Radiomics for rectal cancer
Introduction
Rectal cancer malignancies represent one of the most challenging aspects in modern oncology, because of the complex intersection among different specialties needed to treat this kind of cancer. The treatment workflow involves mainly surgeons (at diagnosis and surgical treatment time), radiation oncologists (for neoadjuvant treatment management) and clinical medical oncologists (for managing chemotherapy administration during radiation treatment delivery and/or distant metastases therapy). In each treatment step, the involvement of diagnostic findings and imaging contribution for characterization of malignant tumors represent key factors to direct patients to the optimal treatment pathway. At this moment, only “qualitative” imaging features and simple findings related to tumor infiltration characteristics, as anatomic involvement of pelvic structures (for pelvis limited tumors), are being used as validated standards. Novel aspects of imaging, such as the “quantitative” imaging and the radiomics approach, are being included in the tumor characterization to better direct patients to a more appropriate and tailored clinical pathway. Currently there is a lack of shared solutions to be considered as “standard” for the characterization of radiomics for rectal cancer. One of the most exciting aspects of tumor characterization is the definition of “biomarkers” able to correlate with defined outcome [e.g., survival or tumor regression grade (TRG) (1) after neoadjuvant treatment]. The ability to biologically characterize the tumor is improving due the discovery of different genetic pathways involved in tumor progression and coupling with possible contributions offered by modern imaging techniques. “Radiomics” represents the high-throughput extraction of large amounts of image features from radiographic images and is one of the approaches that hold great promises but need further validation in multi-centric settings and in the laboratory (2) for a wide shared application. Image heterogeneity is now considered a potential biomarker regarding multiple clinical settings and a recent review has been conducted to investigate the use and performance of different heterogeneity imaging biomarkers extracted from diagnostic tumor images (3). One of the most important issues in radiomics analysis is the availability of wide series of features to be correlated to a given number of observed cases. This situation can raise the need to adopt “alternative” model fitting procedures that primarily have to solve the features selection process in order to finalize the modeling process. Interesting tools adopted in this scenario can be for example the “elastic net” (4) or unsupervised clustering (5). This interesting perspective is being applied also to rectal cancer, starting from the need to characterize the features of primary lesions and is being moved to nodal and metastases classification and definition.
Definition of primary tumor and treatment monitoring
The study of primary rectal cancer in radiomics literature has often been dealt analyzing at the same time rectal and colon cancer patients. These two kinds of malignancies are completely different, because of the different treatment pathways that usually involve these patients: rectal cancer is often treated by using chemo-radiotherapy, more often in neoadjuvant setting, and subsequently by surgery that is performed trying to remove the residual tumor (if any), and trying to spare (if possible) the anal sphincter complex to avoid the abdominal-perineal amputation (6). On the other hand the treatment of colon cancer is mostly performed by surgery (as primary treatment) and subsequently (if required) adjuvant chemotherapy (7). For this reason the oncologist’s and radiation oncologist’s perspectives in tumor characterization about rectal and colon cancer can differ significantly. This scenario, containing some “blurred” aspects and overlaps between these two kinds of tumor has to be clearly taken into account in almost all papers that will be shown in this review. Starting from the definition of the primary tumor the literature already offers some interesting proofs that radiomics can be helpful in describing the pathological characterization of the lesions: Song and collaborators (8) created an interesting modeling procedure using a machine learning approach in order to distinguish among benign and malignant lesions in CT colonography exams. The performance of their model is fair with an AUC of the ROC (9,10) of 0.8525, but we have to consider, as a limitation in this paper, the lack of an external validation of the model [hopefully needed in all modeling works (11)] and subsequently the potential enhancement of overfitting issues typical of machine learning techniques as support vector machine (12) used in this paper. Regarding the primary tumor characterization, it is very interesting to observe the contribution of textural analysis as implemented by Ng et al. (13) and its correlation with the overall survival outcome. In this paper the authors studied the contribution of CT scan with contrast medium in order to characterize the tumor. They used different levels of filtration of the raw images, implementing the Laplacian of Gaussian (LoG) filter in order to smooth the high frequency noise and enhance the variation of values among adjacent pixels in the images. LoG filter can return images with different appearance according the value of σ parameter that is used in LoG filter Eq. [1]:
This formula returns the values of a convolution kernel that is a matrix with values that have to be convolved above the initial pixels values of an image. The returned values of a convolution kernel matrix are similar to the Table 1, where x and y represent the coordinates respect to the target pixel (the center grey colored one) and σ=1.

Full table
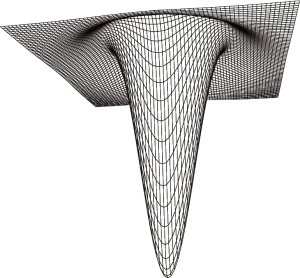
The shape of the LoG filter convolution matrix is similar to a “reversed Mexican hat” as shown in Figure 1.
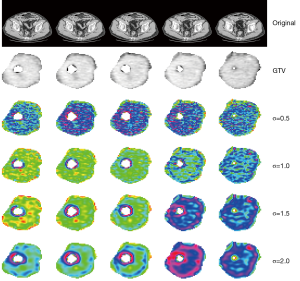
An example of the result of application of LoG filter on a rectal cancer CT scan is given in Figure 2. The upper line shows the initial CT scan over five different levels of the tumor. The second line shows the delineated gross tumor volume (GTV), the following lines show the appearance of processed images using LoG filter at different values of σ (0.5, 1.0, 1.5 and 2.0 pixels). It is evident, with growing the σ value, the appearance of the texture shows larger variations as the σ value grows up. It is interesting to understand as the use of filtering process can be an important prerequisite to achieve significant result in radiomics analysis. In this paper, indeed, only LoG processed images returned significant correlations with observed outcome while raw images didn’t: the features that showed significant prediction value for overall survival after Cox multivariate analysis were entropy, kurtosis, uniformity, skewness and standard deviation (SD) of pixel distribution histogram: among these features, entropy, kurtosis and skewness are mathematically invariant for pixels values when these are scaled linearly. This characteristic will show its value in the analysis of MR images, as it will be shown hereafter.
The application of MR for radiomics has always been considered affected by many issues due to the intrinsic difficulty in generalizing the analysis of signal in MR images because of the problem of normalization and regularization of MR images (14). On the other hand the characterization of primary tumor given by MR has been treated in literature starting from the early 1990’s up to our days (15-19), so the application of radiomics could result in interesting outcome if applied. One of the most important outcomes considered in the treatment of rectal cancer is the pathological complete response (pCR). It has been proven that patients showing pCR usually show better survival outcome than others (1). MR has been already used for determining the probability of pCR by comparing pre-treatment staging MR and pre-surgery (after chemoradiotherapy) MR (20) but without using a proper “radiomics” approach. In a small cohort of patients, De Cecco et al. (21), using a 3T MR device, showed that texture parameters and their changes during treatment could predict histopathological tumour response (P values respectively 0.016 and 0.038). Heterogeneity (kurtosis, skewness, entropy and mean value of positive pixels, MPP) was assessed using histogram parameters extracted from T2 weighted MRI pre-treatment and mid-treatment studies, computed with and without LoG image filtration. In our experience (22) we used the textural analysis of pre-treatment T2 high resolution MR scans (performed with a 1.5T MR scanner) in order to predict the probability of PCR in a cohort of 173 patients. Analysis and models have been obtained by using in-house radiomics analysis software (23). The final model returned a good discrimination capability (AUC of ROC 0.73) and at the same time an optimal prediction accuracy (mean prediction absolute error =0.018). In this radiomics model the use of cited features (skewness, entropy) that are invariant for different scaling factor in the overall signal of MR has been proven to be able (coupled with clinical T stage) in predicting PCR. Maybe such kind of models will result in effective and reliable prediction for future tailoring of patients’ treatments. Much work will be necessary to create MR radiomics models able to be applied on patients coming from environments different from the ones where the models have been created: this seems to be one of the most important challenges for future directions in radiomics applied on MR imaging. Regarding PET-CT, it is extensively used for staging and response monitoring in rectal cancer (24-31). From the radiomics perspective PET-CT was also used in order to extract predictive features: Bundschuh et al. (32) evaluated the correlation between pathological response and textural features obtained from 18F-FDG PET/CT examinations. In 27 patients, conventional and textural parameters (coefficient of variation COV, calculated by dividing SD for the mean value of the activity, skewness and kurtosis), changes of the parameters during and after neoadjuvant chemoradiotherapy (nCRT—early response) before surgery (late response) were extracted from pre-therapeutic co-registered PET/CT images: the COV showed a statistically significant predictive capability regarding pre-therapeutic response (AUC =0.73) and in the assessment of early response (AUC =0.89). In late response COV, skewness, and kurtosis showed statistically significant predictive capabilities (with an AUC of respectively 0.89, 0.74 and 0.74). In 74 patients diagnosed with rectal cancer Bang et al. (33) calculated metabolic and textural features from pre-treatment 18F FDG PET/CT scans. Response to nCRT was assessed by TRG after surgery. Textural parameters from histogram-based and co-occurrence analysis were significantly correlated with TRG, however with no significance after multivariate analysis.
Definition of primary lymph nodes and distant metastases
Quantitative analysis has been reported improving the prediction of nodal status in rectal cancer: Cui et al. (34) evaluated contrast enhanced CT scans in 228 patients with newly diagnosed rectal cancer, and showed that fractal dimension obtained by the Minkowski box-counting approach was higher in malignant nodes than in benign nodes, and there was a significant difference in heterogeneity between metastatic and not-metastatic lymph nodes (model accuracy =88%). For distant metastases special attention was placed over liver metastases: several studies showed that texture analysis of an apparently metastases free-area of the liver in patients with colorectal cancer correlates with hepatic hemodynamic and metabolism, indirectly revealing metastatic status also in the absence of any visible morphological changes. Hepatic metastases are known to be associated with changes in hepatic blood flow in adjacent apparently disease-free areas of the liver, determining both reduced portal perfusion and pro-angiogenic changes with increased arterial perfusion. Ganeshan et al. (35) demonstrated that textural parameters obtained during the portal phase of contrast enhanced CT (derived from perfusion dynamic study) correlate with patients hepatic perfusion index (HPI, ratio of arterial flow to total blood flow) and patients survival. The best correlation values where obtained by entropy values after image filtration, inversely correlated with HPI and directly correlated with survival; an entropy value lower than 2.0 provided a diagnostic threshold that identified patients who died within 36 months with 100% sensitivity and 65% specificity. This fact could be explained by the reduced portal flow in presence of micro-metastases, resulting in lower portal veins enhancement with consequent reduced entropy on filtered images as opposite to higher HPI. Moreover the same authors (36) showed that changes in hepatic entropy and uniformity measurements during the arterial phase of contrast enhanced CT scans (derived from perfusion dynamic study) are tumor-related. Indeed apparently disease-free areas of liver in patients with hepatic metastases from colorectal cancer demonstrated significantly increased uniformity and decreased entropy values during arterial phase compared with patients with no evidence of tumor. Greater uniformity, and the opposite reduced entropy, could relate to vascular dilatation (reduced number of vascular “spot” from small vessels) and/or increased enhancement of the hepatic parenchyma, also in the presence of tumors too small to be directly visualized. Also Rao et al. (37) found higher entropy and corresponding lower uniformity in the not-diseased part of the liver of patients with synchronous metastatic disease as compared to those without. The Authors explained the results with higher heterogeneity due to both the presence of micro-metastases and induced vascular changes. These conflicting results could derive from: (I) different imaging protocol used (phase derived from perfusion dynamic study vs. standard contrast enhanced protocol) with different contrast doses and injection rates, resulting in variable timing of portal venous phase; (II) assessment of mid-liver axial section (35) or whole volume of the liver (37); (III) differences in study group characteristics. Ganeshan et al. (35) included patients during surveillance after primary rectal resection, while Rao et al. (37) included patients at the time of primary staging (before any treatment). Presence vs. absence of the primary tumor or chemotherapy could result in variable hepatic hemodynamic: it’s important however to underline that textural parameters derived from dynamic contrast-enhanced CT could offer additional parameter apart that of perfusion (38). An interesting result is that texture analysis in non-contrast enhanced CT is also useful in revealing changes in apparently disease-free areas of metastatic liver, as demonstrated by different value in patients with hepatic metastases compared to patients with no tumor (entropy) and patients with extra-hepatic disease (uniformity) (39). The use of non-contrast enhanced images could avoid variability related to technical or patient factors affecting contrast enhancement. Moreover it could allow the evaluation in patients with contra-indication to contrast medium administration. Ganeshan et al. (40) also supposed a relation between textural parameter of portal contrast enhanced CT with glucose metabolism, and consequent liver fat content. Colorectal cancer patients have documented insulin resistance independent of patient weight that is reversed by removal of the tumor. On the other hand it is well recognized that tumors exhibit increased glucose metabolism, even microscopically. Lubner et al. (41) showed that in patients with untreated liver metastases from colon-rectal cancer entropy, mean positive pixels (MPP) and SD of pixel distribution histogram were negatively associated with tumor grade, moreover skewness was negatively associated with KRAS mutation. This is another biological correlate, suggesting that tumors that are more homogeneous (less entropy, smaller SD, higher in attenuation/higher mean of positive pixels) are potentially more aggressive in their biology.
Conclusions
The use of radiomics for analysis and characterization of tumors is a “trend topic” that is being increasingly explored in modern oncological and radiological sciences. The literature gives a wide and fragmented series of publications dealing mainly with the need to assess what are the technical pathways to be defined and used for creating a reliable “radiomics workflow”. An important issue about these workflows is the need to assess the external validation, applicability and re-usability of a given radiomics model. If many efforts have been already done in terms of basic research and exploratory findings, now the time for seeking shared confirmations that can establish the “rules” in the radiomics field. This step is not trivial at all: the intrinsic value of clinical prediction model is mainly based on the possibility to apply externally the model itself, as recently established in the TRIPOD publication (11). In the radiomics of rectal cancer, we do not have any model published with a reliable external validation process yet. Hopefully in the coming years we will see new publications able to create a more reproducible workflow that will offer the chance to apply worldwide the potential of radiomics findings in the classification and categorization of cancer patients. Another key point will be the possibility of using MR data for radiomics research. Great efforts have to be made to create valid external validation processes. The role of MR certainly will increase, since this imaging modality already proven its validity in the characterization of tumor in more traditional fashion, as stated before. The first findings in MR radiomics topic seem promising so our expectation is that MRI will provide the first reliable results in this field, being sure that they will exploit the potential of radiomics in rectal cancer better than traditional CT scan made in the recent past.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Laurence E. Court, Arvind Rao and Sunil Krishnan) for the series “Radiomics in Radiation Oncology” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.08). The series “Radiomics in Radiation Oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752-60. [Crossref] [PubMed]
- Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441-6. [Crossref] [PubMed]
- Alic L, Niessen WJ, Veenland JF. Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. PLoS One 2014;9:e110300 [Crossref] [PubMed]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Statist Soc B 2005;67:301-20. [Crossref]
- Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Song B, Zhang G, Lu H, et al. Volumetric texture features from higher-order images for diagnosis of colon lesions via CT colonography. Int J Comput Assist Radiol Surg 2014;9:1021-31. [Crossref] [PubMed]
- Bradley AP. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit 1997;30:1145-59. [Crossref]
- Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett 2006;27:861-74. [Crossref]
- Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. [Crossref] [PubMed]
- Cawley GC, Talbot NL. On over-fitting in model selection and subsequent selection bias in performance evaluation. J Mach Learn Res 2010;11:2079-107.
- Ng F, Ganeshan B, Kozarski R, et al. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 2013;266:177-84. [Crossref] [PubMed]
- Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 2004;22:81-91. [Crossref] [PubMed]
- Allen SD, Padhani AR, Dzik-Jurasz AS, et al. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol 2007;188:442-51. [Crossref] [PubMed]
- Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 1999;211:215-22. [Crossref] [PubMed]
- Vliegen RF, Beets GL, Lammering G, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology 2008;246:454-62. [Crossref] [PubMed]
- Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum 2005;48:23-8. [Crossref] [PubMed]
- de Lange EE, Fechner RE, Edge SB, et al. Preoperative staging of rectal carcinoma with MR imaging: surgical and histopathologic correlation. Radiology 1990;176:623-8. [Crossref] [PubMed]
- Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 2009;250:730-9. [Crossref] [PubMed]
- De Cecco CN, Ganeshan B, Ciolina M, et al. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol 2015;50:239-45. [Crossref] [PubMed]
- Dinapoli N, Barbaro B, Gatta R, et al. MR radiomics predicting complete response in radiochemotherapy (RTCT) of rectal cancer (LARC). ESTRO 2016;35:E35-0909.
- Dinapoli N, Alitto AR, Vallati M, et al. Moddicom: a complete and easily accessible library for prognostic evaluations relying on image features. Conf Proc IEEE Eng Med Biol Soc 2015;2015:771-4.
- Capirci C, Rubello D, Pasini F, et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys 2009;74:1461-9. [Crossref] [PubMed]
- Chennupati SK, Quon A, Kamaya A, et al. Positron emission tomography for predicting pathologic response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Am J Clin Oncol 2012;35:334-9. [Crossref] [PubMed]
- Maffione AM, Ferretti A, Grassetto G, et al. Fifteen different 18F-FDG PET/CT qualitative and quantitative parameters investigated as pathological response predictors of locally advanced rectal cancer treated by neoadjuvant chemoradiation therapy. Eur J Nucl Med Mol Imaging 2013;40:853-64. [Crossref] [PubMed]
- Huh JW, Min JJ, Lee JH, et al. The predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiation. Am J Clin Oncol 2012;35:340-4. [Crossref] [PubMed]
- Capirci C, Rampin L, Erba PA, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging 2007;34:1583-93. [Crossref] [PubMed]
- Li C, Lan X, Yuan H, et al. 18F-FDG PET predicts pathological response to preoperative chemoradiotherapy in patients with primary rectal cancer: a meta-analysis. Ann Nucl Med 2014;28:436-46. [Crossref] [PubMed]
- Maffione AM, Chondrogiannis S, Capirci C, et al. Early prediction of response by 18F-FDG PET/CT during preoperative therapy in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol 2014;40:1186-94. [Crossref] [PubMed]
- Maffione AM, Marzola MC, Capirci C, et al. Value of (18)F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2015;204:1261-8. [Crossref] [PubMed]
- Bundschuh RA, Dinges J, Neumann L, et al. Textural Parameters of Tumor Heterogeneity in 18F-FDG PET/CT for Therapy Response Assessment and Prognosis in Patients with Locally Advanced Rectal Cancer. J Nucl Med 2014;55:891-7. [Crossref] [PubMed]
- Bang JI, Ha S, Kang SB, et al. Imaging biomarker for predicting neoadjuvant radiation chemotherapy response and survival using pretreatment F-18 FDG PET/CT scan in locally advanced rectal cancer. J Nucl Med 2015;56:576.
- Cui C, Cai H, Liu L, et al. Quantitative analysis and prediction of regional lymph node status in rectal cancer based on computed tomography imaging. Eur Radiol 2011;21:2318-25. [Crossref] [PubMed]
- Ganeshan B, Miles KA, Young RC, et al. Hepatic enhancement in colorectal cancer: texture analysis correlates with hepatic hemodynamics and patient survival. Acad Radiol 2007;14:1520-30. [Crossref] [PubMed]
- Ganeshan B, Miles KA, Young RC, et al. Hepatic entropy and uniformity: additional parameters that can potentially increase the effectiveness of contrast enhancement during abdominal CT. Clin Radiol 2007;62:761-8. [Crossref] [PubMed]
- Rao SX, Lambregts DM, Schnerr RS, et al. Whole-liver CT texture analysis in colorectal cancer: Does the presence of liver metastases affect the texture of the remaining liver? United European Gastroenterol J 2014;2:530-8. [Crossref] [PubMed]
- Ganeshan B, Burnand K, Young R, et al. Dynamic contrast-enhanced texture analysis of the liver: initial assessment in colorectal cancer. Invest Radiol 2011;46:160-8. [Crossref] [PubMed]
- Ganeshan B, Miles KA, Young RC, et al. Texture analysis in non-contrast enhanced CT: impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol 2009;70:101-10. [Crossref] [PubMed]
- Ganeshan B, Miles KA, Young RC, et al. In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol 2007;14:1058-68. [Crossref] [PubMed]
- Lubner MG, Stabo N, Lubner SJ, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging 2015;40:2331-7. [Crossref] [PubMed]