- Department of Neurosurgery, Hospital Clínico Regional de Concepción, Concepción, Bío Bío, Chile.
DOI:10.25259/SNI_222_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Juan P. Cabrera, Sebastián Vigueras, Rubén Muñoz, Eduardo López. Double neurophysiological certification of the filum terminale during sectioning surgery in pediatric population. 01-Aug-2020;11:229
How to cite this URL: Juan P. Cabrera, Sebastián Vigueras, Rubén Muñoz, Eduardo López. Double neurophysiological certification of the filum terminale during sectioning surgery in pediatric population. 01-Aug-2020;11:229. Available from: https://surgicalneurologyint.com/surgicalint-articles/10183/
Abstract
Background: Surgery of thickened-fibrolipoma filum terminale (FT) is performed routinely and without conflict but is not a risk-free surgical procedure. Intraoperative neurophysiological monitoring with mapping techniques can help to certify the FT before sectioning. However, a tailored surgical approach to cauda equina and a low threshold of surrounding nerve roots can confuse the final surgical decision. The aim is to demonstrate the usefulness of this double methodology for FT certification.
Methods: A prospective study collected and reviewed retrospectively, from 2015 to 2018, 40 patients undergoing an FT surgery section were included in the study. After opening the dura mater and under the microscope, the cauda equina mapping is performed and the recording of muscles of the lower limbs and the external anal sphincter. In addition, a high-intensity stimulation of constant current of an isolated FT for a short period of time and in a dry surgical field, obtaining a bilateral-polyradicular-symmetrical response of cauda equina nerve roots.
Results: Traditional motor mapping identified FT in 65% (26/40) of patients. Although, 35% (14/40) of the patients still have low-intensity stimuli response (
Conclusion: This proposed methodology accurately confirms the FT so that it can be safely found and cut. The Double Neurophysiological Certification improves the gap of the traditional mapping techniques of cauda equina and can be used in a variety of more complex surgeries in this area.
Keywords: Cauda equina, Conus medullaris, Filum terminale, Intraoperative neurophysiological monitoring, Sectioning surgery
INTRODUCTION
The conus medullaris is connected to the dural sac through intradural filum terminale (FT), a not-well defined fibrous ligament which has received relatively limited and not recent attention in the literature regarding its anatomical and histological characteristics.[
Thickened FT or FT fibrolipoma require surgical section for prevention or treatment of neurological, musculoskeletal, and urological abnormalities,[
In spite of FT can be anatomically recognized, mapping techniques, that is, stimulating motor nerve roots to identify and locate the FT before cutting, are recommended.[
MATERIALS AND METHODS
The prospective study collected and reviewed retrospectively, from 2015 to 2018, 40 patients undergoing a sectioning surgery for FT. We considered patients with magnetic resonance imaging (MRI) showing fibrolipoma FT or thickened FT aged from 6 months to 18 years old. The main indications of surgery were patients with urodynamic abnormalities ruling out other causes, neurogenic bowel/ bladder, before scoliosis correction and FT abnormality, FT pathology with syringomyelia, progressive lower extremities orthopedic deformities, conus medullaris low inserted, and clinical symptoms of TCS. We excluded patients with other causes of TCS and patients who had previously undergone lumbosacral spine surgery to prevent the need for isolation of scar tissue in neural structures.
Anesthesia was performed with total intravenous anesthesia, avoiding neuromuscular blockade. Gauze bite blocks were intraorally used in all cases.
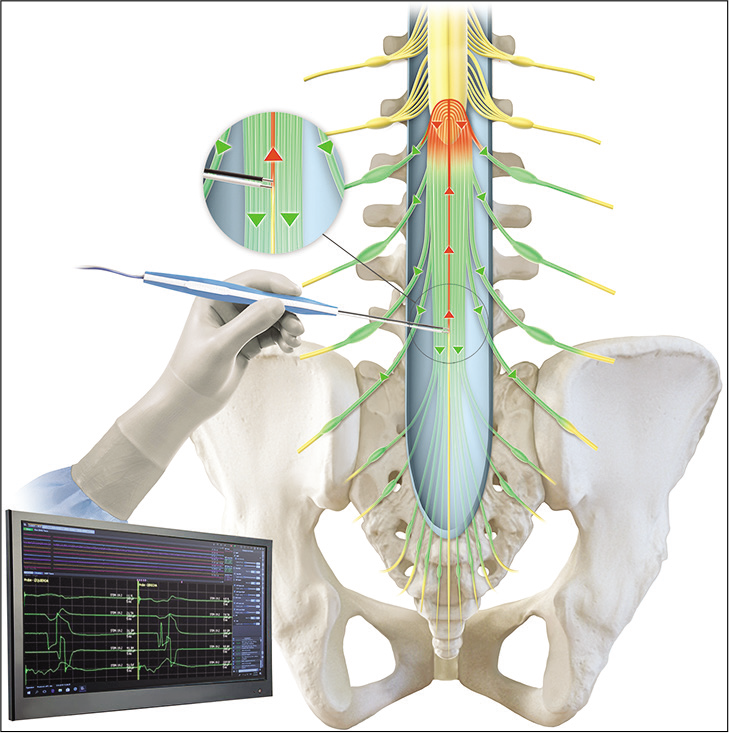
Intraoperative neurophysiological monitoring
We used the NIM Eclipse Medtronic IOM system, with disposable subdermal double needle electrodes, which are 12 mm in length and 0.4 mm in diameter (27 gauge).
The needle electrodes are placed in groups of muscles innervated by the nerve roots which had the greatest risk of injury during release. These muscles included the bilateral quadriceps femoris, tibialis anterior, gastrocnemius medial, abductor hallucis, and anal sphincter.
Recording parameters for free-run and triggered EMG are set with filters between 20 and 3.000 Hz, gain 100 μV/Div, and time base 5 m/div.
Traditional mapping technique
Single-pulse stimulation is performed with repetitive constant current, 200 μS pulse duration at 1–2 Hz, using a monopolar flush-tip/ball tip probe, or bipolar probe when is available preferably. Mapping technique comprises stimulating the motor nerve roots of cauda equina and getting a unilateral, asymmetrical, and single radicular response from muscles studied according to its dominance and participation. When the FT is stimulated, non-response is expected. Mapping of each motor or sensory nerve roots was not performed.
High intensity stimulation
This proposed methodology consists in stimulation of an isolated FT using high intensity current as much as 20 mA, obtaining a bilateral, symmetrical, and polyradicular response from all cauda equina nerve roots studied. This technique complements the traditional technique when stimulation of the FT with low intensity evokes a response from any muscle, despite proper separation and dissection of the surrounding neural elements. When the FT is stimulated, an all-response is expected. Latencies of the responses were not assessed and not compared.
Surgical technique
Minimally invasive posterior approach to L5-S1 level is performed and under microscopical view, the dura mater opened, the nerve roots are recognized, and any of them is stimulated for obtaining motor threshold. The FT is identified anatomically followed by arachnoidal dissection, isolated by vascular a band, maintaining a dry surgical field, and placing cottonoids over surrounding nerve roots. If the mapping technique does not evoke any muscle response using ≥3 mA, the FT is coagulated and cut. Alternatively, if any muscle response is obtained, the region surrounding the FT is explored certifying absence of neural elements; if the response persists, the current intensity is increased until 20 mA. When this stimulation evokes response from all muscles studied, the FT is coagulated and cut. During this study, we performed double neurophysiological certification in all cases, despite no response with traditional technique.
RESULTS
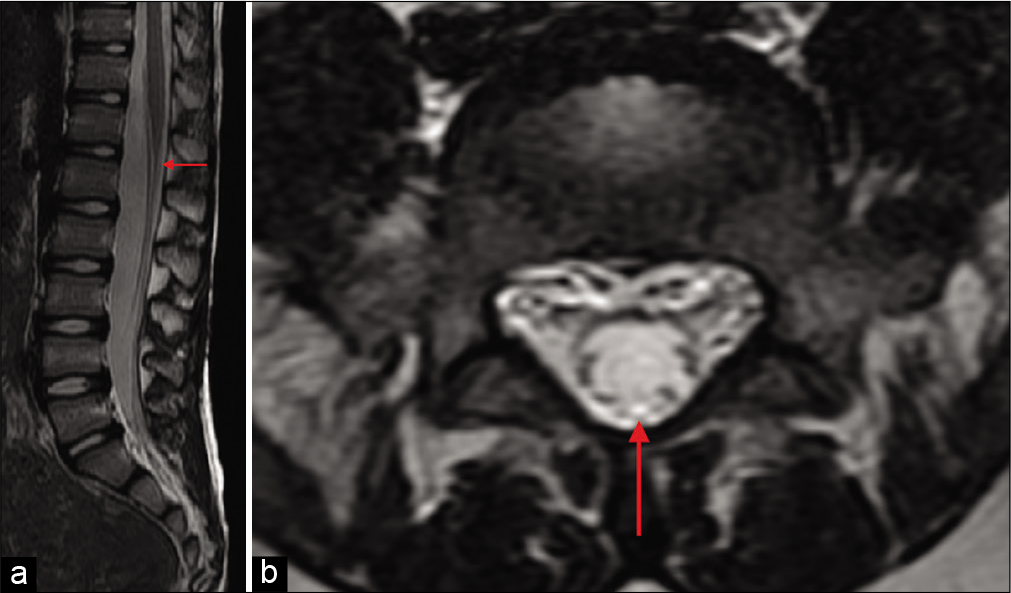
In all cases (40/40), patients were studied with MRI showing kind of FT and if conus medullaris is normally located [
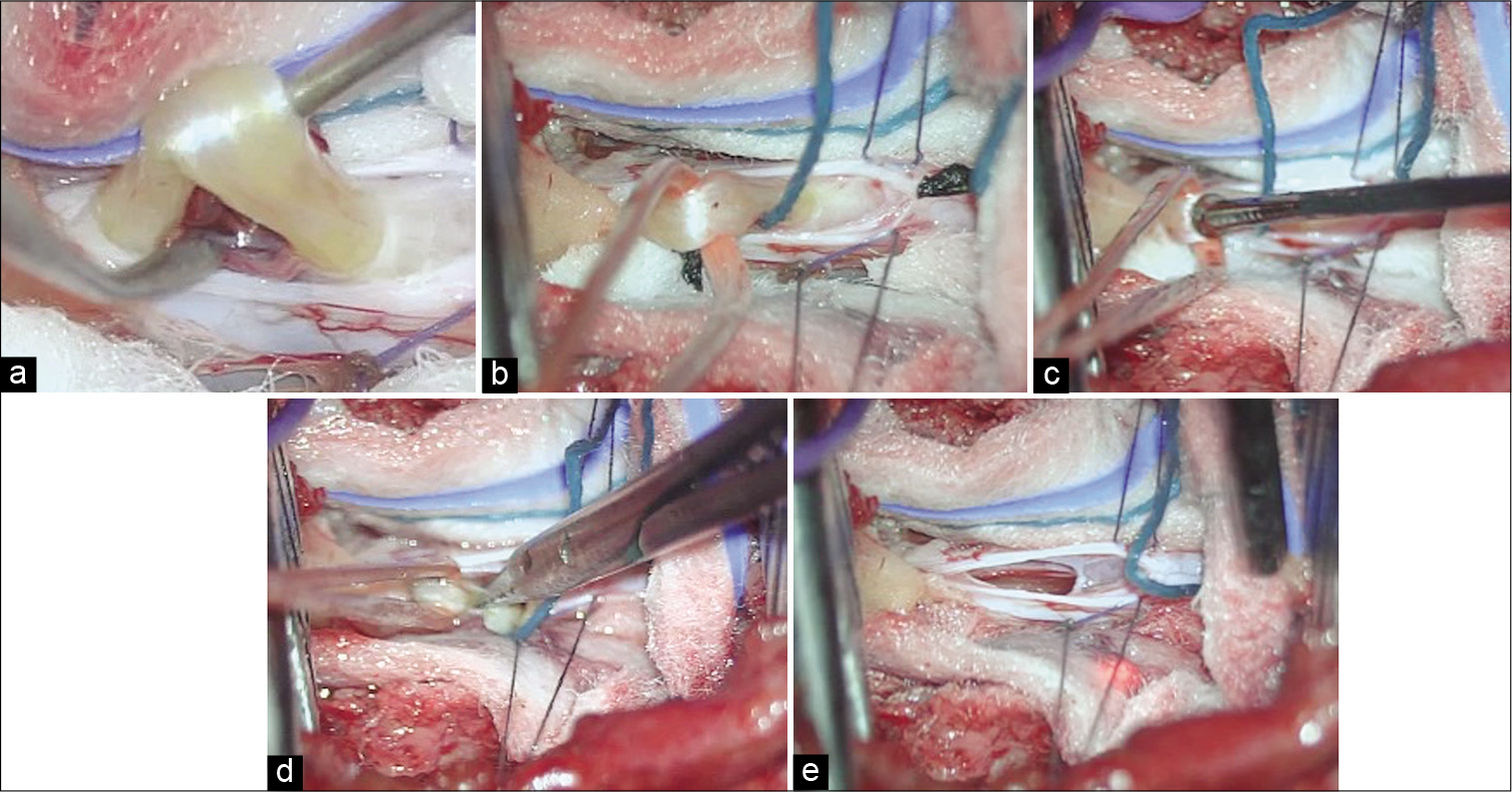
Figure 2:
Surgical steps during filum terminale (FT) sectioning. (a) FT is exposed and separated of the surrounding nerve roots. (b) FT is carried outside the dura mater by a vascular band. (c) Isolated FT is stimulated after arachnoid dissection in a dry surgical field. (d) FT is cut with safety. (e) Dura mater is already to close with the pathology of FT resolved.
Traditional motor mapping technique could identify FT in 65% (26/40) of patients, using intensity >3 mA without eliciting a response, increasing stimulation intensity up to 5 mA to ensure absence of nerve roots before sectioning, while threshold of motor nerve roots was elicited with <1 mA.
Despite low-intensity stimulation (<1 mA), muscle response was still present in 35% (14/40) of cases and was almost always an anal sphincter response, either unilateral or bilateral. When this happens, optimization of the dissection surrounding the FT is performed. In this scenario, 25% (10/40) of patients still have a muscle response despite an isolated FT.
After improved the dissection and isolation, an increment of the stimulation intensity up to 20 mA evoked response of the cauda equina in all cases (40/40), obtaining a bilateral- polyradicular-symmetrical response in all muscles included in the recording. This double certification allowed safely cut FT in all patients [
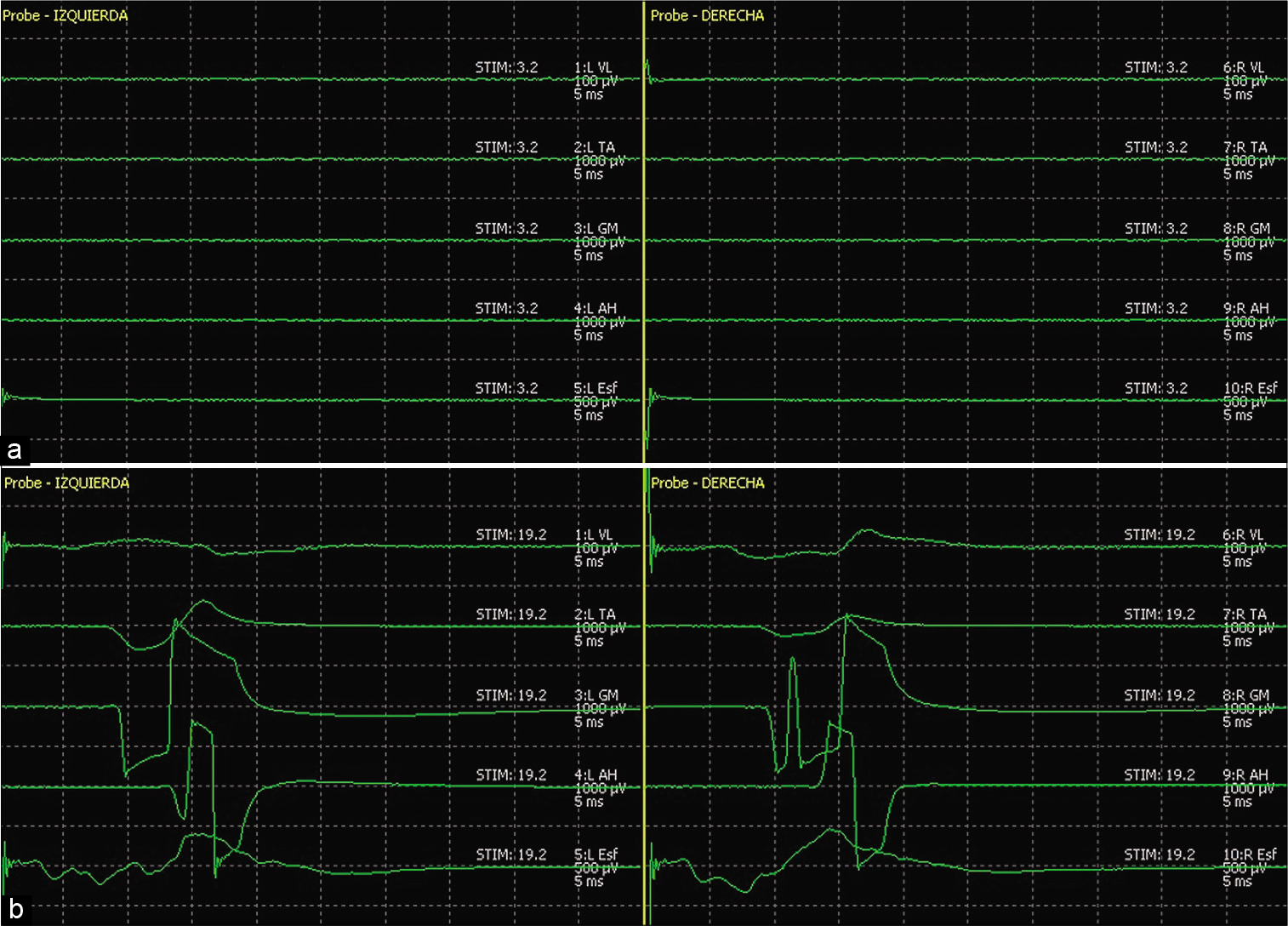
Figure 3:
Double neurophysiological certification of filum terminale (FT). (a) Traditional mapping technique, stimulation of FT at 3.2 mA without motor nerve roots response, confirming FT. (b) High-intensity stimulation of the same FT. Note the bilateral, polyradicular, and symmetrical response in all muscles studied, confirming FT. Quadriceps femoris (vastus lateralis), tibialis anterior, gastrocnemius medial, abductor hallucis, anal sphincter.
Stimulating a non-isolated nerve root at 1 mA threshold was obtained and at 20 mA evokes a mainly unilateral- polyradicular-asymmetrical response [
Figure 4:
Stimulation of intradural, wet and non-isolated right sided nerve root. (a) Close to the threshold <1 mA, obtaining a single muscle response from the same side (arrow). (b) High-intensity stimulation at 20 mA of the same nerve root. Note the response preferably unilateral, polyradicular for current spreading, and highly asymmetrical. Quadriceps femoris (vastus lateralis), tibialis anterior, gastrocnemius medial, abductor hallucis, and anal sphincter.
Notably, all patients of this case series did not develop a new neurological worsening.
DISCUSSION
As an integral methodology of intraoperative neurophysiological monitoring, motor root mapping techniques can accurately differentiate neurological tissues from other non-neurological surrounding structures.[
FT is a midline non-neurological structure. However, in accordance with their common embryological origin, a true anatomical and histological continuum was found between the conus medullaris and FT.[
Some authors have reported that a 1:100 ratio between the motor root and the filum threshold for FT identification predicts that, if all nerve roots are sufficiently separated from FT in the adult[
CONCLUSION
From a practical point of view, this methodology is proposed to accurately confirm FT, locate it, and safely cut it. There is no doubt that future research is needed to rule out current spreading and understand the exact mechanism of the response. The Double Neurophysiological Certification improves the gap of the traditional motor mapping technique of the cauda equina in a tailored surgical approach and can be used in various more complex surgeries in this area.
Declaration of patient consent
Patient’s consent not obtained as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Hayashi T, Kimiwada T, Kohama M, Shirane R, Tominaga T. Minimally invasive surgical approach to filum lipoma. Neurol Med Chir (Tokyo). 2018. 58: 132-7
2. Husain AM, Shah D. Prognostic value of neurophysiologic intraoperative monitoring in tethered cord syndrome surgery. J Clin Neurophysiol. 2009. 26: 244-7
3. Kothbauer KF, Deletis V. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst. 2010. 26: 247-53
4. Picart T, Barritault M, Simon E, Robinson P, Barrey C, Meyronet D. Anatomical and histological analysis of a complex structure too long considered a simple ligament: The filum terminale. World Neurosurg. 2019. 129: e464-71
5. Quiñones-Hinojosa A, Gadkary CA, Gulati M, von Koch CS, Lyon R, Weinstein PR. Neurophysiological monitoring for safe surgical tethered cord syndrome release in adults. Surg Neurol. 2004. 62: 127-33
6. Sala F, Tramontano V, Squintani G, Arcaro C, Tot E, Pinna G. Neurophysiology of complex spinal cord untethering. J Clin Neurophysiol. 2014. 31: 326-36
7. Schirmer CM, Shils JL, Arle JE, Cosgrove GR, Dempsey PK, Tarlov E. Heuristic map of myotomal innervation in humans using direct intraoperative nerve root stimulation: Clinical article. J Neurosurg Spine. 2011. 15: 64-70
8. Steinbok P, MacNeily AE, Hengel AR, Afshar K, Landgraf JM, Hader W. Filum section for urinary incontinence in children with occult tethered cord syndrome: A randomized, controlled pilot study. J Urol. 2016. 195: 1183-8
9. Von Koch CS, Quinones-Hinojosa A, Gulati M, Lyon R, Peacock WJ, Yingling CD. Clinical outcome in children undergoing tethered cord release utilizing intraoperative neurophysiological monitoring. Pediatr Neurosurg. 2002. 37: 81-6