
- Rahman MS, Kwon WS, Lee JS, et al. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci Rep 2015;5:9169. [Crossref] [PubMed]
- Isaksson R, Tiitinen A. Present concept of unexplained infertility. Gynecol Endocrinol 2004;18:278-90. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril 2006;86:S111-4. [Crossref] [PubMed]
- Hamada A, Esteves SC, Nizza M, et al. Unexplained male infertility: diagnosis and management. Int Braz J Urol 2012;38:576-94. [Crossref] [PubMed]
- Spano M, Seli E, Bizzaro D, et al. The significance of sperm nuclear DNA strand breaks on reproductive outcome. Curr Opin Obstet Gynecol 2005;17:255-60. [Crossref] [PubMed]
- Samplaski MK, Dimitromanolakis A, Lo KC, et al. The relationship between sperm viability and DNA fragmentation rates. Reprod Biol Endocrinol 2015;13:42. [Crossref] [PubMed]
- Lopes S, Sun JG, Jurisicova A, et al. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 1998;69:528-32. [Crossref] [PubMed]
- Irvine DS, Twigg JP, Gordon EL, et al. DNA integrity in human spermatozoa: relationships with semen quality. J Androl 2000;21:33-44. [PubMed]
- Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil 2014;15:2-14. [PubMed]
- Giwercman A, Richthoff J, Hjøllund H, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril 2003;80:1404-12. [Crossref] [PubMed]
- Spanò M, Kolstad AH, Larsen SB, et al. The applicability of the flow cytometric sperm chromatin structure assay in epidemiological studies. Asclepios. Hum Reprod 1998;13:2495-505. [Crossref] [PubMed]
- Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 2008;89:823-31. [Crossref] [PubMed]
- Dumoulin JC, Land JA, Van Montfoort AP, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod 2010;25:605-12. [Crossref] [PubMed]
- Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int 2005;95:503-7. [Crossref] [PubMed]
- Lewis SE. The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertil Soc J 2013;18:78-82. [Crossref]
- Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet 2009;26:427-32. [Crossref] [PubMed]
- Gosálvez J, González-Martínez M, López-Fernández C, et al. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil Steril 2011;96:1083-6. [Crossref] [PubMed]
- Sharma R, Kattoor AJ, Ghulmiyyah J, et al. Effect of sperm storage and selection techniques on sperm parameters. Syst Biol Reprod Med 2015;61:1-12. [Crossref] [PubMed]
- Gosálvez J, Migueles B, López-Fernández C, et al. Single sperm selection and DNA fragmentation analysis: The case of MSOME/IMSI. Nat Sci 2013;5:7-14.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Alvarez JG. DNA fragmentation in human spermatozoa: significance in the diagnosis and treatment of infertility. Minerva Ginecol 2003;55:233-9. [PubMed]
- Ollero M, Gil-Guzman E, Lopez MC, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod 2001;16:1912-21. [Crossref] [PubMed]
- Bedford JM, Bent MJ, Calvin H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil 1973;33:19-29. [Crossref] [PubMed]
- Koppers AJ, De Iuliis GN, Finnie JM, et al. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008;93:3199-207. [Crossref] [PubMed]
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol 2006;18:325-32. [Crossref] [PubMed]
- Das M, Al-Hathal N, San-Gabriel M, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet 2013;30:843-8. [Crossref] [PubMed]
- ASH. Smoking statistics: illness and death. Available online: http://ash.org.uk/information-and-resources/fact-sheets/smoking-statistics-illness-and-death/
- Esteves SC, Agarwal A, Sharma R, et al. Reply to Eugenio Ventimiglia, Montorsi Francesco, and Andrea Salonia's Letter to the Editor Re: Reecha Sharma, Avi Harlev, Ashok Agarwal, Sandro C. Esteves. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. In Press. [Crossref] [PubMed]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 2008;20:281-91. [Crossref] [PubMed]
- Oyeyipo IP, Maartens PJ, du Plessis SS. In vitro effects of nicotine on human spermatozoa. Andrologia 2014;46:887-92. [Crossref] [PubMed]
- Oliveira H, Spanò M, Santos C, et al. Adverse effects of cadmium exposure on mouse sperm. Reprod Toxicol 2009;28:550-5. [Crossref] [PubMed]
- Pant N, Kumar G, Upadhyay AD, et al. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res Int 2014;21:11066-74. [Crossref] [PubMed]
- Perrin J, Tassistro V, Mandon M, et al. Tobacco consumption and benzo(a)pyrene-diol-epoxide-DNA adducts in spermatozoa: in smokers, swim-up procedure selects spermatozoa with decreased DNA damage. Fertil Steril 2011;95:2013-7. [Crossref] [PubMed]
- Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010;7:153-61. [Crossref] [PubMed]
- Dupont C, Faure C, Sermondade N, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl 2013;15:622-5. [Crossref] [PubMed]
- Chavarro JE, Toth TL, Wright DL, et al. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222-31. [Crossref] [PubMed]
- Wu DH, Leung YK, Thomas MA, et al. Bisphenol A (BPA) confers direct genotoxicity to sperm with increased sperm DNA fragmentation. Fertil Steril 2011;96:S5-6. [Crossref]


