Robotic surgery for gastric tumor: current status and new approaches
Introduction
Despite initial concerns with oncological safety, laparoscopic surgery has penetrated various fields of surgery, owing to improved surgical outcomes of good cosmesis, early bowel recovery, and better quality of life. To maximize the benefits of laparoscopic surgery, clinicians and researchers have sought to advance instruments important to performing laparoscopic procedures. Among such advances, robot systems have been introduced to minimize the limitations of laparoscopic surgery by providing technical advantages of a high-resolution 3D surgical view, instrumentation with a higher degree of freedom in movement, and a more ergonomic posture for the surgeon. Indeed, with these advantages, the use of robotic systems has permeated various surgical fields, including gastrectomy, on which clinical application and investigations are increasing (Figure 1A,B).
Nevertheless, studies have yet to show that the technical superiority of robotic systems provides superior surgical outcomes in gastrectomy. In the meantime, overestimation of the benefits of technological advances without sound evidence can increase the cost of medical services without improvement in outcomes. Underestimation, however, in the early phases of implementing new technology can deprive researchers the opportunity to validate the benefits thereof.
In the present review, we attempted to provide a balanced perspective on the current state of robotic gastrectomy, outlining evidence and opportunities for its use.
Current evidence
Feasibility
The feasibility and safety of robotic gastrectomy have been extensively tested (1-5). Most studies have been designed as single-arm case series, focusing on short-term surgical outcomes. Currently, only one prospective study on the feasibility of robotic surgery has been reported (1); it showed excellent surgical outcomes with a median hospital stay of 8 days and no major complications or mortality. Indeed, most studies have found the feasibility and safety of robotic gastrectomy to be acceptable.
Learning curve
The learning curve associated with robotic surgery could be of great concern, as robot surgery can be regarded as a variant of laparoscopic surgery, which has a long learning curve. Thus, adaptation of robot systems has been extensively analyzed: According to a learning curve analysis of three surgeons, operation times were stabilized after 9.6, 9.6, and 6 cases of robotic gastrectomy, respectively (6). Another report comparing an initial 20 robotic gastrectomies with 80 thereafter showed satisfactory surgical outcomes in the latter robotic gastrectomies (7). A report applying multi-dimensional analysis showed that operation times stabilize after 95 and 121 cases, according to moving average and non-linear regression analysis (8). In that report, a fewer number of robotic cases than laparoscopies was required to reach stabilized operation times. Also, a CUSUM analysis showed that robotic surgeries are successful even for initial cases. In the report, surgical failure was defined as conversion to laparoscopic or open surgery, failure to harvest an adequate number of lymph nodes for staging, resection margin involvement, and major postoperative complications including mortality. Thus, the literature suggests that a fewer number of cases is needed to stabilize operation times for robotic surgery than for laparoscopy and that surgical outcomes following robotic gastrectomy are acceptable even during its initial implementation.
Comparison of surgical outcomes
The most important and frequently asked questions concerning robotic surgery are related to its benefits over laparoscopic surgery. Laparoscopic surgery provided tremendous advantages over open surgery, such as good cosmesis, reduced pain, and shorter hospital stay. On the contrary, studies seem to suggest that no perceptible benefit is provided by robotic surgery over laparoscopic surgery, especially to patients (9-14). A recent multicenter prospective trial comparing robot and laparoscopic gastrectomy confirmed the lack of substantial benefits (15). Despite longer operation time and higher costs, which are the major pitfalls of robotic surgery, perioperative surgical outcomes, such as bleeding, number of retrieved lymph nodes, gas passing, and hospital stay, as well as all complications and major complications rates, are not greatly different.
Opportunities
Advantages of robotic surgery in relation to classical perioperative parameters
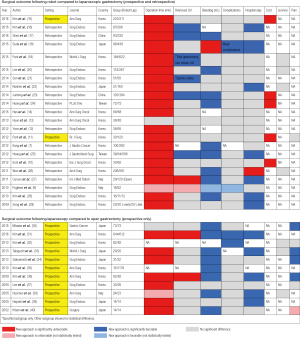
Figure 2 compares robotic gastrectomy versus laparoscopic gastrectomy and laparoscopic gastrectomy versus open gastrectomy. Parameters in which the newer technology shows statistical benefit over the older are noted in blue, while statistical detriments are noted in red. Other parameters that were not statistically analyzed are noted as weak blue or weak red. Although operation time and cost show no benefit, other parameters highlight areas in which robotic gastrectomy can be of benefit.
Higher number of retrieved lymph nodes
Compared with open surgery, laparoscopy exhibits comparable or poorer retrieval of lymph nodes (31,36). On the contrary, robotic surgery shows comparable or better retrieval of lymph nodes than laparoscopy (16,17,19,21,23,25). The reduced number of retrieved lymph nodes is the one single weakness of laparoscopic surgery, compared with open surgery, for which robotic systems can compensate. This strength of robot systems is especially apparent in difficult operations requiring total gastrectomy or D2 dissection (16,19,21). The working space within the supra-pancreatic area, hiatus, and splenic hilum is quite far from the trocar site in laparoscopic surgery. This introduces problems with physiologic tremor. Meanwhile, robotic systems automatically compensate for any physiologic tremors and provide enhanced dexterity of surgical instruments, facilitating retrieval of a higher number of lymph nodes.
Less bleeding
A well-known feature, less bleeding is typically recorded for laparoscopic procedures, compared to open surgery. In robotic gastrectomy, about half of all publications report less bleeding than that in laparoscopic surgery (7,17,20,23-28). Statistically, less bleeding may have no impact on the clinical course of the patients; however, it implies that robots offer more precise dissection of the lymph nodes following the surgical plane. Checking long-term survival in relation to whether the reduced bleeding associated with more precise dissection could affect cancer recurrence is warranted (41).
Fewer complications
Most publications comparing robotic over laparoscopic and laparoscopic over open surgery show similar complication rates. Only a few publications have reported fewer complications for laparoscopic surgery than for open surgery (31,32). Currently, only one publication has shown fewer complications for robot surgery in comparison to laparoscopic surgery (18). The authors of the report proposed that the increased dexterity and maneuverability offered by the robotic system facilitated less pancreatic fistula.
Shorter hospital stay
As seen in Figure 2, newer technology seems to provide shorter hospital stay, which is likely associated with less trauma and faster recovery. Laparoscopy shortened hospital stays, compared to open surgery (31,33,36,38,39). While robotic surgery further decreased hospital stays over laparoscopy, although the clinical relevance thereof is uncertain (16,18,22,25,27-29).
New approaches using the technological advantages of robotic surgical systems
Image-guided surgery
Using robotic systems, preoperative CT images can reportedly be used to guide anatomical dissection of lymph nodes during gastrectomy (42). This navigation surgery is based on the concept that although the stomach is flexible and vascular structures are subject to change, in accordance with the position of the patient during an operation, the length of the vessel is fixed. After reconstruction of preoperative CT images for surgery, they can be used to aid in dissection. Not only CT images, but also real-time endoscopic images can be visualized in the console view (43). This additional visual information allows for easier manipulation of targeted tissues and materials.
Also, robot surgical systems can be equipped with near-infrared detectors for visualization of fluorescent indocyanine green (ICG), allowing for more complete removal of lymph nodes. Contrary to a previous report on the use of ICG for sentinel lymph node mapping (44), a new concept utilizing ICG for more radical surgery, with complete lymph node dissection, is currently undergoing validation (NCT01926743).
Mentoring system
Training young surgeons to use robotic systems is of great importance. The Tilepro® system can also be used to guide novice surgeons (NCT01319084). Using prepared video clips, novice surgeons can be reminded of critical points in the procedure before continuing on to the next step. Also, as previously reported, dual console systems can also be used to train young surgeons (45,46).
Reduced-port surgery
The Single-Site® system was initially developed for single-site cholecystectomy or hysterectomy (47,48). Currently, reduced-port robotic gastrectomy using the Single-Site port with an additional third robotic arm is currently under investigation (NCT02347956). Therewith, reduced-port surgery can be performed enjoying a similar degree of freedom as that for conventional robotic gastrectomy.
Conclusions
Currently, perioperative surgical outcomes comparable to those for laparoscopy are reported for robot gastrectomy, along with longer operation time and high cost. Nevertheless, robot surgery is still in its primitive stage, merely seeking to replicate surgical tasks performed by laparoscopic surgery. Thus, we offer two strategies for the continued development of robotic surgery: First would be to develop less invasive procedures (Figure 3A). Image-guided surgery and reduced-port gastrectomy can potentially lessen the invasiveness of gastrectomy. Second would be to expand robotic surgery to more radical procedures (Figure 3B). ICG-guided lymph node dissection and image-guided surgery could be of use therein.
Acknowledgements
We would like to thank Anthony Thomas Milliken, ELS (Editing Synthase, Seoul, Korea) for his help with the editing of this manuscript.
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1007706).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tokunaga M, Sugisawa N, Kondo J, et al. Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer 2014;17:542-7. [Crossref] [PubMed]
- Anderson C, Ellenhorn J, Hellan M, et al. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 2007;21:1662-6. [Crossref] [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [PubMed]
- Yoshimura F, Inaba K, Kawamura Y, et al. Clinical outcome and clinicopathological characteristics of recurrence after laparoscopic gastrectomy for advanced gastric cancer. Digestion 2011;83:184-90. [Crossref] [PubMed]
- Uyama I, Kanaya S, Ishida Y, et al. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 2012;36:331-7. [Crossref] [PubMed]
- Park SS, Kim MC, Park MS, et al. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 2012;26:60-7. [Crossref] [PubMed]
- Kang BH, Xuan Y, Hur H, et al. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer 2012;12:156-63. [Crossref] [PubMed]
- Kim HI, Park MS, Song KJ, et al. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol 2014;40:1346-54. [Crossref] [PubMed]
- Pugliese R, Maggioni D, Sansonna F, et al. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc 2010;24:2594-602. [Crossref] [PubMed]
- Eom BW, Yoon HM, Ryu KW, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol 2012;38:57-63. [Crossref] [PubMed]
- Park JY, Jo MJ, Nam BH, et al. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg 2012;99:1554-61. [Crossref] [PubMed]
- Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 2012;26:1377-81. [Crossref] [PubMed]
- Hyun MH, Lee CH, Kwon YJ, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol 2013;20:1258-65. [Crossref] [PubMed]
- Han DS, Suh YS, Ahn HS, et al. Comparison of Surgical Outcomes of Robot-Assisted and Laparoscopy-Assisted Pylorus-Preserving Gastrectomy for Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol 2015;22:2323-8. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
- Kim YW, Reim D, Park JY, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc 2015. [Epub ahead of print]. [PubMed]
- Shen W, Xi H, Wei B, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc 2016;30:574-80. [Crossref] [PubMed]
- Suda K, Man-I M, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673-85. [Crossref] [PubMed]
- Park JY, Ryu KW, Reim D, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg 2015;39:1789-97. [Crossref] [PubMed]
- Lee J, Kim YM, Woo Y, et al. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc 2015;29:3251-60. [Crossref] [PubMed]
- Son T, Lee JH, Kim YM, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606-15. [Crossref] [PubMed]
- Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc 2014;28:1180-7. [Crossref] [PubMed]
- Junfeng Z, Yan S, Bo T, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc 2014;28:1779-87. [Crossref] [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One 2014;9:e111499. [Crossref] [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1303-10. [Crossref] [PubMed]
- Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086-92. [Crossref] [PubMed]
- Caruso S, Patriti A, Marrelli D, et al. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot 2011;7:452-8. [Crossref] [PubMed]
- Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 2010;24:610-5. [Crossref] [PubMed]
- Song J, Kang WH, Oh SJ, et al. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 2009;23:1204-11. [Crossref] [PubMed]
- Misawa K, Fujiwara M, Ando M, et al. Long-term quality of life after laparoscopic distal gastrectomy for early gastric cancer: results of a prospective multi-institutional comparative trial. Gastric Cancer 2015;18:417-25. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Kim YW, Yoon HM, Yun YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301). Surg Endosc 2013;27:4267-76. [Crossref] [PubMed]
- Takiguchi S, Fujiwara Y, Yamasaki M, et al. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg 2013;37:2379-86. [Crossref] [PubMed]
- Sakuramoto S, Yamashita K, Kikuchi S, et al. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc 2013;27:1695-705. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [Crossref] [PubMed]
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172-6. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [Crossref] [PubMed]
- Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011;18:2818-25. [Crossref] [PubMed]
- Kim YM, Baek SE, Lim JS, et al. Clinical application of image-enhanced minimally invasive robotic surgery for gastric cancer: a prospective observational study. J Gastrointest Surg 2013;17:304-12. [Crossref] [PubMed]
- Woo Y, Choi GH, Min BS, et al. Novel application of simultaneous multi-image display during complex robotic abdominal procedures. BMC Surg 2014;14:13. [Crossref] [PubMed]
- Miyashiro I, Hiratsuka M, Kishi K, et al. Intraoperative diagnosis using sentinel node biopsy with indocyanine green dye in gastric cancer surgery: an institutional trial by experienced surgeons. Ann Surg Oncol 2013;20:542-6. [Crossref] [PubMed]
- Crusco S, Jackson T, Advincula A. Comparing the da Vinci si single console and dual console in teaching novice surgeons suturing techniques. JSLS 2014;18.
- Morgan MS, Shakir NA, Garcia-Gil M, et al. Single- versus dual-console robot-assisted radical prostatectomy: impact on intraoperative and postoperative outcomes in a teaching institution. World J Urol 2015;33:781-6. [Crossref] [PubMed]
- Vizza E, Corrado G, Mancini E, et al. Robotic single-site hysterectomy in low risk endometrial cancer: a pilot study. Ann Surg Oncol 2013;20:2759-64. [Crossref] [PubMed]
- Wren SM, Curet MJ. Single-port robotic cholecystectomy: results from a first human use clinical study of the new da Vinci single-site surgical platform. Arch Surg 2011;146:1122-7. [Crossref] [PubMed]
Cite this article as: Lim SH, Lee HM, Son T, Hyung WJ, Kim HI. Robotic surgery for gastric tumor: current status and new approaches. Transl Gastroenterol Hepatol 2016;1:28.