Abstract
The tetracycline class of antimicrobials exhibit a broad-spectrum of activity against numerous pathogens, including Gram-positive and Gram-negative bacteria, as well as atypical organisms. These compounds are bacteriostatic, and act by binding to the bacterial 30S ribosomal subunit and inhibiting protein synthesis. The tetracyclines have been used successfully for the treatment of a variety of infectious diseases including community-acquired respiratory tract infections and sexually transmitted diseases, as well in the management of acne. The use of tetracyclines for treating bacterial infections has been limited in recent years because of the emergence of resistant organisms with efflux and ribosomal protection mechanisms of resistance. Research to find tetracycline analogues that circumvented these resistance mechanisms has lead to the development of the glycylcyclines.
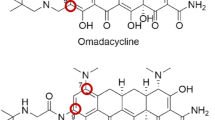
The most developed glycylcycline is the 9-tert-butyl-glycylamido derivative of minocycline, otherwise known as tigecycline (GAR-936). The glycylcyclines exhibit antibacterial activities typical of earlier tetracyclines, but with more potent activity against tetracycline-resistant organisms with efflux and ribosomal protection mechanisms of resistance. The glycylcyclines are active against other resistant pathogens including methicillin-resistant staphylococci, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci.
Tigecycline is only available in an injectable formulation for clinical use unlike currently marketed tetracyclines that are available in oral dosage forms. Tigecycline has a significantly larger volume of distribution (>10 L/kg) than the other tetracyclines (range of 0.14 to 1.6 L/kg). Protein binding is approximately 68%. Presently no human data are available describing the tissue penetration of tigecycline, although studies in rats using radiolabelled tigecycline demonstrated good penetration into tissues. Tigecycline has a half-life of 36 hours in humans, less than 15% of tigecycline is excreted unchanged in the urine. On the basis of available data, it does not appear that the pharmacokinetics of tigecycline are markedly influenced by patient gender or age.
The pharmacodynamic parameter that best correlates with bacteriological eradication is time above minimum inhibitory concentration. Several animal studies have been published describing the efficacy of tigecycline. Human phase 1 and 2 clinical trials have been completed for tigecycline. Phase 2 studies have been conducted in patients with complicated skin and skin structure infections, and in patients with complicated intra-abdominal infections have been published as abstracts. Both studies concluded that tigecycline was efficacious and well tolerated. Few human data are available regarding the adverse effects or drug interactions resulting from tigecycline therapy; however, preliminary data report that tigecycline can be safely used, is well tolerated and that the adverse effects experienced were typical of the tetracyclines (i.e. nausea, vomiting and headache).
Tigecycline appears to be a promising new antibacterial based on in vitro and pharmacokinetic/pharmacodynamic activity; however more clinical data are needed to fully evaluate its potential.













Similar content being viewed by others
References
Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001; 65(2): 232–60
Sum PE, Lee VJ, Testa RT, et al. Glycylcyclines, 1: a new generation of potent antibacterial agents through modification of 9-aminotetracyclines. J Med Chem 1994; 37: 184–8
Hunter PA, Castaner J. GAR-936. Drugs Future 2001; 26(9): 851–8
Chopra I. Glycylcyclines: third-generation tetracycline antibiotics. Curr Opin Pharmacol 2001; 1(5): 464–9
Martin AR. Antibacterial antibiotics. In: Delgado JN, Remers WA, editors. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. 10th ed. Philadelphia (PA): Lippincott-Raven Publishers, 1998: 210–5
Rasmussen BA, Gluzman Y, Tally FP. Inhibition of protein synthesis occurring on tetracycline-resistant, TetM-protected ribosomes by a novel class of tetracyclines, the glycylcyclines. Antimicrob Agents Chemother 1994; 38(7): 1658–60
Tuckman M, Petersen PJ, Projan SJ. Mutations in the interdomain loop region of the tetA (A) tetracycline resistance gene increase efflux of minocycline and glycylcyclines. Microb Drug Resist 2000; 6(4): 277–82
Sum PE, Sum FW, Projan SJ. Recent developments in tetracycline antibiotics. Curr Pharm Des 1998; 4: 119–32
Barden TC, Buckwalter BL, Testa RJ, et al. ‘Glycylcyclines’. 3: 9-Aminodoxycyclinecarboxamides. J Med Chem 1994; 37: 3205–11
Tally FT, Ellestad GA, Testa RT. Glycylcylines: a new generation of tetracyclines. J Antimicrob Chemother 1995; 35: 449–52
Testa RT, Petersen PJ, Jacobus NV, et al. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob Agents Chemother 1993; 37(11): 2270–7
Bronson JJ, Barrett JF. Quinolone, everninomycin, glycylcycline, carbapenem, lipopeptide and cephem antibacterials in clinical development. Curr Med Chem 2001; 8: 1775–93
Sum PE, Petersen P. Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936. Bioorg Med Chem Lett 1999; 9(10): 1459–62
Projan SJ. Preclinical pharmacology of GAR-936, a novel glycylcycline antibacterial agent. Pharmacotherapy 2000; 20 (9 Pt 2): 219S–23S
Bergeron J, Ammirati M, Danley D, et al. Glycylcyclines bind to the high-affinity tetracycline ribosomal binding site and evade Tet (M)- and Tet (O)-mediated ribosomal protection. Antimicrob Agents Chemother 1996; 40(9): 2226–8
Orth P, Schnappinger D, Sum PE, et al. Crystal structure of the tet repressor in complex with a novel tetracycline, 9-(N,N-dimethylglycylamido)-6-demethyl-6-deoxy-tetracycline. J Mol Biol 1999; 285(2): 455–61
Someya Y, Yamaguchi A, Sawai T. A novel glycylcycline, 9-(N,N-dimethylglycylamido)-6-demethyl-6-deoxytetracycline, is neither transported nor recognized by the transposon Tn10-encoded metal-tetracycline/H+ antiporter. Antimicrob Agents Chemother 1995; 39(1): 247–9
Boucher HW, Wennersten CB, Eliopoulos GM. In vitro activities of the glycylcycline GAR-936 against gram-positive bacteria. Antimicrob Agents Chemother 2000; 44(8): 2225–9
Betriu C, Rodriguez-Avial I, Sanchez BA, et al. In vitro activities of Tigecycline (GAR-936) against recently isolated clinical bacteria in Spain. Antimicrob Agents Chemother 2002; 46(3): 892–5
Betriu C, Sanchez BA, Rodriguez-Avial I, et al. Comparative in vitro activities of GAR-936 and other antimicrobial against Stenotrophomonas maltophilia. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Biedenbach DJ, Beach ML, Jones RN. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant grampositive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn Microbiol Infect Dis 2001; 40(4): 173–7
Boucher HW, Wennosten CB, Moellering RC, et al. Studies on a Glycylcycline GAR-936. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Citron DM, Goldstein EJC. Comparative in vitro activity of GAR-936, a novel glycylcycline, against 422 strains of unusual aerobic bacteria isolated from infected bite wounds. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Edlund C, Nord CE. In vitro susceptibility of anaerobic bacteria to GAR-936, a new glycylcycline. Clin Microbiol Infect 2000; 6(3): 159–63
Eliopoulos GM, Wennersten CB, Cole G, et al. In vitro activities of two glycylcyclines against gram-positive bacteria. Antimicrob Agents Chemother 1994; 38(3): 534–41
Finch RG, Mandragos K. Tetracyclines, in antibiotic and chemotherapy. In: Lambert HP, O’Grady FW, editors. Antibiotics and chemotherapy. New York: Churchill Livingstone Ltd, 1992: 277–90
Gales AC, Jones RN. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn Microbiol Infect Dis 2000; 36(1): 19–36
Goldstein EJ, Citron DM, Merriam CV, et al. Comparative in vitro activities of GAR-936 against aerobic and anaerobic animal and human bite wound pathogens. Antimicrob Agents Chemother 2000; 44(10): 2747–51
Henwood CJ, Gatward T, Warner M, et al. Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936). J Antimicrob Chemother 2002; 49(3): 479–87
Henwood CJ, Gatwood T, James D, et al. Antibiotic resistance among clinical isolates of Acinetobacter spp. in the United Kingdom, and in vitro activity of tigilcycline. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Hoban DJ, Bellyou Tm, Polatnick L, et al. GAR-936 (GAR) demonstrates potent in vitro activity against multi-drug resistant streptococcus pneumoniae (SPN). 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Hoellman DB, Pankuch GA, Jacobs MR, et al. Antipneumococcal activities of GAR-936 (a new glycylcycline) compared to those of nine other agents against penicillin-susceptible and-resistant pneumococci. Antimicrob Agents Chemother 2000; 44(4): 1085–8
Hoellman DB, Jacobs MR, Appelbaum PC. Antipneumococcal activity of GAR 936, a new glycylcycline, by agar dilution MIC. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Jones RN, Gales AC, Deshpane LM, et al. Antimicrobial activity of the novel glycylcycline, GAR-936, tested against over 1000 recent clinical isolates including multiresistant gram-positive cocci. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Joshi N, Miller D. Doxycycline revisited. Arch Intern Med 1997; 157(13): 1421–8
Kenny GE, Cartwright FD. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob Agents Chemother 2001; 45(9): 2604–8
Kenny GE, Cartwright FD. The susceptibilities of human mycoplasmas to a new glycylcycline, GAR 936. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Kucers A, Bennett NM. The use of antibiotics. 3rd ed. London: William Heinemann Medical Books Ltd, 1979
Mahalingam E, Trepeski L, Pong-Porter S, et al. In vitro activity of new glycylcycline, Gar 936 against methicillin-resistant and-susceptible Staphylococcus aureus isolated in North America. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Mikels SM, Brown AS, Breden L, et al. In vivo activities of GAR-936 (GAR), gentamicin (GEN), piperacillin (PIP), alone and in combination in a murine model of pseudomonas aeruginosa pneumonia. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Milatovic D, Fluit AC, Verhoef J. In vitro activity of GAR-936 against gram-positive and gram-negative bacteria. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Murakami K, Tateda K, Matsumoto T, et al. Efficacy of a novel tetracycline derivative, glycylcycline, against penicillin-resistant Streptococcus pneumoniae in a mouse model of pneumonia. J Antimicrob Chemother 2000; 46: 629–31
Murphy TM, Deitz JM, Petersen PJ, et al. Therapeutic efficacy of GAR-936, a novel glycylcycline, in a rat model of experimental endocarditis. Antimicrob Agents Chemother 2000; 44(11): 3022–7
Petersen PJ, Jacobus NV, Weiss WJ, et al. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylg-lycylamido derivative of minocycline (GAR-936). Antimicrob Agents Chemother 1999; 43(4): 738–44
Piper KE, Rouse MS, Wilson WR, et al. In vitro activity of GAR-936 against vancomycin resistant enterococci, methicillin-resistant staphylococcus aureus, and penicillin-resistant pneumococci. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Robbins M, Marais R, Felmingham D, et al. The in vitro activity of doxycycline and minocycline against anaerobic bacteria. J Antimicrob Chemother 1987; 20: 379–82
Roblin PM, Hammerschlag MR. In vitro activity of GAR-936 against Chlamydia pneumoniae and Chlamydia trachomatis. Int J Antimicrob Agents 2000; 16(1): 61–3
Schulin T, Wennersten CB, Ferraro MJ, et al. Susceptibilities of Legionella spp. to newer antimicrobials in vitro. Antimicrob Agents Chemother 1998; 42(6): 1520–3
Shonekan D, Handwerger S, Mildvan D. Comparative in vitro activities of RP59500 (quinupristin/dalfopristin), CL 329,998, CL 331,002, trovafloxacin, clinafloxacin, teicoplanin and vancomycin against Gram-positive bacteria. J Antimicrob Chemother 1997; 39: 405–9
Wexler HM, Molitoris E, Finegold SM. In vitro activities of two new glycylcyclines, N,N-dimethylglycylamido derivatives of minocyclines and 6-demethyl-6-deoxytetracycline, against 339 strains of anaerobic bacteria. Antimicrob Agents Chemother 1994; 38(10): 2513–5
Whittington WL, Roberts MC, Hale J, et al. Susceptibilities of Neisseria gonorrhoeae to the Glycylcyclines. Antimicrob Agents Chemother 1995; 39(8): 1864–5
Williams DN. Tetracyclines. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. Philadelphia (PA): WB Saunders Company, 1992: 227–31
Wise R, Andrews JM. In vitro activities of two glycylcyclines. Antimicrob Agents Chemother 1994; 38(5): 1096–102
National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Twelfth Information Supplement, M100-512. Wayne (PA): National Committee for Clinical Laboratory Standards, 2002
Heine HS, Dicks R, Andrews G. In vitro activity of oritavancin (LY333328), levofloxacin, meropenem, GAR936 and linezolid against strains of bacillus anthracis. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Abramowicz M, editor. The medical letter handbook of antimicrobial therapy. New Rochelle (NY): The Medical Letter Inc., 2000
Hedberg M, Nord CE. In vitro activity of anaerobic bacteria to GAR-936, a new glycylcycline. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Verderame M, editor. CRC handbook of chemotherapeutic agents. Vol. 1. Boca Raton (FL): CRC Press Inc, 1986
United States pharmacopeia drug information for the health care professional. 20th ed. Vol. 1. Englewood (CO): Micromedex Inc., 2000
Compendium of Pharmaceuticals and specialties. Ottawa (ON): Canadian Pharmacists Association, 2002
Kapusnik-Uner JE, Sande MA, Chambers HF. The pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill, 1996
Lacy CF. Drug information handbook. 8th ed. Hudson (OH): Lexi-Comp Inc., 2001
Rathbun RC. Parasitic infections. In: Herfindal ET, Gourley DR, editors. Textbook of therapeutics drug and disease management. Baltimore (MD): Lippincott Williams and Wilkins, 2000: 1634
Muralidharan G, Getsy J, Mayer P, et al. Pharmacokinetics (PK), safety and tolerability of GAR-936, a novel glycylcycline antibiotic, in healthy subjects. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Tombs NI. Tissue distribution of Gar-936, a broad-spectrum antibiotic, in male rats. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Troy SM, Muralidharan G, Micalizzi M, et al. The effects of renal disease on the pharmacokinetics of tigecycline (GAR-936). 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL)
Gin A, Zhanel GG. Tetracycline. In: McCormack J, editor. Drug therapy decision making guide. Philadelphia (PA): WB Saunders Company, 1996: 513–4
Muralidharan G, Mojaverian P, Micalizzi M, et al. The effects of age and gender on the pharmacokinetics, safety and tolerability of GAR-936, a novel glycylcycline antibiotic, in healthy subjects. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–12
Vogelman B, Craig WA. Kinetics of antimicrobial activity. J Pediatr 1986; 108(2): 835–40
Rotschafer JC, Zabinski RA, Walker KJ. Pharmacodynamic factors of antibiotic efficacy. Pharmacotherapy 1992; 12 (6 pt 2): 62S–70S
Zhanel GG, Hoban DJ, Harding GKM. The postantibiotic effect: a review of in vitro and in vivo data. Ann Pharmacother 1991; 25: 153–63
van Ogtrop ML, Andes D, Stamstad TJ, et al. fa vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob Agents Chemother 2000; 44(4): 943–9
Lefort A, Massias L, Saleh-Mghir A, et al. Pharmacodynamics of GAR 936 (GAR) in experimental endocarditis due to VanA-type enterococcus faecium. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sep 17–20; Toronto
Cunha BA, Domenico P, Cunah CB. Pharmacodynamics of doxycycline. Clin Microbiol Infect 1999; 6(5): 270–3
Postier R, Klein S, Green S. Results of a phase 2, open label safety and efficacy study of tigecycline to treat complicated skin and skin structure infections in hospitalized patients. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL)
Murray J, Wilson S, Klein S, et al. The clinical response to tigecycline in the treatment of complicated intra-abdominal infections in hospitalized patients, a phase 2 clinical trial. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL)
Edelstein PH, Weiss W, Edelstein M. Tigecycline (GAR-936) activity vs Legionella in vitro, and in a cell and animal model of Legionnaires’ disease. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 Sep 26–30; San Diego (CA)
Nannini EC, Singh KV, Murray BE. Efficacy of GAR-936, a novel glycylcycline, against enterococci in a mouse peritonitis model. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago (IL)
Lefort A, Lefaurie M, Saleh-Mghir, et al. Activity and diffusion of GAR-936 (GAR) in experimental enterococcal endocarditis. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 Sep 26–29; San Francisco (CA)
Mikels SM, Brown AS, Breden L, et al. Therapeutic efficacy of GAR-936 (GAR), a novel glycylcycline, in murine infections. 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1998 Sep 24–27; San Diego (CA)
Hansten PD, Horn JR. Drug interactions analysis and management. St Louis (MO): Facts and Comparisons Publishing Group, 2001
Brearley LJ, Storey E. Tetracycline-induced tooth changes, 2: prevalence, localization and nature of staining in extracted diciduous teeth. Med J Aust 1968 Oct 26; 2(17): 714–9
Dodd MA, Dole EJ, Troutman WG, et al. Minocycline-asso-ciated tooth staining. Ann Pharmacother 1998; 32: 887–9
Parkins FM, Furnish G, Bernstein M. Minocycline use discolors teeth. J Am Dent Assoc 1992; 123: 87–9
Jones HE, Lewis CW, Reisner JE. Photosensitive lichenoid eruption associated with demeclocycline. Arch Dermatol 1972; 106: 58–62
Frost P, Weinstein GD, Gomez EC. Phototoxic potential of minocycline and doxycycline. Arch Dermatol 1972; 105: 681–3
Cullen SI, Catalano PM, Helfman RJ. Tetracycline sun sensitivity [letter]. Arch Dermatol 1966; 93: 77
Lasser AE, Steiner MM. Tetracycline photo-onycholysis. Pediatrics 1978; 61: 98–9
Kestel Jr JL. Tetracycline-induced onycholysis unassociated with photosensitivity [letter]. Arch Dermatol 1972; 106: 766
Lindembaum J, Rund DG, Butler Jr VP, et al. Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. N Engl J Med 1981; 305: 789–94
Acknowledgements
The authors would like to express their sincere thanks to Drs Alice Marshall, Ray Testa and Andrew Trofa of Wyeth (Canada and US) for their assistance in obtaining information used in this review. Drs Zhanel, Embil and Hoban have received research funding from Wyeth.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhanel, G.G., Homenuik, K., Nichol, K. et al. The Glycylcyclines. Drugs 64, 63–88 (2004). https://doi.org/10.2165/00003495-200464010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464010-00005