Abstract
Background
Febuxostat is a novel non-purine selective inhibitor of xanthine oxidase currently being developed for the management of hyperuricemia in patients with gout.
Objective
To investigate the pharmacokinetics, pharmacodynamics and safety of febuxostat over a range of oral doses in healthy subjects.
Methods
In a phase I, dose-escalation study, febuxostat was studied in dose groups (10, 20, 30, 40, 50, 70, 90, 120, 160, 180 and 240mg) of 12 subjects each (10 febuxostat plus 2 placebo). In all groups, subjects were confined for 17 days and were administered febuxostat once daily on day 1, and days 3–14. During the course of the study, blood and urine samples were collected to assess the pharmacokinetics of febuxostat and its metabolites, and its pharmacodynamic effects on uric acid, xanthine and hypoxanthine concentrations after both single and multiple dose administration. Safety measurements were also obtained during the study.
Results
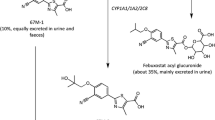
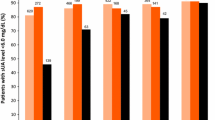
Orally administered febuxostat was rapidly absorbed with a median time to reach maximum plasma concentration following drug administration of 0.5–1.3 hours. The pharmacokinetics of febuxostat were not time dependent (day 14 vs day 1) and remained linear within the 10–120mg dose range, with a mean apparent total clearance of 10–12 L/h and an apparent volume of distribution at steady state of 33–64L. The harmonic mean elimination half-life of febuxostat ranged from 1.3 to 15.8 hours. The increase in the area under the plasma concentration-time curve of febuxostat at doses >120mg appeared to be greater than dose proportional, while the febuxostat maximum plasma drug concentration was dose proportional across all the doses studied. Based on the urinary data, febuxostat appeared to be metabolised via glucuronidation (22–14% of the dose) and oxidation (2–8%) with only 1–6% of the dose being excreted unchanged via the kidneys. Febuxostat resulted in significant decreases in serum and urinary uric acid concentrations and increases in serum and urinary xanthine concentrations. The percentage decrease in serum uric acid concentrations ranged from 27% to 76% (net change: 1.34–3.88 mg/dL) for all doses and was dose linear for the 10–120 mg/day dosage range. The majority of adverse events were mild-to-moderate in intensity.
Conclusion
Febuxostat was well tolerated at once-daily doses of 10–240mg. There appeared to be a linear pharmacokinetic and dose-response (percentage decrease in serum uric acid) relationship for febuxostat dosages within the 10–120mg range. Febuxostat was extensively metabolised and renal function did not seem to play an important role in its elimination from the body.














Similar content being viewed by others
References
Roubenoff R, Klag MJ, Mead LA, et al. Incidence and risk factors for gout in white men. JAMA 1991; 266: 3004–7
Wortmann RL, Kelley WN. Gout and hyperuricemia. In: Ruddy S, Harris Jr ED, Sledge CB, editors. Kelley’s textbook of rheumatology. 6th ed. Philadelphia (PA): WB Saunders Company, 2001: 1339–1376
Emmerson BT. The management of gout. N Engl J Med 1996; 334: 445–51
Wyngaarden JB, Kelly WN. Gout. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, et al., editors. Metabolic basis of inherited disease. 5th ed. New York: McGraw-Hill Book Co, 1982: 1043
Benedict JD, Forsham PH, Stetten D. The metabolism of uric acid in normal and gouty human studies with the aid of isotopic uric acid. J Biol Chem 1949; 181: 183–93
Benedict JD, Roche M, Yu TF, et al. Incorporation of glycine nitrogen into uric acid in normal and gouty man. Metabolism 1952; 1: 3–12
Scott JT, Holloway VP, Glass HI, et al. Studies of uric acid pool size and turnover rate. Ann Rheum Dis 1969 Jul; 28(4): 366–73
Scott JT. Gout: the last 50 years. J R Soc Med 1996; 89: 634–7
Star VL, Hochberg MC. Prevention and management of gout. Drugs 1993; 45: 212–22
De Bont M, Pieters R. Management of hyperuricemia with rasburicase review. Nucleosides Nucleotides Nucleic Acids 2004; 23: 1431–40
Okamoto K, Eger BT, Nishino T, et al. An extremely potent inhibitor of xanthine oxidoreductase: crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem 2003; 278: 1848–55
Takano Y, Hase-Aoki K, Horiuchi H, et al. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/ xanthine dehydrogenase. Life Sci 2005; 76: 1835–47
Osada Y, Tsuchimoto M, Fukushima H, et al. Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodents. Eur J Pharmacol 1993; 241: 183–8
Komoriya K, Osada Y, Hasegawa M, et al. Hypouricemic effect of allopurinol and the novel xanthine oxides inhibitor TEI-6720 in chimpanzees. Eur J Pharmacol 1993; 250: 455–60
Horiuchi H, Ota M, Kobayashi M, et al. A comparative study on the hypouricemic activity and potency in renal xanthine calculus formation of two xanthine oxidase/xanthine dehydrogenase inhibitors: TEI-6720 and allopurinol in rats. Res Commun Mol Pathol Pharmacol 1999; 104: 307–19
Becker MA, Kisicki J, Khosravan R, et al. Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids 2004; 23: 1111–6
Kubo J, Yonezawa H, Mizuno H, et al. Pharmacodynamics of TMX-67 (TEI-6720), a novel xanthine dehydrogenase/oxidase inhibitor, in man. Clin Biochem 1997; 30: 265
Komoriya K, Hoshide S, Takeda K, et al. Pharmacokinetics and pharmacodynamics of febuxostat (TMX-67), a non-purine selective inhibitor of xanthine oxidase/xanthine dehydrogenase (NP-SIXO) in patients with gout and/or hyperuricemia. Nucleosides Nucleotides Nucleic Acids 2004; 23: 1119–22
Ishiwata Y, Kubo J, Komoriya K, et al. TMX-67 a novel xanthine oxidase/xanthine dehydrogenase (XOD) inhibitor, shows strong uric acid lowering action in patients with hyperuricemia and gout [abstract]. Arthritis Rheum 2001; 44: S129
Kamatani N, Fujimori S, Hada T, et al. Phase II dose response clinical trial using febuxostat (TMX-67), a novel-type xanthine oxidase/xanthine dehydrogenase inhibitor, for gout and hyperuricemia [abstract]. Arthritis Rheum 2003; 48: S530
Kondo S, Nishimura S, Mochizuki T, et al. Metabolic fate of [14C]-TEI-6720, a novel xanthine oxidase inhibitor: tissue distribution after oral administration in rats, protein binding and metabolism in vivo and in vitro [abstract]. Drug Metab Rev 1995; 8: 56
Hoshide S, Nishimura S, Ishii S, et al. Metabolites of TMX-67, a new pharmaceutical entity for the treatment of gout or hyperuricemia, and their pharmacokinetic profiles in human [abstract]. Drug Metab Rev 2000; 32: 269
Grabowski B, Khosravan R, Vernillet L, et al. Pharmacokinetics, pharmacodynamics, and safety of febuxostat (TMX-67), a non-purine selective inhibitor of xanthine oxidase, in healthy subjects [abstract]. J Clin Pharmacol 2004; 44: 1196
Khosravan R, Bopp BA, Cox MA, et al. Dose-related decreases in uric acid observed in a multiple-dose safety, pharmacokinetic, and pharmacodynamic study of TMX-67, anovel xanthine oxidase/dehydrogenase inhibitor, in healthy subjects [abstract]. Arthritis Rheum 2000; 43: S401
Mayer MD, Khosravan R, Vernillet L, et al. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther 2005; 12: 22–34
Khosravan R, Grabowski B, Mayer MD, et al. Effect of mild and moderate hepatic impairment on pharmacokinetics, pharmacodynamics, and safety of febuxostat [abstract]. Clin Pharmacol 2005; 45: 1083
Khosravan R, Kukulka B, Wu JT, et al. Effects of age and gender on febuxostat pharmacokinetics, pharmacodynamics, and safety in healthy subjects [abstract]. Clin Pharmacol Ther 2005; 77: P50
Hende KR, Noone RM, Stome WJ. Severe allopurinol toxicity: description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984; 76: 47–56
Turnheim K, Privanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young. Br J Clin Pharmacol 1999; 48: 501–9
Khosravan R, Erdman K, Vernillet L, et al. Effect of febuxostat on pharmacokinetics of desipramine, a CYP2D6 substrate, in healthy subjects [abstract]. Clin Pharmacol Ther 2005; 77: P43
Khosravan R, Mayer MD, Wu JT, et al. Effect of concomitant administration of febuxostat and colchicine on pharmacokinetics of febuxostat and colchicine at steady state [abstract]. Arthritis Rheum 2005; 52: S102–3
Khosravan R, Wu JT, Joseph-Ridge, et al. Effect of concomitant administration of febuxostat with naproxen or indomethacin on pharmacokinetics of febuxostat, naproxen, or indomethacin at steady state [abstract]. Arthritis Rheum 2005; 52: S103
Khosravan R, Wu JT, Lademacher C, et al. Effect of febuxostat on pharmacokinetics and pharmacodynamics of warfarin [abstract]. J Clin Pharmacol 2005; 45: 1084
Cotran RS, Kumar V, Robbins SL. Robbins pathologic basis of disease. 4th ed. Philadelphia (PA): WB Saunders, 1989: 1359
Seegmiller JE. Xanthine stone formation. Am J Med 1968; 45: 780–3
Mandel GS, Mandel NS. Analysis of stones. In: Coe FL, Favous MJ, Pak CYC, et al. editors. Renal stones: medical and surgical management. Philadelphia (PA): Lippincott-Raven, 1996: 323–335
Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum 2005; 52: 916–23
Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005; 353: 2450–61
Yu P, Khosravan R, MacDonald P, et al. Effect of febuxostat, a novel non-purine, selective inhibitor of xanthine oxidase, on the QT interval in healthy subjects [abstract]. J Clin Pharmacol 2004; 44: 1195
Acknowledgements
The authors would like to thank James Kisicki, MD, and Irving Weston, MD, for performing the clinical studies; William Palo, MS, and Galen Witt, MS, for their statistical assistance; Patricia MacDonald, RN, NP, and Christopher Lademacher, MD, for their review of the manuscript; and MDS Pharma Services for performing the bioanalytical sample analyses. The study was funded by TAP Pharmaceutical Products Inc. Part of the results were presented at the 64th and 68th Annual Scientific Conference of American College of Rheumatology and the 11th International and 9th European Symposium on Purines and Pyrimidines in Man.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khosravan, R., Grabowski, B.A., Wu, JT. et al. Pharmacokinetics, Pharmacodynamics and Safety of Febuxostat, a Non-Purine Selective Inhibitor of Xanthine Oxidase, in a Dose Escalation Study in Healthy Subjects. Clin Pharmacokinet 45, 821–841 (2006). https://doi.org/10.2165/00003088-200645080-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645080-00005