Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening
Introduction
Lung cancer remains a leading cause of cancer-related death worldwide (1). Various efforts have been made to contain the extent of the disease and an early detection of lung cancer is crucial for successful treatment and prolonged survival (2,3). Lung cancer screening studies using low-dose computed tomography (LDCT) were set up all over the world, to assess the feasibility of detecting lung cancer in high-risk individuals as early as possible (4-6). The National Lung Screening Trial, which is the largest randomized-controlled LDCT lung cancer screening trial, reported a relative reduction in lung cancer-specific mortality of 15–20% when comparing chest X-ray and LDCT screening (7). Currently, lung cancer screening by LDCT is widely recommended for high-risk individuals by US guidelines (8-15). However, there still is an ongoing debate if screening should be recommended for high-risk individuals in Europe, and further evidence is needed (16). Nevertheless, the vast data on (small) pulmonary nodules provided by the lung cancer screening trials enable further insights into the clinical management of pulmonary nodules and the development of future screening guidelines.
Most LDCT lung cancer screening trials present results for baseline and incidence screening rounds separately and elaborate reviews of this data were published before (4,5,17,18). Although comparing screening rounds provides valuable information about a trial’s lung cancer screening performance in general, this approach does not appreciate the possible differences among nodules firstly detected during baseline and incidence screening rounds and is heavily influenced by the methodological differences of the respective LDCT lung cancer screening trials. For instance, a lung cancer screening trial with an aggressive baseline screening follow-up strategy may report lower cancer rates during incidence screening rounds, than a trial with a less aggressive strategy at baseline screening, even though the overall lung cancer rate is similar. However, only limited evidence concerning the different groups of pulmonary nodules identified is provided. Non-calcified pulmonary nodules detected at baseline screening consist of a combination of nodules that may have been present for years and a fewer number of more recently developed nodules. Non-calcified pulmonary nodules firstly detected during incidence screening may be entirely new (not present on a previous screen), not new (missed on a previous screen), or below the detection threshold of the respective LDCT lung cancer screening trial on the previous screen (hence, these are growing nodules). Unfortunately, lung cancer screening trials present their data concerning lung cancer rates in the various groups of non-calcified pulmonary nodules differently and the definitions of incidence nodules vary widely (4,5,10,16). The recently released British Thoracic Society Guidelines for the Investigation and Management of Pulmonary Nodules addresses this issue by stating that there is little evidence for the management of new incident nodules that appear on follow-up CTs (19).
This review intends to create a basis for assessing non-calcified pulmonary nodules detected during lung cancer screening in a more clinical relevant manner. The aim is to present detection rates of non-calcified pulmonary baseline nodules and non-calcified pulmonary incident nodules not present on a previous scan (thus new) without clustering them together. Furthermore, lung cancer probabilities of non-calcified baseline and new non-calcified incident pulmonary nodules will be assessed, as well as the lung cancer risk for participants with such nodules. As the majority of trials do not explicitly state rates concerning new non-calcified pulmonary incident nodules, only limited evidence is available for this nodule group. This review focusses mainly on the following European lung cancer screening trials: United Kingdom lung screening (UKLS) trial, Italian detection and screening of early lung cancer by novel imaging technology and molecular assays (DANTE) trial, Danish lung cancer screening trial (DLCST), Dutch-Belgian lung cancer screening trial (NELSON), Italian lung study (ITALUNG), German lung cancer screening intervention study (LUSI); American lung cancer screening trials: National lung screening study (NLST), early lung cancer action project (ELCAP), Mayo CT Screening study (Mayo trial), Pittsburg Lung Screening Study (PLuSS); and the international early lung cancer action project (IELCAP) trial.
Pulmonary nodules in baseline screening rounds of LDCT lung cancer screening
Prevalence of non-calcified pulmonary nodules at baseline rounds of LDCT lung cancer screening
The prevalence of pulmonary nodules at baseline rounds of LDCT lung cancer screening depends on the methodology of the respective screening approach, such as the CT protocol or the use of an artificial detection limit. Additionally, a higher prevalence of certain diseases, such as histoplasmosis, may influence the number of detected solitary lung nodules (20). Most European and American trials with no detection limit (PLuSS and Mayo trial) or detection limit of 3 mm or 15 mm3 (NELSON and UKLS) reported non-calcified pulmonary nodules in between 41–51% of baseline participants (Tables 1,2) (21,24,31,32). However, the ELCAP and DLCST trial, which both did not employ a detection limited, reported lower non-calcified pulmonary nodule rates in participants at baseline [23% (233/1,000) and 22% (447/2,052) respectively] (23,30). These differences could be explained by a plethora of factors, such as differences in methodology, patient population, infectious disease prevalence, etc. For instance, the difference between the Mayo trial [51% (780/1,520) non-calcified pulmonary nodule baseline prevalence] and ELCAP trial [23% (233/1,000) non-calcified pulmonary nodule baseline prevalence] has been attributed to differences in slice thickness during CT detection (5,30,33). Furthermore, the ELCAP trial only reported nodules of participants with less than six nodules, possibly reducing the non-calcified pulmonary nodule baseline prevalence (30).
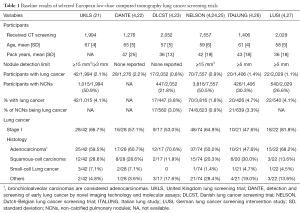
Full table
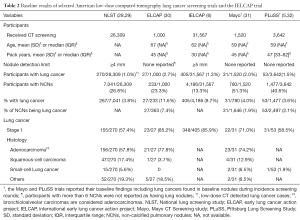
Full table
Strengthening the case for a higher non-calcified pulmonary nodule prevalence at baseline, at least in the European smoker or former smoker population, are the recently released results of the UKLS trial’s baseline round. This trial shared an analogous methodology with the NELSON trial and confirmed a non-calcified pulmonary nodule baseline prevalence in 51% of the participants for the respective screening setting (21,24). Trials with a detection limit of 4 mm or greater (IELCAP, NLST, ITALUNG, LUSI) reported a lower non-calcified pulmonary nodule rate of between 13–30% at baseline (6,26-28). This suggests that a great number of non-calcified pulmonary nodules at baseline are small pulmonary nodules. Of the trials with no or a low detection limit, the Mayo trial reported that 39% (307/780) of participants only had non-calcified pulmonary nodules smaller than 4 mm and the NELSON trial found that 56% (4,861/8,623) of the non-calcified pulmonary nodules detected at baseline were smaller than 50 mm3 (roughly 4.7 mm) (24,31,33). Within the baseline round of the DLCST trial, 66% (371/560) of the non-calcified pulmonary nodules were below 5 mm and in baseline participants of the ELCAP trial, the largest non-calcified pulmonary nodule was smaller than 5 mm in 58% (136/233) (23,30,34).
Concluding, evidence from trials with no or a low detection limit indicates that 22–51% of heavy smokers and former heavy smokers have non-calcified pulmonary nodules at baseline screening. Of the non-calcified pulmonary nodules detected at baseline, possibly up to 56% are small pulmonary nodules below 50 mm3 or 5 mm.
Lung cancer risk of participants with non-calcified pulmonary nodules at baseline and lung cancer probability of non-calcified pulmonary baseline nodules
Unfortunately, data regarding the overall lung cancer risk of participants with baseline nodules is not frequently described. Trials rather report how many participants are diagnosed with lung cancer per round, irrespective in which round the nodule was found initially. However, information about the overall lung cancer risk is crucial, since it could directly influence the clinical practice approach of incidentally found lung nodules in smokers and provide essential information for the development of new guidelines.
The Mayo trial (5-year results) and PluSS trial (3-year result) report that 4% (31/780 and 53/1,477 respectively) of participants with a non-calcified pulmonary nodule at baseline developed cancer in such a nodule within their screening program (31,32). Both trials did not employ a detection limit. The NELSON trial, which used a 15 mm3 (roughly 3 mm) detection limit, reported a 2-year lung cancer risk of 3% (94/3,189) for Dutch participants with baseline nodules (35).
Regarding the probability of a non-calcified pulmonary baseline nodule being diagnosed as lung cancer eventually, the Mayo trial (5-year results) and PLuSS trial (3-year results) reported that 2% (31/1,646 and 53/2,497 respectively) of the non-calcified baseline nodules turned out to be lung cancer (31,32).
The other trials included here, only reported the baseline detection rate, thus the number of lung cancers found in participants at baseline, ranging between 1–3% for all participants (6,21,22,24-27,30,34), and 2–11% for participants with non-calcified pulmonary baseline nodules (6,21,24-27,29,30,34). During baseline screening, the probability of a non-calcified pulmonary baseline nodule being detected as lung cancer ranged between 1–7.4% (23,24,26,35). In particular, the ELCAP and IELCAP trial reported very high lung cancer rates [12% (27/233) and 10% (405/4,186) respectively] for participants with non-calcified pulmonary nodules during baseline screening (6,30). However, as demonstrated previously, these trials also reported a low non-calcified pulmonary nodule overall detection rate (6,30). Apparently, the screening methodology of these studies enabled an efficient manner of recognizing individuals with high-risk pulmonary nodules, while potentially not detecting or registering unsuspicious nodules.
As mentioned before, the UKLS and NELSON trial share a similar screening methodology; however, the participant recruiting strategy differed significantly. While inclusion in the NELSON trial was mainly based on age and smoked pack-years (24,36), the UKLS trial used a multivariate conditional logistic regression model (including: smoking duration, selected prior respiratory diseases, occupational exposure to asbestos, prior diagnosis of malignant tumors and early onset family history of lung cancer) based on the Liverpool Lung Project (21,37,38). The UKLS trial included participants only if their calculated 5-year lung cancer risk was more or equal to 5% (21). This difference in selection methodology resulted in an older screening population in the UKLS if compared to the NELSON trial (mean age: 67 vs. 59 years) and an increased lung cancer baseline detection rate in participants with non-calcified pulmonary baseline nodules [4.1% (42/1,015) vs. 1.8% (70/3,816)] (21,24,25). This unique comparison, which is made possible due to the similar screening methodology, demonstrates the impact of pre-test probability and the limited comparability even of methodologically similar lung cancer screening trials.
Concluding, the sparse existing evidence from the Mayo, PLuSS and NELSON trial indicates that 3–4% of heavy smokers or former heavy smokers with non-calcified pulmonary nodules at baseline screening will be diagnosed with lung cancer in such a nodule within 2–5 years (assuming similar epidemiology as in these trials). However, as demonstrated through baseline lung cancer detection rates of the other mentioned trials, depending on screening protocol and disease prevalence within the screened population, the number may be significantly higher. The translation from lung cancer screening trials to clinical management of incidentally detected nodules relies on careful assessment of the study population from which the data was generated.
Stage and histology of lung cancers found in non-calcified pulmonary baseline nodules
Only the Mayo and PLuSS trial reported data in a way enabling assessment of lung cancers found in non-calcified pulmonary baseline nodules across all screening rounds. Most lung cancers detected in a non-calcified pulmonary baseline nodule were stage I [Mayo: 71% (22/31), PLuSS: 59% (31/53)] (31,32). Only the Mayo trial provided information concerning the histology of lung cancer found in non-calcified pulmonary baseline nodules during all screening rounds. The majority [74% (23/31)] of lung cancers were adenocarcinomas, followed by squamous- cell carcinomas [13% (4/31)] and small-cell lung cancer [7% (2/31)].
The results concerning stage and histology at baseline screening are equivocal. The ELCAP trial, IELCAP trial, and LUISI trial reported a very high proportion of stage I lung cancer at baseline (82–86%) (6,27,30). The other trials, including the two largest, randomized controlled trials (NLST and NELSON), reported lower numbers regarding stage I lung cancers (48–67%) (7,21,22,24,26,27). There is no data available about differences in stage or histology distribution between non-calcified pulmonary baseline nodules identified as lung cancers at baseline compared to non-calcified pulmonary baseline nodules identified as lung cancers in later rounds. Differences between lung cancers found at baseline and incidence rounds, as published for instance by the ELCAP trial (39), cannot be used for the here performed assessment, since observed variances may be due to lung cancers found in newly detected nodules.
Concluding, lung cancers detected in non-calcified pulmonary baseline nodules are mostly adenocarcinomas. Current evidence suggests that only a small fraction is small-cell lung cancer. At baseline, lung cancers are stage I in 48–86% of the cases. Data concerning stage distribution of lung cancers detected in baseline nodules at subsequent rounds is sparse.
New non-calcified pulmonary nodules in incidence screening rounds of LDCT lung cancer screening
Prevalence of new non-calcified pulmonary nodules in incidence rounds of LDCT lung cancer screening
As pointed out by several studies and the recently released British Thoracic Society guidelines for the Investigation and Management of Pulmonary Nodules, little evidence exists concerning pulmonary incident nodules that appear after baseline screening and are not visible in retrospect (10,19,40). In 2005, the Fleischner society reported, citing the Mayo trial, that 10% of screening participants develop a new nodule not present in retrospect within a 1-year interval, and the PLuSS trial described that 7% (256/3,423) of their participants developed a new nodule in the same interval (32,33,41). Numbers from the ELCAP and IELCAP publications suggest annual new nodule rates of 3% (40/1,184) and 5% (1,460/27,456) respectively in LDCT lung cancer screening (6,42). In the annual screening round of the NELSON trial, 5% (344/7,295) of participants developed a new non-calcified solid nodule, while a total of 11% (787/7,295) of participants developed a new non-calcified solid nodule within the first two incidence screening rounds (3 years after baseline) (40).
The NELSON trial reported that 57% (697/1,222) of the newly detected nodules were small pulmonary nodules with a volume less than 50 mm3 (roughly 4.7 mm) (40). The ELCAP trial reported that in the 30 participants with high-resolution CT confirmed new non-calcified pulmonary incident nodules, the largest nodule had had a diameter less than 5 mm in 53% (16/30) of participants (42), and in 37% (70/191) of participants with new non-calcified pulmonary incident nodules in the Mayo trial, the nodules were smaller than 4 mm (33).
Concluding, current evidence suggests that 3–10% of LDCT lung cancer screening participants may develop a new non-calcified pulmonary incident nodule annually and up to 57% of these nodules are pulmonary nodules smaller than 50 mm3 or 5 mm.
Lung cancer risk of participants with new non-calcified pulmonary incident nodules and lung cancer probability of new non-calcified pulmonary incident nodules
The evidence regarding lung cancer probability of new non-calcified pulmonary incident nodules is scarce. Furthermore, differing methodologies of trials make the numbers hardly comparable.
The NELSON trial recently reported that 6% (49/787) of participants with a new non-calcified solid nodule developed lung cancer in such a nodule, with 4% (50/1,222) of the new non-calcified solid incident nodules proving to be lung cancer (40). The ELCAP trial reported that 10% (4/40) of participants with new non-calcified pulmonary incident nodules on LDCT had lung cancer in a new nodule, and the IELCAP reported this was the case for 5% (74/1,460) of its participants (6,32,42). The Mayo trial found a lower rate of 1.6% (3/191) (33). However, the Mayo trial reported a substantially higher new nodule rate than the other trials (see above) and the clinic where the trial was performed is located in an area with a high prevalence of histoplasmosis (20). This may explain why the Mayo trial found the highest new nodule rate, but the lowest cancer rate in new non-calcified pulmonary incident nodules. Without providing numbers, the NLST reported that detection of new non-calcified pulmonary incident nodules in the second incidence screening round was predictive for cancer if compared to stable nodules (43).
Concluding, there is only little evidence concerning the lung cancer risk of participants with new non-calcified pulmonary incident nodules. The two large studies that provide data (IELCAP and NELSON trial) show that in 5–6% of participants with new non-calcified pulmonary incident nodules, such a nodule proves to be lung cancer. The only available numbers concerning lung cancer probability of new (solid) incident nodules come from the NELSON trial, where 4% of the new solid non-calcified pulmonary incident nodules proved to be lung cancer.
Stage and histology of lung cancers found in new non-calcified pulmonary incident nodules
The only trial to provide explicit data concerning lung cancer stage, as well as histology for new incident nodule lung cancer, is the NELSON trial. It was found that 68% (34/50) of the new incident nodule lung cancers were detected at stage I (40). Of the detected lung cancers 38% (19/50) were adenocarcinomas, 22% (11/50) were squamous-cell carcinoma, and 10% (5/50) were small-cell lung cancer. The IELCAP trial reported that 86% (64/74) of lung cancers in patients with new non-calcified pulmonary incident nodules was detected at stage I (6).
Concluding, it appears that thorough LDCT lung cancer screening can detect most new nodule lung cancer at an early and still treatable stage. There is insufficient data to make definite statements about cancer histology of new nodule lung cancer detected in incidence screening rounds of LDCT lung cancer screening.
Comparing lung cancer probability of small pulmonary nodules detected at baseline and newly detected during LDCT incidence screening
Due to the differences in screening methodology, baseline nodules and new incident nodules should not be compared across lung cancer screening trials. Valid conclusions can only be reached through analysis within one screening trial. Furthermore, because only a subgroup of participants develops new incident nodules, trials have to be large enough to provide a significant sample size of new nodule lung cancers.
The IELCAP trial reported a cancer rate of 10% (405/4,186) in participants with baseline nodules and a cancer rate of 5% (74/1,460) for participants with new non-calcified pulmonary incident nodules (6). However, it is crucial to note that the screening method for baseline and incidence screening deviated significantly. While during the baseline screening round only nodules greater or equal to 5 mm were registered, there was no detection limit for new non-calcified pulmonary incident nodules at incidence screening rounds (6). The cancer rate at baseline excluded participants who only had nodules smaller than 5 mm, which as seen in other trials comprise the largest group of nodules, but the cancer rate for new incident nodules included them, rendering the numbers incomparable.
The ongoing NELSON trial did not yet provide the cancer rate of nodules detected at baseline for the overall screening. A comparison of cancer probability of new non-calcified pulmonary incident nodules and non-calcified pulmonary baseline nodules has to be made indirectly. In the baseline screening round of the NELSON trial, 1% (70/7,557) of participants were detected with lung cancer (24), and within the first three screening rounds, 3% (200/7,582) participants had screen-detected lung cancer (including 44 cancers detected in new solid non-calcified pulmonary incident nodules) (25,40). As mentioned before, the 2-year cancer risk of participants detected with baseline nodules in the NELSON trial has been reported to be 3% (94/3,189). The cancer risk of participants detected with new solid non-calcified pulmonary incident nodules was 6% (49/749). Comparing these numbers, new solid non-calcified pulmonary incident nodules appear to have a higher lung cancer probability than do non-calcified baseline nodules. This is underlined by the fact, that the overall cancer risk of participants with a new solid non-calcified pulmonary incident nodule was similar to the risk of participants with a suspicious nodule at baseline that required further follow-up (40).
New incident nodules are considered fast-growing and some lung cancer screening trials and screening guidelines anticipated this by using different cut-off values for baseline nodules and new incident nodules (6,44,45). The NELSON trial showed that there is a significant difference in the lung cancer probability of small pulmonary non-calcified nodules already present at baseline and new non-calcified pulmonary incident nodules. Within the NELSON trial, baseline nodules that were smaller than 100 mm3 (roughly 5.8 mm) had a lung cancer probability of about 0.5–0.7%, which statistically did not differ from participants without baseline nodules (35). It was concluded that these nodules do not necessitate follow-up. However, this does not apply in case of new solid non-calcified pulmonary incident nodules, where 3% of participants whose largest new nodule was smaller than 100 mm3 (roughly 5.8 mm) were eventually diagnosed with lung cancer, with 2% (15/819) of new solid non-calcified incident nodules smaller than 100 mm3 (roughly 5.8 mm) found to be lung cancer (40). These findings caused the NELSON investigators to propose different cut-off values for the follow-up of baseline nodules and new solid non-calcified pulmonary incident nodules. Based on the results of the NELSON trial, non-calcified baseline nodules smaller than 100 mm3 (0.6% lung cancer probability) or 5 mm (0.4% lung cancer probability) may continue in regular screening, non-calcified baseline nodules 100–300 mm3 (2.4% lung cancer probability) or 5–10 mm (1.3% lung cancer probability) represent an indeterminate subgroup requiring follow-up with volume doubling time measurement (<600 days necessitates further follow-up), and, non-calcified baseline nodules greater than 300 mm3 (16.9% lung cancer probability) or 10 mm (15.2% lung cancer probability) should be referred for immediate diagnostic evaluation (35). New non-calcified pulmonary incident nodules require a more aggressive follow-up strategy and only a new non-calcified solid incident nodule smaller than 27 mm3 (0.5% lung cancer probability) or 3.7 mm (0.6% lung cancer probability) should continue regular screening, new non-calcified solid incident nodules between 27–206 mm3 (3.1% lung cancer probability) or 3.7–8.2 mm (3.0% lung cancer probability) represent an indeterminate subgroup requiring follow-up and volume doubling time measurement, and new non-calcified pulmonary incident nodules greater or equal 206 mm3 (16.9% lung cancer probability) or 8.2 mm (14.2% lung cancer probability) should be referred for immediate diagnostic evaluation (40). This verifies part of the LungRads guidelines as provided by the American College of Radiologists (44). It has been suggested that the findings regarding new nodules may be translated directly into routine clinical practice for the respective risk group (i.e., smokers or former heavy smokers) outside a screening program, if the nodule can be proven to be newly developed within 1–2 years (40,46).
The explanation for the different lung cancer probabilities at smaller sizes of non-calcified baseline and new non-calcified pulmonary incident nodules could be the fact that compared to new incident nodules, baseline nodules had more time to grow before their first detection. Therefore, growing baseline nodules which possess a higher lung cancer probability are larger, while even fast growing new nodules may still be relatively small at initial detection. Furthermore, new non-calcified pulmonary incident nodules may be inherently more likely to be cancer than non-calcified baseline nodules. Nevertheless, more evidence is necessary to expand existing evidence.
Conclusions
Reporting lung cancer screening results per round, without providing overall cancer risks of participants detected with non-calcified pulmonary nodules at baseline or with new non-calcified pulmonary incident nodules at subsequent screening rounds, only provides limited information on lung cancer probabilities of the respective nodule groups. Much evidence is to gain from a more standardized manner of reporting, including subgrouping of the detected nodules according to the moment of the first detection, such as baseline nodule or new incident nodule. This would also simplify the translation to the current clinical practice of incidentally detected nodules.
Around half of heavy smokers or former heavy smokers may present with non-calcified pulmonary nodules at baseline screening. Though there only is limited evidence, it can be expected that at least 3–4% of these individuals will be diagnosed with lung cancer in a non-calcified pulmonary baseline nodule within the next 2–5 years. The majority of non-calcified pulmonary nodules detected at baseline are pulmonary nodules smaller than 50 mm3 or 5 mm and possess a low lung cancer probability.
Furthermore, 3–10% of heavy smokers or former heavy smokers develop a new non-calcified pulmonary incident nodule annually, and these nodules prove to be lung cancer in 5–6% of participants. Internal comparison of the NELSON trial provided evidence that new non-calcified pulmonary incident nodules possess a greater lung cancer probability than baseline nodules at a smaller size. This may be due to the reduced time they had to grow before first nodule detection, or due to an inherently increased cancer probability. Therefore, small pulmonary non-calcified nodules detected newly at lung cancer incidence screening rounds should be followed up more aggressively than small pulmonary non-calcified nodules detected at baseline screening. Additionally, for the respective risk population, the findings may be extrapolated for the management of incidentally detected nodules in routine clinical care, outside a screening program.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 1988;96:440-7. [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [Crossref] [PubMed]
- Field JK, van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: Post NLST. J Surg Oncol 2013;108:280-6. [Crossref] [PubMed]
- Midthun DE, Jett JR. Screening for lung cancer: the US studies. J Surg Oncol 2013;108:275-9. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [Crossref] [PubMed]
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012;10:240-65. [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S.
- Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin 2015;65:30-54. [Crossref] [PubMed]
- Moyer VA, U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- American Lung Association. Providing Guidance on Lung Cancer Screening to Patients and Physicians 2015. Available online: http://www.lung.org/assets/documents/lung-cancer/lung-cancer-screening-report.pdf. Accessed 1 Sep 2016.
- de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:311-20. [Crossref] [PubMed]
- Coureau G, Salmi LR, Etard C, et al. Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: A systematic methodological appraisal of published randomised controlled trials. Eur J Cancer 2016;61:146-56. [Crossref] [PubMed]
- Gopal M, Abdullah SE, Grady JJ, et al. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010;5:1233-9. [Crossref] [PubMed]
- Fu C, Liu Z, Zhu F, et al. A meta-analysis: is low-dose computed tomography a superior method for risky lung cancers screening population? Clin Respir J 2016;10:333-41. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-ii54. [Crossref] [PubMed]
- Starnes SL, Reed MF, Meyer CA, et al. Can lung cancer screening by computed tomography be effective in areas with endemic histoplasmosis? J Thorac Cardiovasc Surg 2011;141:688-93. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355-63. [Crossref] [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848-54. [Crossref] [PubMed]
- Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. [Crossref] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956-61. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [Crossref] [PubMed]
- Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120:868-74. [Crossref] [PubMed]
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270-6. [Crossref] [PubMed]
- Raji OY, Duffy SW, Agbaje OF, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012;157:242-50. [Crossref] [PubMed]
- Carter D, Vazquez M, Flieder DB, et al. Comparison of pathologic findings of baseline and annual repeat cancers diagnosed on CT screening. Lung Cancer 2007;56:193-9. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92:153-9. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- American College of Radiology. LungRADS Version 1.0 Assessment Categories Release date: April 28, 2014. Available online: http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/LungRADS/AssessmentCategories.pdf. Accessed 1 Sep 2016.
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- Baldwin DR, Devaraj A. Lung cancer risk in new pulmonary nodules: implications for CT screening and nodule management. Lancet Oncol 2016;17:849-50. [Crossref] [PubMed]


