Abstract
Synopsis
Amisulpride is a substituted benzamide. It is a dopamine antagonist with high selectivity for dopamine D2 and D3 receptors. In high doses, amisulpride exhibits dopaminergic blocking activity similar to that induced by classical antipsychotic agents, whereas in low doses it appears to facilitate dopaminergic transmission.
In well controlled studies of patients with primary negative symptoms of schizophrenia who had high negative and low positive symptoms scores, amisulpride 50 to 300 mg/day was more effective than placebo. The drug was at least as effective as low-dose fluphenazine (2 to 12 mg/day) in less rigorous trials which included patients with negative symptoms of schizophrenia.
At higher dosages (600 to 1200 mg/day), amisulpride exhibits efficacy similar to that of haloperidol 5 to 40 mg/day or flupenthixol 25 mg/day in patients with positive symptoms of schizophrenia.
In low and high dosages, amisulpride is generally well tolerated. Extrapyramidal symptoms (EPS) induced by amisulpride can occur at both low and high dosages and are dose-dependent. Symptoms are generally mild or moderate. In comparative trials, amisulpride caused an incidence of EPS similar to that with placebo and lower than that caused by haloperidol, flupenthixol or fluphenazine. Neuroendocrine adverse events were reported rarely with low-dose amisulpride and at similar incidences with amisulpride ≥600 mg/day and haloperidol 16 mg/day. Insomnia and excitation occurred rarely.
As classical antipsychotic drugs are not generally effective in reducing negative symptoms of schizophrenia, amisulpride should be considered a promising agent in the management of patients with schizophrenia who have predominantly negative symptoms. While amisulpride does not offer superior efficacy over classical antipsychotics in the management of patients with positive symptoms of schizophrenia, its lower potential for causing EPS justifies consideration of its use, especially in patients intolerant of classical antipsychotics.
Pharmacodynamic Properties
Like classical antipsychotic drugs, amisulpride is an antagonist of dopamine D2 and D3 receptors; however, the D3 to D2 receptor selectivity of amisulpride is greater than that of classical antipsychotic drugs. Amisulpride has little affinity for other neurotransmitter receptors or dopamine receptor subtypes. Low doses of amisulpride in animals facilitate presynaptic dopamine receptormediated behaviours, whereas higher doses decrease behaviours associated with postsynaptic receptor activation.
No deleterious effects on psychometrics or memory performance were observed after single oral doses up to 200mg in healthy volunteers. Like classical antipsychotic drugs, amisulpride affects dopaminergic control of plasma hormone levels. Amisulpride produced a significant increase in daytime and nocturnal prolactin levels in healthy volunteers. Daytime, but not nocturnal, thyroid-stimulating hormone levels were increased significantly and a significant decrease in the sleep-onset growth hormone peak was recorded. Other endocrine effects were minor.
Pharmacokinetic Properties
Observed peak plasma concentrations after single oral doses of ami sui pride 50 and 200mg, respectively, are approximately 54 and 443 μg/L at 3 hours. An earlier, lower peak concentration is detectable 1 hour after drug administration. The drug distributes widely and rapidly to the tissues and is minimally bound to plasma protein. Clearance is principally through renal elimination of unchanged drug, although 10 to 15% of a dose is metabolised to inactive metabolites. Elimination is biphasic with a terminal elimination half-life of approximately 12 hours.
Therapeutic Efficacy
Amisulpride has demonstrated efficacy against both positive and negative symptoms of schizophrenia. At low dosages (≤300 mg/day) in randomised, double-blind trials which included patients with high negative symptom scores and low positive symptom scores, amisulpride reduced negative symptoms as measured by the Scale for Assessment of Negative Symptoms (SANS). Scores were reduced by 32 to 46% from baseline compared with 8 to 23% in placebo-treated patients. In 2 double-blind trials with less rigorous inclusion and exclusion criteria, low-dose amisulpride (≤300 mg/day) and fluphenazine 2 to 12 mg/day reduced Brief Psychiatric Ratings Scale (BPRS) scores and SANS scores to a similar extent (BPRS 40 and 29% and SANS 18 and 22%, respectively).
In patients with positive symptoms of schizophrenia, amisulpride 400 to 1200 mg/day reduced BPRS scores by approximately 50%, a response rate not significantly different from that produced by haloperidol or flupenthixol in doubleblind, randomised trials.
Tolerability
In low and high dosages, amisulpride is generally well tolerated. At low dosages (50 to 300 mg/day), amisulpride was as well tolerated as placebo. Adverse events reported in comparative trials included extrapyramidal symptoms (EPS), endocrine adverse events, insomnia, agitation and dryness of the mouth.
EPS can occur at both low and high dosages of amisulpride, but the incidence is dose-related, with increases at dosages greater than 800 mg/day. Symptoms are usually mild or moderate and occur less frequently in amisulpride-treated patients than in comparable patients receiving haloperidol, flupenthixol or fluphenazine.
The incidence of neuroendocrinological adverse events associated with low dosages of amisulpride is similar to that associated with placebo, and with high dosages the incidence is similar to that observed in haloperidol-treated patients.
Dosage and Administration
Amisulpride is administered orally twice daily. The recommended dosages of amisulpride are 50 to 300 mg/day for the treatment of patients with predominantly negative symptoms of schizophrenia and 400 to 800 mg/day for those with positive symptoms. Dosages as high as 1200 mg/day have been studied, but were not clearly superior to amisulpride 800 mg/day. Dosage reduction may be necessary in patients with creatinine clearance below 30 ml/min.
Similar content being viewed by others
References
Sokoloff P, Giros B, Martres MP, et al. Localization and function of the D3 dopamine receptor. Arzneimittel Forschung 1992; 42 (2)A: 224–30
Schwartz J-C, Levesque D, Martres M-P, et al. Dopamine D3 receptor: basic and clinical aspects. Clin Neuropharmacol 1993; 16(4): 295–314
Scatton B, Perrault G, Sanger DJ, et al. Pharmacological profile of amisulpride, an atypical neuroleptic which preferentially blocks presynaptic D2/D3 receptors [abstract]. Neuropsychopharmacology 1994 May; 10 Suppl.1: 242S
Chivers JK, Gommeren W, Leysen JE, et al. Comparison of the in-vitro receptor selectivity of substituted benzamide drugs for brain neurotransmitter receptors. J Pharm Pharmacol 1988; 40(6): 415–21
Scatton B, Perrault G, Gonon F, et al. Novel neuroleptics acting on presynaptic dopaminergic receptors [abstract no. S-19-1]. Neuropsychopharmacology 1995; 5(Spec. Iss.): 214
Maitre M, Ratomponirina C, Gobaille S, et al. Displacement of [3H]γ-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics. Eur J Pharmacol 1994 Apr 21; 256: 211–4
Vasse M, Protais P, Costentin J, et al. Unexpected potentiation by discriminant benzamide derivatives of stereotyped behaviours elicited by dopamine agonists in mice. Naunyn Schmiedebergs Arch Pharmacol 1985 Apr; 329: 108–16
Vasse M, Protais P. Potentiation of apomorphine-induced stereotyped behaviour by acute treatment with dopamine depleting agents: a potential role for an increased stimulation of D1 dopamine receptors. Neuropharmacology 1989 Sep; 28: 931–9
Vasse M, Protais P. Increased grooming behaviour is induced by apomorphine in mice treated with discriminant benzamide derivatives. Eur J Pharmacol 1988 Oct 26; 156: 1–11
Guyon A, Assouly-Besse F, Biala G, et al. Potentiation by low doses of selected neuroleptics of food-induced conditioned place preference in rats. Psychopharmacology 1993 Mar; 110: 460–6
Martres MP, Sokoloff P, Delandre M, et al. Selection of dopamine antagonists discriminating various behavioural responses and radioligand binding sites. Naunyn Schmiedebergs Arch Pharmacol 1984; 325: 102–15
Protais P, Hermier C, Costentin J. The discriminant dopamine antagonist property of benzamides is observed at various times after their systemic or intracerebroventricular administration. Neuropharmacology 1985 Sep; 24: 861–7
Martinot JL, Paillère-Martinot ML, Poirier MF, et al. In vivo characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology 1996; 124: 154–8
Farde L, Nordström A-L, Wielel F-A, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 1992 Jul; 49: 538–44
Farde L, Nordström A-L, Karlsson P, et al. Positron emission tomography studies on dopamine receptors in schizophrenia. Clin Neuropharmacol 1995; 18 Suppl.1: S121–9
Wetzel H, Wiesner J, Hiemke C, et al. Acute antagonism of dopamine D2-like receptors by amisulpride: effects on hormone secretion in healthy volunteers. J Psychiatr Res 1994 Sep-Oct; 28: 461–73
Szegedi A, Wetzel H, Hiemke C, et al. Effects of the dopamine antagonist amisulpride on the nocturnal secretion of prolactin, melatonin and TSH in man [abstract]. Acta Endocrinol 1992 Mar; 126 Suppl.4: 121
Wetzel H, Gründer G, Riemann M, et al. Amisulpride: effects on nocturnal hormone secretion and sleep EEG parameters in healthy young volunteers [abstract]. Pharmacopsychiatry 1992 Mar; 25: 93
Perault M, Bergougnan L, Paillat A, et al. Lack of interaction beteen amisulpride and lorazepam on psychomotor performance and memory in healthy volunteers. 9th Congress of the European College of Neuropsychopharmacology; 1996, Amsterdam.
Mattila MJ, Seppala T, Patat A, et al. Oral single doses of amisulpride do not interact with ethanol on performance and memory [abstract]. Br J Clin Pharmacol 1995; 41: 454
Dufour A, Desanti C. Pharmacokinetics and metabolism of amisulpride [in French]. Ann Psychiatr 1988; 3 (3)bis: 298–305
Bianchetti G, Canal M, Rosenzweig P. Amisulpride (IV, part 1): Summary of human pharmacology and pharmacokinetics studies. Synthelabo Groupe (Le Plessis-Robinson, France), 1995, Report no. 95-00679-EN-00. (Data on file)
Charmes JP, Dagonneau M, Strouzma P. Pharmacokinetic parameters of amisulpride in patients with renal impairment and in healthy volunteers after a 50 mg intramuscular administration of amisulpride. Synthèlabo (Le Plessis-Robinson, France), Report no. 95-00680-EN-OI. (Data on file)
Panis-Rouzier R, Fourcade J. Study of amisulpride dialysance in patients with chronic renal impairment undergoing haemodialysis. Synthelabo (Le Plessis-Robinson, France), Report no. 95-00968-EN-OI. (Data on file)
Piette F, Hamon B, Ahtoy P. Amisulpride study of pharmacokinetics and safety following a single administration (50 mg tablets) in the elderly. Synthèlabo (Le Plessis-Robinson, France), Report no. 95-00596-EN-01. (Data on file)
Costa e-Silva JA. Treatment of dysthymic disorder with lowdose amisulpride. A comparative study of 50 mg/d amisulpride versus placebo [in French]. Ann Psychiatr 1990; 5(3): 242–9
Lecrubier Y, Boyer P, Rein W, et al. The treatment of dysthymics with a dopaminergic presynaptic blocker [abstract]. Neuropsychopharmacology 1994 May; 10 Suppl.1 (Pt 1): 302S
Leon CA, Vigoya J, Conde S, et al. Therapeutic efficacy of amisulpride and viloxazine in the treatment of dysthymia: a comparison [in Spanish]. Acta Psiquiatr Psicol Am Lat 1994 Mar; 40: 41–9
Lecrubier Y, Puech AJ, Aubin F, et al. Improvement by amisulpride of the negative syndrome in non-psychotic subjects: a preliminary study. Psychiatr Psychobiol 1988; 3(5): 329–33
Lemoine P, Margot F. Effectiveness of ami sui pride (Solian® 50) in chronic psychoemotional negative symptoms [in French]. Sem Hop 1989; 65(15): 965–70
Robillard J. A preliminary study of amisulpride (Solian® 50) in negative psychoemotional symptoms in elderly patients [in French]. Sem Hop 1989 Oct 5; 65: 2147–50
Dollfus S, Petit M, Menard JF, et al. Amisulpride versus bromocriptine in infantile autism: a controlled crossover comparative study of two drugs with opposite effects on dopaminergic function. J Autism Dev Disord 1992 Mar; 22: 47–60
Miller DD. Schizophrenia: its etiology and impact. Pharmacotherapy 1996 Jan/Feb; 16(1 Pt 2): 2S–5S
Andreasen NC, Olsen S. Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39: 789–94
Kay SR, Opler LA, Fiszbein A. Significance of positive and negative syndromes in chronic schizophrenia. Br J Psychiatry 1986; 149: 439–48
Buchanan RW, Carpenter Jr WT. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis 1994; 182: 193–204
Carpenter Jr WT. The deficit syndrome. Am J Psychiatry 1994; 151(3): 327–9
Carpenter WT Jr. Treatment of negative symptoms: pharmacologic and methodologic issues. Neuropsychopharmacology 1994 May; 10 Suppl.(Pt 1): 369S
Rao ML, Moller H-J. Biochemical findings of negative symptoms in schizophrenia and their putative relevance to pharmacologic treatment. A review. Neuropsychobiology 1994; 30(4): 160–72
Möller H-J, van Praag-HM, Aufdembrinke B, et al. Negative symptoms in schizophrenia: considerations for clinical trials. Working group on negative symptoms in schizophrenia. Psychopharmacology 1994; 115(1-2): 221–8
Opler LA, Albert D, Ramirez PM. Psychopharmacologic treatment of negative schizophrenic symptoms. Compr Psychiatry 1994; 35 (1): 16–28
Carpenter Jr WT. Psychopathology and common sense: where we went wrong with negative symptoms [editorial]. Biol Psychiatry 1991; 29: 735–7
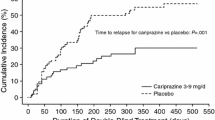
Loo H, Poirier-Littré MF, Fleurot O, et al. Amisulpride versus placebo in the long-term treatment of negative schizophrenia [abstract]. Eur Psychiatry 1994; 9 Suppl.1: 154S
Synthèlabo. Long-term study of safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Interim report. Report no. 95-00869-EN-OO. (Data on file)
Benker O, Gerbaldo H, Wetzel H. Negative symptomatology: Psychopharmacological strategies and methodological aspects for drug evaluation. Eur Neuropsychopharmacol 1993; 3 (3): 204–5
Carpenter Jr WT, Conley RR, Buchanan RW, et al. Patient response and resource management: another view of c10zapine treatment of schizophrenia. Am J Psychiatry 1995; 152: 827–32
Meltzer HY. Clozapine: is another view valid? Am J Psychiatry 1995; 152: 821–5
Bogetto F, Fonzo V, Maina G, et al. Adjunctive fluoxetine or amisulpride improves schizophrenic negative symptoms. Eur J Psych 1995; 9(2): 119–26
Lehman AF, Carpenter Jr WT, Goldman HH, et al. Treatment outcomes in schizophrenia: implications for practice, policy, and research. Schizophr Bull 1995; 21 (4): 669–75
Terra JL, Chatard JP, Dufour H, et al. Value of ami sui pride in schizophrenia with negative symptoms. Results of an open, multicenter trial as single-drug therapy [in French]. Sem Hop 1990 Feb 1; 66: 251–3
Singer L, Danion JM. Therapeutic value of low doses of amisulpride in the treatment of schizophrenia where negati ve symptoms predominate [in French]. J Med Strasbourg 1990; 21(6): 344–8
Mecheri G, Marie Cardine-M, Terra J-L. Open trial of amisulpride on negative symptoms of schizophrenia: report of nine cases [in French]. Psychol Med 1993; 25 (4): 347–55
Vaiva G, Thomas P, Dutoit D, et al. Low doses of amisulpride in negative schizophrenia: a 99m-Tc HMPAO SPECT study. Eur Neuropsychopharmacol 1995 Sep; 5 (special issue): 336
Clerc G. Double-blind study of amisulpride (Solian® 50) in various dosages in patients with negative schizophrenic symptoms [in French]. Sem Hop 1989; 65(17): 1079–82
Boyer P, Lecrubier Y, Puech AJ, et al. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995 Jan; 166: 68–72
Paillère-Martinot M-L, Lecrubier Y, Martinot J-L, et al. Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. Am J Psychiatry 1995 Jan; 152: 130–3
Pichot P, Boyer P. A double blind, controlled, multicenter trial of low dose amisulpride (Solian® 50) versus low dose fluphenazine in the treatment of negative symptoms in chronic schizophrenia [in French]. Ann Psychiatr 1988; 3(3) bis: 312–20
Saletu B, Küfferle B, Grünberger J, et al. Clinical, EEG mapping and psychometric studies in negative schizophrenia: comparative trials with amisulpride and fluphenazine. Neuropsychobiology 1994; 29(3): 125–35
Rein W, Favennec-Meidinger C. Amisulpride clinical textual summary (IV B). Synthèlabo Groupe (Le Plessis-Robinson, France), 1995, Report no. 95-00854-EN-OO. (Data on file)
Puech AJ, Fleurot O, Rein W, et al. Amisulpride in the treatment of acute exacerbations of subchronic or chronic schizophrenia: a dose range finding study. Annual Meeting of the Royal College of Psychiatrists (Joint Meeting with the Association of European Psychiatrists); 1996 Jul 7-12; London, England.
Costa e-Silva JA. A comparative double-blind trial of amisulpride versus haloperidol in the treatment of acute psychotic disorders [in French]. Ann Psychiatr 1990; 5(1): 71–8
Delcker A, Schoon ML, Oczkowski B, et al. Amisulpride versus haloperidol in treatment of schizophrenic patients — results of a double-blind study. Pharmacopsychiatry 1990 May; 23: 125–30
Möller HJ, Boyer P, Turjanski S, et al. Amisulpride in the treatment of subchronic or chronic schizophrenia with acute exacerbation: a double-blind comparison with haloperidol. Annual Meeting of the Royal College of Psychiatrists (Joint Meeting with the Association of European Psychiatrists, 7-12 Jul 1996), London
Pichot P, Boyer P. A double blind, controlled, multicenter trial of amisulpride versus high dose haloperidol in acute psychotic disorders [in French]. Ann Psychiatr 1988; 3(3) Bis: 326–32
Hillert A, Philipp M, Gattaz WF, et al. Amisulpride vs flupentixol in the treatment of schizophrenia with predominant positive symptomatology: a controlled double-blind study [abstract]. Neuropsychopharmacology 1994 May; 10 Suppl.2: 31
Van Putten T, Marder SR, Mintz J. A controlled dose comparison of haloperidol in newly admitted schizophrenic patients. Arch Gen Psychiatry 1990; 47: 754–8
Rifkin A, Doddi S, Karajgi B, et al. Dosage of haloperidol for schizophrenia. Arch Gen Psychiatry 1991; 48: 166–70
Cunningham Owens DG. Adverse effects of antipsychotic agents: do newer agents offer advantages? Drugs 1996; 51(6): 895–930
Kane JM. Schizophrenia. N Engl J Med 1996 Jan 4; 334(1): 34–41
Tracqui A, Mutter-Schmidt C, Kintz P, et al. Amisulpride poisoning: a report on two cases. Hum Exp Toxicol 1995 Mar; 14: 294–8
Terkelson KG, Menikoff A. Measuring the costs of schizophrenia: implications for the post-institutional era in the US. PharmacoEconomics 1995; 8(3): 199–222
Frankenburg FR, Hegarty JD. Cost considerations in the treatment of schizophrenia. CNS Drugs 1996; 5(1): 75–82
Wagstaff AJ, Bryson HM. Clozapine: a review of its pharmacological properties and therapeutic use in patients with schizophrenia who are unresponsive to or intolerant of classical antipsychotic agents. CNS Drugs 1995; 4(5): 370–400
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: W.T. Carpenter Jr, Maryland Psychiatric Research...
W.T. Carpenter Jr, Maryland Psychiatric Research Center, University of Maryland, Baltimore, Maryland, USA; Y. Lecrubier, Psychopathologie et Pharmacologie des Comportements, Institute National de la Santé et de la Recherche Médicale, Hôpital La Salpêtrière, Paris, France; J.-L. Martinot, Service Hospitalier Frédéric Joliot, Institute National de la Santé et de la Recherche Médicale, Orsay, France; H.-J. Möller, Psychiatrische Klinik und Poliklinik mit Konsiliardienst Grosshadem, Friedrich-Willhelms-Universität, Bonn, Germany; L.A. Opler, New York State Psychiatric Institute, New York, New York, USA; P. Protais, U.F.R. de Médecine et de Pharmacie, Université de Rouen—Haute Normandie, Saint Etienne Rouvray, France; P. Sokoloff, Unité de Neurobiologie et Pharmacologie, Centre Paul Broca, Institute National de la Santé et de la Recherche Médicale, Paris, France.
Rights and permissions
About this article
Cite this article
Coukell, A.J., Spencer, C.M. & Benfield, P. Amisulpride. CNS Drugs 6, 237–256 (1996). https://doi.org/10.2165/00023210-199606030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-199606030-00006