Associations of Chronic Kidney Disease Markers with Cognitive Function: A 12-Year Follow-Up Study
Abstract
Background:
The role of chronic kidney disease (CKD) as a risk factor for cognitive impairment independent of their shared antecedents remains controversial.
Objective:
To determine whether kidney damage (indicated by albuminuria) or kidney dysfunction (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2) predict future (12-year) cognitive function independently of their shared risk factors.
Methods:
We studied 4,128 individuals from the 1999/00 population-based Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study who returned in 2011/12 for follow-up cognitive function testing. Albuminuria was defined by urinary albumin:creatinine≥3.5 (women) or≥2.5 mg/mmol (men). Kidney dysfunction was indicated by eGFR <60 ml/min/1.73 m2. Cognitive function domains assessed included memory (California Verbal Learning Test [CVLT]) and processing speed (Symbol Digit Modalities Test [SDMT]).
Results:
Baseline albuminuria and kidney dysfunction were identified in 142 (3.4%) and 39 (0.9%) individuals, respectively, with minimal overlap (n = 7). Those with albuminuria demonstrated concurrently reduced 12-year SDMT (p = 0.084) and CVLT scores (p = 0.005) following adjustment for age, sex, and education. However, only CVLT performance remained worse (p = 0.027) following additional adjustment for myocardial infarction, stroke, and related risk factors (hypertension, diabetes, dyslipidemia, smoking, BMI, physical activity, and alcohol intake). Indeed, these collective covariates were responsible for 47% of the effect of albuminuria on SDMT, but only 21% of its effect on CVLT. Kidney dysfunction was not associated with either SDMT or CVLT performance (p > 0.10).
Conclusions:
Albuminuria predicted worse memory function at 12 years follow-up, whereas its effect on processing speed was driven largely by differences in cardiovascular risk. Kidney dysfunction based on eGFR predicted neither cognitive domain.
INTRODUCTION
Cognitive impairment preceding dementia is common in older adults and projected to continue to escalate in line with ever-increasing longevity [1]. Screening of high-risk individuals and early therapeutic intervention are obviously desirable in this context, but a prerequisite of this remains a more complete understanding of antecedent risk factors and risk markers. Cognitive impairment is highly prevalent in end-stage kidney disease [2] and associated with more prolonged hospitalization, greater time demands on healthcare professionals, and excess mortality in this setting [3–5]. Over-representation of cognitive impairment in earlier stages of chronic kidney disease (CKD) is also recognized—an association found in several cross-sectional studies to be independent of traditional cardiovascular risk factors and other potential covariates [2]. While these data collectively suggest CKD as a contributor to cognitive impairment and progressive cognitive decline toward dementia, relevant prospective studies have produced conflicting evidence. A 2012 meta-analysis reported ∼1.4 times higher odds of worse cognition in those with CKD, compared to those without, but with significant between-study heterogeneity (from six longitudinal studies spanning 2 to 7 years) [2]. Given emerging evidence that kidney damage (e.g., albuminuria) may affect cognitive function independently of the effects of kidney function [6], a common limitation of these studies has been their reliance on estimates of glomerular filtration in isolation. Concern has also been raised about lack of adjustment for known determinants of cognitive impairment that frequently coexist with CKD [2]. Accordingly, studies of longer-term exposure characterizing both kidney damage and kidney dysfunction, appropriately adjusted for all key confounders, are required to better define the respective roles of these indicators in subsequent cognitive decline.
The population-based, Australian Diabetes, Obesity and Lifestyle Study (AusDiab) quantified kidney function and kidney damage as part of broader cardiometabolic risk profiling at baseline and then again at 5 and 12 years of follow-up. Cognitive function, including the domains of processing speed, memory, and verbal ability, was additionally assessed at 12 years. In this study, we tested the hypothesis that the constituent subtypes of CKD (i.e., kidney damage indicated by albuminuria, and kidney dysfunction based on reduced estimated glomerular filtration rate [eGFR]) would independently predict worse cognitive function measured 12 years later.
MATERIALS AND METHODS
AusDiab is a national population-based survey directed at the epidemiology of diabetes and related chronic diseases and risk factors in adults aged≥25 years. Detailed sampling and data collection methods have been described elsewhere [7]. In brief, 11,247 individuals completed the baseline survey in 1999/00, 6,537 of whom returned for a 5-year follow-up assessment in 2004/05 and 4,615 for a 12-year follow-up in 2011/12. Blood and urine biochemical markers of kidney damage and kidney function were measured at all time points. Cognitive function testing was introduced at the 2011/12 follow-up. The AusDiab study was approved by human research ethics committees of the International Diabetes Institute, Monash University and Alfred Hospital. All individuals provided written informed consent.
Exposures (kidney damage and kidney dysfunction)
Albuminuria (encompassing macro- and microalbuminuria) was defined by albumin:creatinine ratio≥3.5 mg/mmol (women) or≥2.5 mg/mmol (men) from an early morning random spot urine sample. Kidney dysfunction was indicated by eGFR <60 ml/min/1.73 m2 (where eGFR was calculated from serum creatinine according to the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation [8]). The primary analyses were based on albuminuria/kidney dysfunction characterized at baseline (1999/00; i.e., given this afforded the longest duration of exposure). However, comparisons were also made on the basis of the 2004/05 classification to explore potential differences according to exposure duration (i.e., 12 years versus 7 years).
Since cystatin C (an alternate filtration biomarker that may hold advantages over creatinine for eGFR calculation) was also measured in 1999/00, we repeated the 12-year exposure analyses after redefining kidney dysfunction using two cystatin C-based eGFR equations (one using cystatin C only; the other both cystatin C and serum creatinine combined; available in 95% of the final sample) [9].
Outcome measures (cognitive function)
Two domains of cognitive function comprised the primary outcome measures [10]:
1) Memory (California Verbal Learning Test [CVLT]; one immediate and one delayed [20-min] trial) [11]. Scores range from 0 to 16 based on recall of 16 common shopping list items.
2) Processing speed (Symbol Digit Modalities Test [SDMT]) [12]. Score (unbounded) based on the number of correct symbol-to-digit substitutions made within 90 s, using a supplied reference key.
Since CVLT and SDMT scores were based on different scales of measurement (i.e., making it difficult to evaluate differences in memory and processing speed relative to each other), these data were re-expressed as z-scores (i.e., standardized values based on number of standard deviations from the mean).
Premorbid/age-independent cognition was estimated by level of educational attainment in all individuals; however, a formal assessment—the Spot-The-Word (STW) test of verbal ability [13]—was undertaken in a subgroup of 4,037 individuals (scored from 0 to 60 based on the number of ‘real’ words identified from 60 pairs of real versus non-words). This afforded sensitivity analyses of between-group differences adjusted for STW score, rather than education level, in 98% of the final sample.
Individuals 60 years and older were additionally screened for global cognitive impairment using the Mini-Mental State Examination (MMSE; <24 out of 30 indicating cognitive impairment) [14].
Covariates
Age, sex, education level, medical history, medication use, and clinical/behavioral risk factors were derived from a combination of interviewer-administered questionnaires and clinical/ biochemical data. Diabetes was deemed to be present in cases of a self-reported previous diagnosis and/or use of hypoglycemic medication, or if World Health Organization (WHO) oral glucose tolerance testing (OGTT) criteria were met [15]. Pre-diabetes was also classified on the basis of WHO OGTT criteria. Hypertension was defined by blood pressure≥140/90 mmHg (seated rest; mean of at least two recordings) and/or use of antihypertensive medication. Physical activity was quantified using a validated survey [16], based on which individuals were classified as being sufficiently active (i.e., meeting the public health-recommended volume of≥150 min/week moderate-intensity activity, or an equivalent volume at higher intensity), insufficiently active (i.e., <150 min/week moderate-intensity activity or equivalent), or inactive. Due to its previously reported non-linear relationship with cognition [17], alcohol intake was categorized as follows: abstainers; >0 but <10 g/day; ≥10 to <20 g/day; 20 + g/day (where 10 g defines 1 standard drink in Australia). Dyslipidemia was defined by any abnormality of total cholesterol (≥5.5 mmol/L), HDL cholesterol (<1.0 mmol/L), LDL cholesterol (≥3.5 mmol/L), or triglycerides (≥2.0 mmol/L), or use of lipid-lowering medication. Other covariates included body mass index (BMI; standard calculation from height and weight), smoking status (current versus former smoker versus never-smoked), and depressive symptoms (based on a Center for Epidemiologic Studies Short Depression Scale [18] score of≥10). All covariates were assessed at each time point except for depressive symptoms (2011/12 follow-up only).
Laboratory methods
Urinary albumin was analyzed by rate nephelometry with the Beckman Array (Beckman/Coulter, Fullerton, USA). Urinary creatinine in 1999/00 was derived from the modified kinetic Jaffé reaction (Olympus AU600 autoanalyzer; Olympus Optical Co. Ltd., Tokyo, Japan), whereas at follow-up it was based on the spectrophotometric-Jaffe alkaline picrate method (Roche Modular; Roche Diagnostics, Indianapolis USA). To ensure standardization of eGFR values across the entire cohort, serum creatinine was measured on stored, frozen (–80°C) samples by enzymatic assay calibrated to the Isotope Dilution Mass Spectrometry method (Roche Modular). Cystatin C was measured using an immunoturbidimetric assay (Roche/Hitachi 917, MODULAR P analyzer, Roche Diagnostics).
Plasma glucose, total cholesterol, HDL-cholesterol, and triglycerides were measured enzymatically using an Olympus AU600 analyzer (Olympus Optical). Glycated hemoglobin (HbA1c) was derived from HPLC (Bio-Rad Variant Hemoglobin Testing System).
Statistical analyses
Data were expressed as mean±standard deviation (continuous variables) or as percentages (categorical variables), with unadjusted group comparisons reflecting a t- or chi-square test, respectively. Transformations were applied to achieve normality where appropriate.
Independent associations of baseline (i.e., 1999/00) albuminuria and kidney dysfunction with cognitive function in 2011/12 were analyzed using a series of ANCOVA models with adjustment for specific sets of covariates. The base model (Model 1) featured age, sex, and education. Model 2 additionally adjusted for atherosclerotic cardiovascular disease (i.e., myocardial infarction or stroke), diabetes, and hypertension (i.e., major causes/consequences of CKD known to contribute to cognitive impairment; where atherosclerotic cardiovascular disease was a composite of previous history at baseline and incident events during the 12-year follow-up) [17]. In model 3, lifestyle and other risk factors influencing cognitive function were also included (i.e., BMI, physical activity, smoking, alcohol intake, and dyslipidemia) [17]. Where between-group differences were observed in model 1, the percentage changes in the standardized β from model 1 ⟶ 2, and model 1 ⟶ 3 in turn, were calculated. These values estimated the proportions of the effect of albuminuria/kidney dysfunction explained by the added covariates (i.e., beyond age, sex, and education).
Sensitivity analyses included the following: 1) additional adjustment for the presence of depressive symptoms at 12 years; and 2) replacement of education level with STW score in the fully adjusted model.
Interactions were tested by re-analyzing the fully adjusted model inclusive of an interaction term for each of the following covariates, in turn: age, sex, education level, atherosclerotic cardiovascular disease, diabetes, and hypertension (where p < 0.10 indicated the presence of an interaction effect). For the categorical outcome variable cognitive impairment, multiple logistic regression was applied to determine its associations with each exposure, independent of confounders. All analyses were undertaken using STATA version 14 (STATA, College Station, USA) and p < 0.05 defined statistical significance.
RESULTS
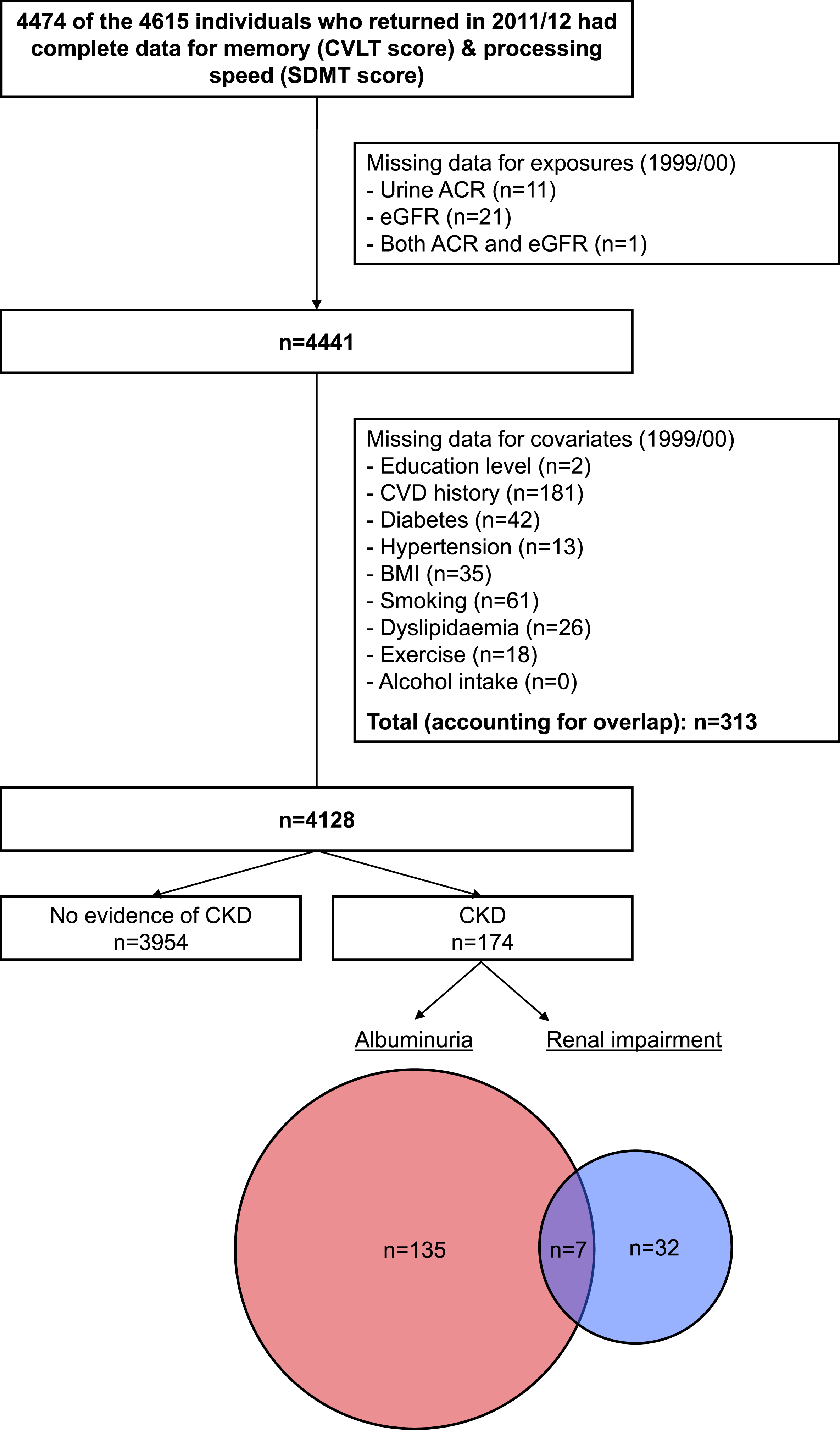
Derivation of the primary analytic sample is depicted in Fig. 1. After taking into account attrition and missing data, we included 4,128 individuals with cognitive function measured in 2011/12, kidney function and kidney damage characterized in 1999/00, and complete data for relevant covariates. CKD was identified in 174 of these individuals (4.2%), of whom 142 had albuminuria (82%) and 39 (22%) had kidney dysfunction (distribution/overlap of the two CKD subtypes is shown in the Venn diagram within Fig. 1). This proportion with CKD was lower than that identified in the original population-based AusDiab sample and in the cohort who returned for the interim 2004/05 assessment (10% for both). Supplementary Table 1 displays a comparison of those with and without evidence of CKD in 1999/00 according to their inclusion/exclusion across subsequent waves of follow-up. The final analytic sample demonstrated a more favorable clinical profile overall (irrespective of CKD status) compared with those groups lost to follow-up in the first (1999/00 to 2004/05) and second (2004/05 to 2011/12) phases, which were relatively similar to each other. However, notable features of the group with CKD included in this study versus those lost to follow-up due to death/withdrawal included their younger age at baseline and lower proportions with a history of myocardial infarction, stroke, and diabetes.
Fig. 1.
Flow diagram showing derivation of the analytic sample. ACR, albumin:creatinine ratio; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; CVLT, California Verbal Learning Test; SDMT, Symbol Digit Modalities Test; eGFR, estimated glomerular filtration rate.
Participant characteristics
Clinical profiles of individuals with and without both albuminuria and kidney dysfunction in 1999/00 are displayed in Table 1. Although each CKD marker was associated with older age, the difference was less marked in the albuminuria group (∼5 years older than controls; mean 53 years) compared with the kidney dysfunction group (∼20 years older than controls; mean 68 years). Each of albuminuria and kidney dysfunction were associated with lower education level, higher prevalence of pre-diabetes/diabetes, hypertension, and myocardial infarction, higher mean values of BMI, systolic blood pressure, total cholesterol, and HbA1c, lower mean HDL cholesterol, and lower alcohol intake. Individuals with albuminuria also reported lower levels of physical activity and were found to have higher diastolic blood pressure (not observed in the kidney dysfunction group). In turn, those with kidney dysfunction tended to have higher LDL cholesterol (not observed in the albuminuria group). Table 1 also displays raw/unadjusted cognitive function data (full analyses described in the sections below). MMSE screening in the subgroup of individuals aged 60 + revealed cognitive impairment in 3.5%.
Table 1
Clinical characteristics at baseline (1999/00)
| Albuminuria (n = 142) | Normal ACR (n = 3986) | p value | Kidney dysfunction (n = 39) | Normal eGFR (n = 4089) | p value | |
| Age (y) | 53±12 | 48±11 | <0.001 | 68±8 | 48±11 | <0.001 |
| Male (%) | 42 | 44 | 0.67 | 44 | 44 | 0.96 |
| Education (%) | 0.004 | 0.12 | ||||
| <12 y | 47 | 34 | 49 | 34 | ||
| Secondary school | 18 | 20 | 10 | 20 | ||
| Tertiary | 35 | 47 | 41 | 46 | ||
| Clinical profile (%) | ||||||
| History of myocardial infarction | 1.4 | 0.5 | – | 5.1 | 0.5 | – |
| History of stroke | 0 | 0.3 | – | 0 | 0.2 | – |
| Diabetes | <0.001 | <0.001 | ||||
| IFG or IGT | 23 | 15 | 44 | 15 | ||
| Diabetes | 15 | 4 | 5 | 4 | ||
| Hypertension | 61 | 22 | <0.001 | 79 | 23 | <0.001 |
| Depressive symptoms (2011/12) | 14 | 10 | 0.14 | 16 | 10 | 0.23 |
| Events during 12-year f/u | ||||||
| Incident stroke | 1.4 | 0.3 | – | 0 | 0.3 | – |
| Incident CAD | 9.2 | 2.4 | <0.001 | 18.0 | 2.5 | <0.001 |
| Lifestyle risk factors (%) | ||||||
| Smoking | 0.54 | 0.32 | ||||
| Former | 29 | 28 | 23 | 28 | ||
| Current | 13 | 11 | 5 | 11 | ||
| Physical activity | 0.001 | 0.55 | ||||
| Inactive | 26 | 15 | 15 | 15 | ||
| Insufficient | 31 | 31 | 23 | 31 | ||
| Sufficient | 43 | 54 | 62 | 54 | ||
| Alcohol intake | 0.035 | 0.004 | ||||
| Abstainer | 19 | 12 | 26 | 12 | ||
| >0 to <10 g/day | 47 | 45 | 38 | 46 | ||
| ≥10 to <20 g/day | 15 | 18 | 28 | 18 | ||
| ≥20 g/day | 19 | 25 | 8 | 25 | ||
| Clinical measures | ||||||
| Body mass index (kg/m2) | 28.0±5.8 | 26.5±4.7 | <0.001 | 27.7±4.2 | 26.5±4.7 | 0.125 |
| Systolic BP (mmHg) | 142±25 | 126±16 | <0.001 | 144±24 | 126±16 | <0.001 |
| Diastolic BP (mmHg) | 77±13 | 70±11 | <0.001 | 73±12 | 70±11 | 0.110 |
| Total cholesterol (mmol/L) | 5.80±1.12 | 5.58±1.01 | 0.013 | 6.00±0.97 | 5.58±1.02 | 0.010 |
| HDL cholesterol (mmol/L) | 1.37±0.35 | 1.45±0.38 | 0.018 | 1.38±0.38 | 1.45±0.38 | 0.300 |
| LDL cholesterol (mmol/L) | 3.55±0.97 | 3.49±0.90 | 0.441 | 3.74±0.80 | 3.49±0.91 | 0.107 |
| HbA1c (%) | 5.4±1 | 5.1±0.5 | <0.001 | 5.4±0.4 | 5.1±0.5 | 0.007 |
| Cognitive function in 2011/12 | ||||||
| SDMT – raw score | 46±14 | 50±11 | <0.001 | 38±13 | 50±11 | <0.001 |
| – z-score | –0.4±1.2 | 0±1.0 | <0.001 | –1.1±1.2 | 0±1.0 | <0.001 |
| CVLT – raw score | 5.7±2.4 | 6.6±2.4 | <0.001 | 4.9±2.4 | 6.6±2.4 | <0.001 |
| – z-score | –0.3±1.0 | 0±1.0 | <0.001 | –0.7±1.0 | 0±1.0 | <0.001 |
| STW score * | 3.3±1 | 3.2±0.8 | 0.188 | 3.0±1 | 3.2±0.8 | 0.102 |
| Cognitive impairment † | 8.1 | 3.3 | 0.028 | 3.9 | 3.5 | – |
Data are mean±standard deviation or %. p values reflect t-test or chi-square (omitted for categorical variables with cells showing n < 5).
[i] * To achieve normality, raw STW scores were reflected (i.e., so that higher values indicated worse function), and then square-root transformed.
[ii] †Screening performed only on the subgroup of individuals aged 60 + years. ACR, albumin:creatinine ratio; BP, blood pressure; CAD, coronary artery disease; CVLT, California Verbal Learning Test; eGFR, estimated glomerular filtration rate; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SDMT, Symbol Digit Modalities Test; STW, Spot-The-Word.
Albuminuria and cognitive function at 12 years
Individuals with albuminuria in 1999/00 had worse memory 12 years later, indicated by a lower mean CVLT score that persisted across all stages of covariate adjustment (Fig. 2). Relative to the base model inclusive of age, sex, and education, the proportion of this effect explained by the collective covariates of atherosclerotic cardiovascular disease, diabetes, and hypertension was 8% (model 2). When lifestyle and other risk factors were included, the proportion mediated increased to 21%.
Fig. 2.
Albuminuria and cognitive function measured at 12 years of follow-up. Data are between-group differences in CVLT and SDMT z-scores measured at the 2011/12 follow-up. All data are estimated marginal means with 95% confidence intervals (error bars). Error bars crossing the zero line indicate no significant difference between individuals with versus without albuminuria (p > 0.05). Models 1 to 3 reflect incremental adjustment for a progressively more covariates; i.e., Model 1 featured age, sex, and education level only; Model 2: Model 1 covariates + atherosclerotic cardiovascular disease, hypertension and diabetes; Model 3: Model 2 covariates + body mass index, physical activity, smoking, alcohol intake, and dyslipidemia. The percentage values indicate the proportion of the effect of albuminuria mediated by these incrementally added covariates (i.e., beyond the Model 1 covariates of age, sex and education). CVLT, California Verbal Learning Test; SDMT, Symbol Digit Modalities Test.
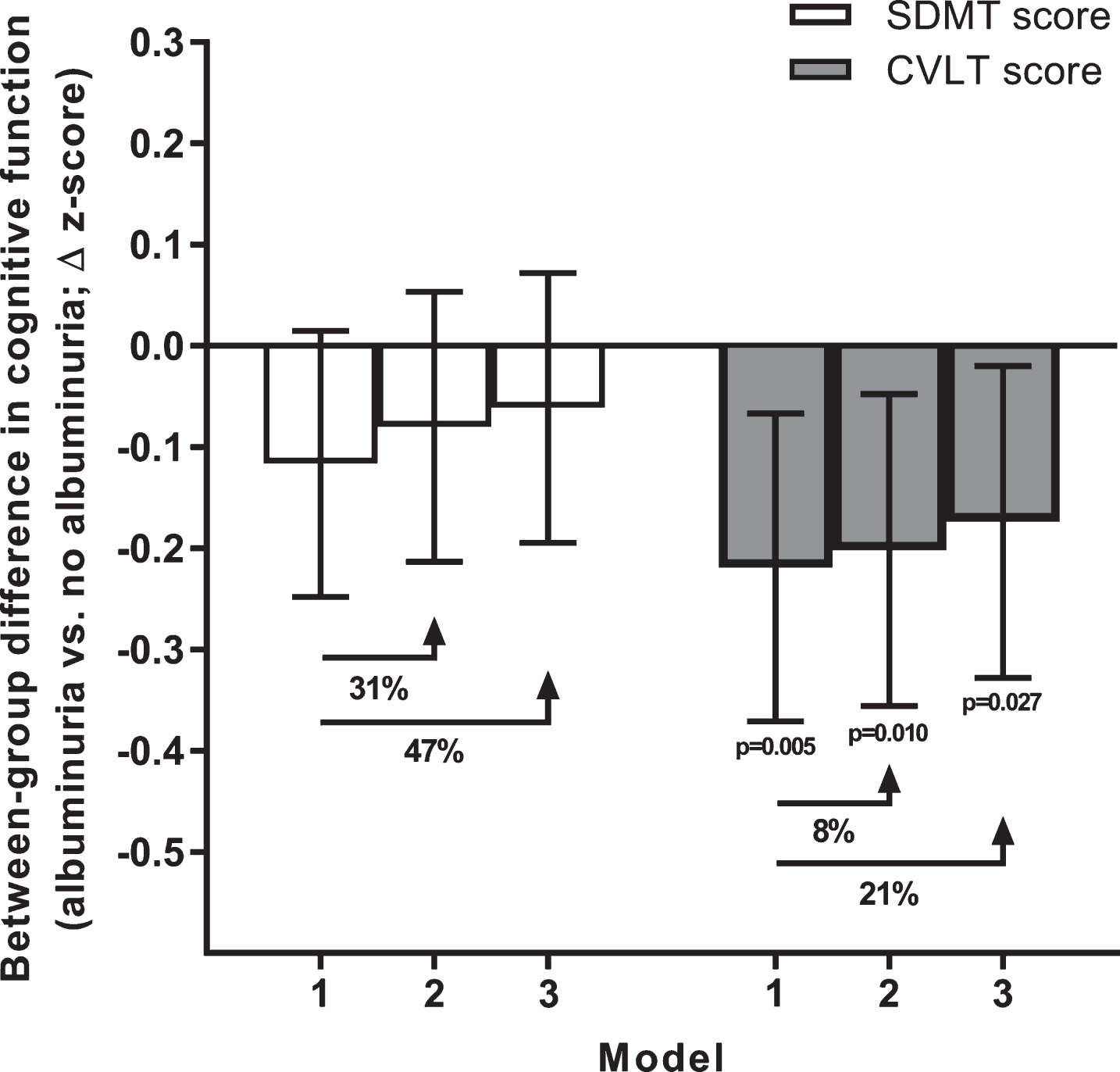
In contrast to memory, worse processing speed in individuals with albuminuria was only borderline significant in the base model, and largely explained by cardiometabolic factors (model 2 proportion mediated: 31%) and lifestyle/other risk factors (model 3 proportion mediated: 47%).
Sensitivity analyses
No interactions between albuminuria and the tested covariates on either CVLT or SDMT scores, were observed. Additional adjustment for the presence of depressive symptoms characterized at the time of cognitive testing (subgroup n = 3985) did not alter group differences in CVLT or SDMT from those described for model 3 (despite depressive symptoms being a significant predictor of both CVLT and SDMT scores; p < 0.05).
In the subgroup of 3,993 individuals whose premorbid function was characterized by verbal ability (STW score), replacement of education level with this marker made no substantive impact on results pertaining to memory (though lower mean CVLT score with albuminuria became borderline significant in the fully adjusted model 3; p = 0.069). Likewise, non-significant differences in processing speed were unaffected by STW adjustment.
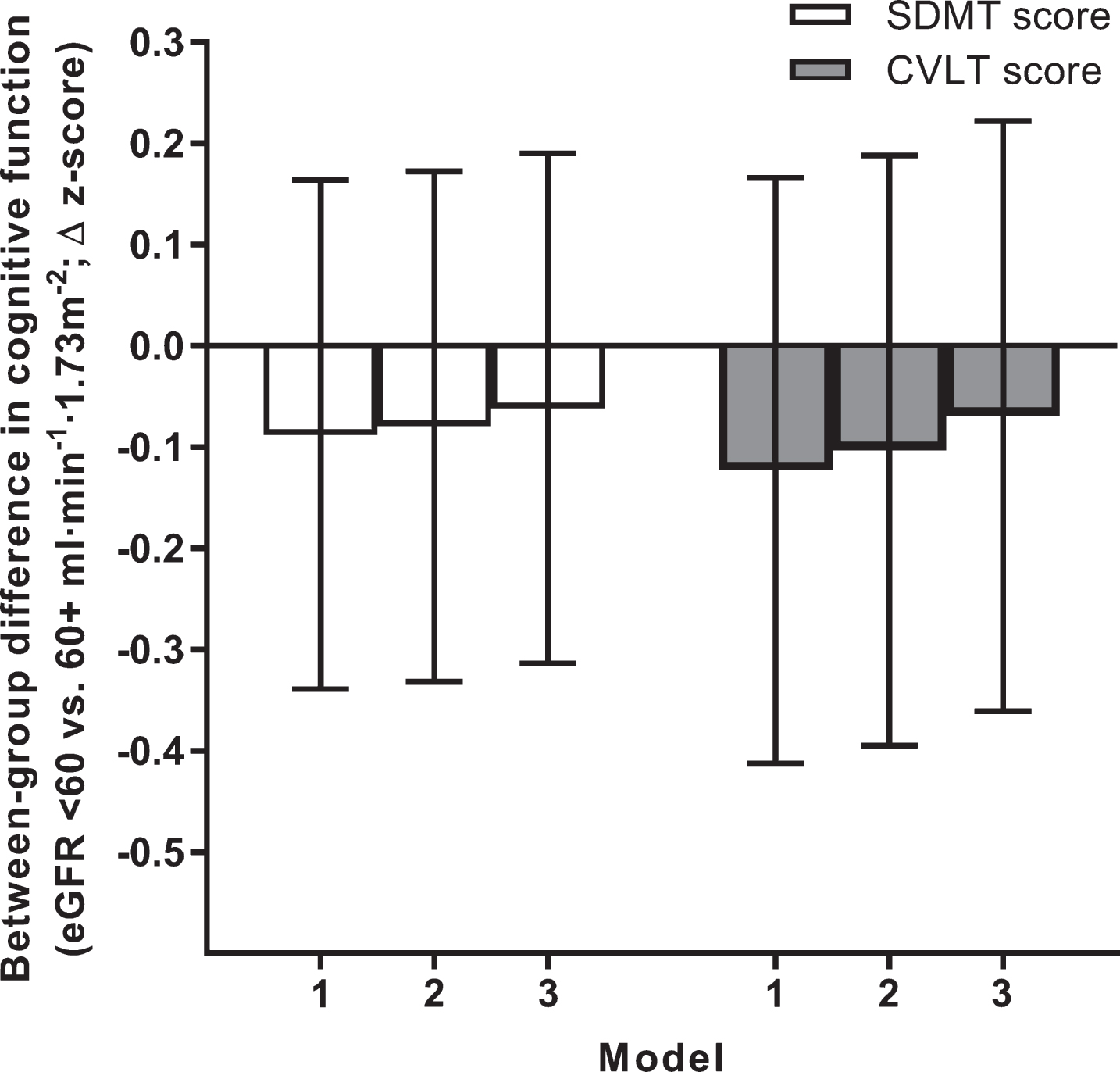
Kidney dysfunction and cognitive function at 12 years
No differences in memory or processing speed at 12 years were identified between individuals with and without kidney dysfunction at baseline (Fig. 3). In the subgroup of 3,867 individuals whose eGFR could be derived from the alternate formulae based on cystatin C alone and cystatin C/creatinine combined, results were similar (data not shown).
Fig. 3.
Kidney dysfunction and cognitive function measured at 12 years of follow-up. Data are between-group differences in CVLT and SDMT z-scores measured at the 2011/12 follow-up. All data are estimated marginal means with 95% confidence intervals (error bars). Error bars crossing the zero line indicate no significant difference between individuals with versus without kidney dysfunction (p > 0.05). Models 1 to 3 reflect incremental adjustment for a progressively more covariates; i.e., Model 1 featured age, sex and education level only; Model 2: Model 1 covariates + atherosclerotic cardiovascular disease, hypertension and diabetes; Model 3: Model 2 covariates + body mass index, physical activity, smoking, alcohol intake, and dyslipidemia. CVLT, California Verbal Learning Test; eGFR, estimated glomerular filtration rate; SDMT, Symbol Digit Modalities Test.
Sensitivity analyses
No interactions between kidney dysfunction and the tested covariates on either CVLT or SDMT scores, were observed. Additional adjustment for the presence of depressive symptoms at 12 years showed no deviation from the above findings. Likewise, models featuring STW score as an alternate to education level were consistent with the primary analyses.
Duration of exposure
Of the primary analytic sample (n = 4,128), 3,333 individuals (81%) participated in the interim (2004/05) CKD screening assessment and had complete data for all model 3 covariates. This in turn enabled comparisons of cognitive function measured in 2011/12 between those with versus without albuminuria/kidney dysfunction from 1999/00 (12-year exposure) and from 2004/05 (7-year exposure). Rates of albuminuria and kidney dysfunction in this subgroup of n = 3,333 in 1999/00 were 3.3% and 1.0%, respectively (i.e., similar to the primary analytic sample). By 2004/05, these rates had increased to 4.0% and 2.3%. Between-group comparisons of CVLT and SDMT scores according to each of the 1999/00 and 2004/05 classifications are displayed in Fig. 4.
Fig. 4.
Between-group differences in cognitive function according to duration of exposure to albuminuria/kidney dysfunction. Between-group differences in CVLT and SDMT z-scores measured at the 2011/12 follow-up are shown in parts A and B, respectively. Data are estimated marginal means with 95% confidence intervals (error bars). Error bars crossing the zero line indicate no significant difference between individuals with versus without albuminuria/kidney dysfunction (p > 0.05). All data reflect adjustment for model 3 covariates (i.e., age, sex, education level, atherosclerotic cardiovascular disease, hypertension, diabetes, body mass index, physical activity, smoking, alcohol intake, and dyslipidemia; n.b. relevant 1999/00 or 2004/05 values were used for time-varying covariates). CKD, chronic kidney disease; CVLT, California Verbal Learning Test; eGFR, estimated glomerular filtration rate; SDMT, Symbol Digit Modalities Test.
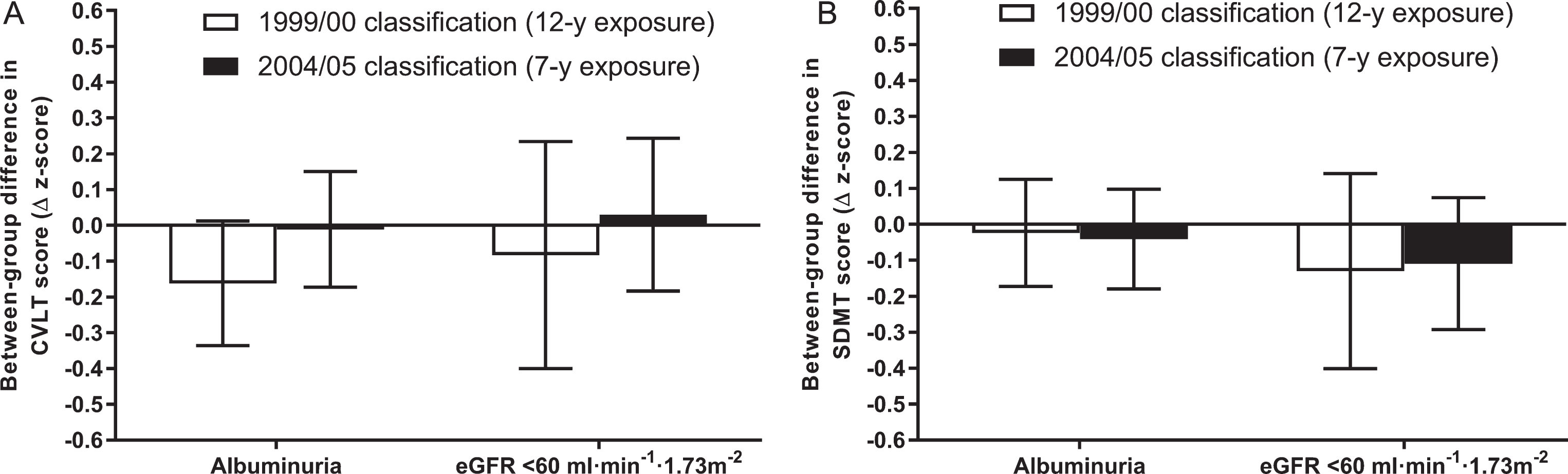
The lower mean CVLT score of individuals with albuminuria from 1999/00 was similar to the primary analysis (n.b. although it was of borderline significance [p = 0.068], the magnitude difference was very similar [i.e., ∼–0.2 units lower]). This difference in CVLT score did not extend to the shorter exposure duration based on 2004/05 classification. In turn, no differences in the between-group comparison of CVLT score were observed based on kidney dysfunction defined in 1999/00 versus its classification in 2004/05. Likewise, SDMT differences were not apparent for albuminuria and kidney dysfunction, irrespective of exposure duration.
Global cognitive impairment
Neither albuminuria at the 1999/00 baseline, nor at 5 years (2004/05) significantly predicted cognitive impairment in the base model adjusted for age, sex, and education (OR 1.7 [95% CI 0.6 to 4.5], p = 0.29 and OR 1.3 [95% CI 0.5 to 3.4], p = 0.56, respectively). The association of kidney dysfunction with cognitive impairment could not be investigated due to too few cases with both conditions (n < 5).
DISCUSSION
In the current study of community-based individuals with mostly preserved global cognitive function, we detected an independent association of albuminuria with memory performance at 12 years of follow-up (CVLT score). However, there was no apparent association of kidney dysfunction (i.e., low eGFR) with memory; likewise, neither albuminuria, nor kidney dysfunction, predicted processing speed at 12 years (SDMT score). Notably, the association of albuminuria with memory persisted following adjustment not only for the major confounders of cardiovascular disease, diabetes, and hypertension, but also for other cardiovascular and lifestyle factors. That these covariates together mediated only 21% of the effect is compelling for kidney damage per se predisposing to future memory impairment. Collectively, our findings advocate albuminuria as a potential risk marker or risk factor for future cognitive impairment that may prove useful in identifying those higher-risk individuals in whom preventive interventions may be most beneficial.
Implications of CKD markers for memory and processing speed
Although excess cognitive impairment in CKD is well-known and—given their shared pathophysiology—unsurprising, the potential for CKD to itself be an antecedent of cognitive impairment remains controversial [19]. Our data suggest that CKD manifesting as albuminuria, but not necessarily as kidney dysfunction, predicts at least one domain of cognitive function in memory. However, we hesitate to conclude differential associations between the two CKD markers given that their cognitive test scores did not deviate substantively, and the smaller kidney dysfunction group (n = 39 for eGFR versus n = 142 for albuminuria) obviously afforded less statistical power. Nevertheless, the trend toward a larger effect for albuminuria was suggestive of this marker having stronger predictive capacity for memory performance. Although it is intuitive to attribute this finding to albuminuria having a stronger basis than eGFR in microangiopathy (and therefore representing a better surrogate of the systemic and cerebral microcirculation), this notion clearly requires evidence beyond the current study. Albuminuria is also associated with more aggressive CKD progression than non-albuminuric kidney dysfunction [20]—the latter phenotype being that exhibited by the majority of patients in the low eGFR group (per Fig. 1). Although the pathophysiology of non-albuminuric CKD remains incompletely understood, it has been reported to be more closely associated with aging, hypertension, and vascular disease [21], key covariates with cognitive function that we adjusted for in our models. A lack of independent predictive capacity of eGFR for cognitive function in this context may therefore not necessarily be surprising, even if this notion is contradicted by the REGARDS study (in which eGFR predicted cognitive impairment only in individuals in the lowest category of albumin excretion [6]). In any case, our findings with respect to eGFR cannot be assumed to be generalizable to individuals with albuminuric kidney dysfunction.
An alternative explanation lies with the age difference between the albuminuria and kidney dysfunction groups. Notwithstanding statistical adjustment, we cannot eliminate the prospect that the younger age of the albuminuria group at baseline (mean 52 years versus 68 years for kidney dysfunction) contributed to their larger deficit in memory 12 years later; i.e., since younger subsamples may be less likely to be biased as a result of attrition due to competing risks (e.g., mortality and incident comorbid disease, which obviously affect older individuals to a greater degree). Consistent with this notion, we previously reported in the AusDiab cohort a stronger association between glycemic control and 12-year cognitive function in younger compared with older individuals (i.e.,<60 versus 60 + years) [10].
CKD and cognitive function
The current study aligns with previous work in some, but not all, aspects. Existent prospective studies have produced mixed findings, though the majority have compared cognitive outcomes in groups defined by eGFR exclusively [2, 19]. Studies incorporating markers of kidney damage (i.e., albuminuria or proteinuria) have been more consistent in reporting associations with cognitive function. Indeed, the Rancho Bernardo Study echoed our own data in reporting albuminuria, but not kidney dysfunction, as a predictor of cognitive function measured ∼7 years later (albeit in men only) [22]. Likewise, proteinuria, but not kidney dysfunction, was a borderline significant predictor of incident vascular dementia in the Three-City (3C) Study [23]. Finally, both albuminuria and kidney dysfunction were associated with cognitive impairment at 4 years in REGARDS (though, as mentioned above, the predictive capacity of each marker appeared to depend on the other being normal) [6].
Notwithstanding these comparisons, it is important to appreciate several methodological factors that distinguish our own study. Firstly, as alluded above, our cohort was ∼1–2 decades younger than those of Rancho Bernardo, 3C, and REGARDS, and was followed for a longer period of time (12 years). Indeed, to our knowledge, no longitudinal studies have examined cognitive outcomes past 7 years of follow-up in CKD. The importance of this was apparent from our results; i.e., no impairment of memory or processing speed was evident for individuals who only showed evidence of CKD from the interim 2004/05 assessment (i.e., 7 years exposure). Of course, while this approach has the advantage of characterizing prolonged exposure effects and minimizing the confounding influence of baseline cognitive impairment, it obviously gives rise to the exclusion of more advanced disease (i.e., due to aforementioned competing risks). Indeed, this was apparent from the more favorable clinical profile of individuals with CKD included the final analytic sample compared with the group with CKD at baseline who had to be excluded (e.g., due to death or study withdrawal before follow-up cognitive function testing).
The other major novel aspect of our study was in its outcome measures. Indeed, we are unaware of any longitudinal studies examining associations between albuminuria and the specific cognitive domains of memory and processing speed (though these are obviously components of tests of global cognition; e.g., MMSE). That we identified a deficit in memory, but not processing speed, in individuals with albuminuria underscores the value of assessing multiple domains. However, confirmation that albuminuria has a greater proclivity to affect memory will require further study since, at this stage, it is unclear whether the finding has a genuine mechanistic basis or simply reflects differences between the CVLT and SDMT in sensitivity and reproducibility.
Clinical relevance
Although the memory impairment suggested by lower CVLT scores in individuals with albuminuria represents a small-to-medium effect according to statistical criteria (Cohen’s d), its clinical relevance is less certain. While it could be considered a form of mild cognitive impairment, the deficit observed in this single domain was obviously not sufficient to manifest in cognitive impairment according to the more commonly applied MMSE-based definition (incorporating multiple cognitive domains). Indeed, with <5% of those aged 60 + years showing evidence of cognitive impairment based on the MMSE, ours was clearly a relatively ‘cognitively healthy’ cohort overall (likely reflecting attrition from what was originally a population-based sample). The prospect that this may have biased our results toward the null should certainly be considered (i.e., a stronger effect for CKD may have been detected with a shift in the distribution of outcome measures toward less favorable values).
Even with the relatively small between-group difference, we can at least be confident that there was an effect on memory of albuminuria per se; i.e., only 21% of the association was contributed by the major antecedents/consequences of CKD known to precipitate cognitive decline (i.e., diabetes, hypertension, and cardiovascular disease) and other relevant risk/lifestyle factors. In adjusting for the covariates that we did, we believe our study addresses some of the potential unmeasured confounding for which previous studies on this topic have been criticized [19]. Attesting to the importance of this was the apparently large contribution of covariates to the difference in processing speed between individuals with and without albuminuria (i.e., 43% from model 1 to model 3).
Relevance to global prevention of dementia
Although some cardiometabolic risk factors for dementia have demonstrated a decline in prevalence in recent decades (e.g., smoking and hypertension), others have failed to improve. The trajectory of CKD, though stable on a global level, still shows an escalation in certain regions and population subgroups [24]. Even if prevalence growth can be stalled or reversed, our ever-increasing lifespan will ensure a burgeoning number of cases worldwide for the foreseeable future. In this context, studies such as ours examining long-term cognitive outcomes in individuals with versus without CKD, and the extent to which these reflect shared cardiometabolic risk factors, are vital in establishing the importance of CKD relative to other modifiable determinants of dementia. In this way, future preventive strategies can be better targeted. The current study suggests that CKD may indeed predispose to worse cognition in the longer term. Although further work is needed to translate these findings more broadly, the implication is that CKD has the potential to be a salient contributor to dementia at a global level.
Study limitations
Cognitive tests were not included in the first wave of testing of the AusDiab cohort. This limitation was mitigated to some extent by the relatively long 12-year follow-up period. Definitions of exposures were based on once-only screening tests, whereas CKD diagnosis usually requires persistence of abnormal ACR and/or low eGFR for at least 3 months [25]. Finally, the battery of cognitive function tests was relatively restricted in encompassing the domains of memory and processing speed only (i.e., beyond global and premorbid function indicated by MMSE and STW, respectively). Future studies would be well-served by the inclusion of tests of other domains known to be affected by cardiometabolic disease (particularly executive function).
CONCLUSIONS
Individuals with albuminuria are predisposed to worse memory and processing speed at 12 years of follow-up, though only the former appears to be independent of concurrent cardiometabolic disease and other shared risk factors. Such predictive capacity did not extend to kidney dysfunction (i.e., eGFR <60 ml/min/1.73 m2). Given the clinical context of this cognitively healthy cohort, albuminuria over a prolonged period at least appears to be an antecedent of a subclinical decline in memory. On this basis, the utility of albuminuria and other CKD indicators for identifying individuals at risk of future cognitive impairment and dementia—particularly relative to other novel and established risk markers—deserves further study.
ACKNOWLEDGMENTS
The AusDiab study, co-coordinated by the Baker Heart and Diabetes Institute, gratefully acknowledges the support and assistance given by: R Atkins, B Balkau, E Barr, A Cameron, M de Courten, D Dunstan, A Kavanagh, S Murray, N Owen, T Welborn, and all the study participants. Funding or logistical support was provided by the National Health and Medical Research Council (NHMRC grants #233200, #1007544, and #1100579; KJA was also supported by a NHMRC Fellowship [#1102694]), the Australian Government Department of Health and Ageing, Abbott Australasia Pty Ltd, Alphapharm Pty Ltd, Amgen Australia, AstraZeneca, Bristol-Myers Squibb, City Health Centre—Diabetes Service—Canberra, Department of Health and Community Services—Northern Territory, Department of Health and Human Services—Tasmania, Department of Health—New South Wales, Department of Health—Western Australia, Department of Health—South Australia, Department of Human Services—Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Eli Lilly Australia, Estate of the Late Edward Wilson, GlaxoSmithKline, Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, Marian & FH Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Pfizer Pty Ltd, Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital—Sydney, Sanofi Aventis, sanofi-synthelabo, and the Victorian Government’s OIS Program. No funding providers played a role in study design/conduct, analysis/interpretation of data, or manuscript preparation.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0498r1).
SUPPLEMENTARY MATERIAL
[3] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180498.
REFERENCES
[1] | Plassman BL , Langa KM , McCammon RJ , Fisher GG , Potter GG , Burke JR , Steffens DC , Foster NL , Giordani B , Unverzagt FW , Welsh-Bohmer KA , Heeringa SG , Weir DR , Wallace RB ((2011) ) Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol 70: , 418–426. |
[2] | Etgen T , Chonchol M , Forstl H , Sander D ((2012) ) Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am J Nephrol 35: , 474–482. |
[3] | Griva K , Stygall J , Hankins M , Davenport A , Harrison M , Newman SP ((2010) ) Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis 56: , 693–703. |
[4] | Raphael KL , Wei G , Greene T , Baird BC , Beddhu S ((2012) ) Cognitive function and the risk of death in chronic kidney disease. Am J Nephrol 35: , 49–57. |
[5] | Sehgal AR , Grey SF , DeOreo PB , Whitehouse PJ ((1997) ) Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 30: , 41–49. |
[6] | Kurella Tamura M , Muntner P , Wadley V , Cushman M , Zakai NA , Bradbury BD , Kissela B , Unverzagt F , Howard G , Warnock D , McClellan W ((2011) ) Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 58: , 756–763. |
[7] | Dunstan DW , Zimmet PZ , Welborn TA , Cameron AJ , Shaw J , de Courten M , Jolley D , McCarty DJ , Australian Diabetes, Obesity and Lifestyle Study (AusDiab) (2002) The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 57: , 119–129. |
[8] | Levey AS , Stevens LA , Schmid CH , Zhang YL , Castro AF, 3rd , Feldman HI , Kusek JW , Eggers P , van Lente F , Greene T , Coresh J , CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) ((2009) ) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: , 604–612. |
[9] | Inker LA , Schmid CH , Tighiouart H , Eckfeldt JH , Feldman HI , Greene T , Kusek JW , Manzi J , van Lente F , Zhang YL , Coresh J , Levey AS , CKD-EPI Investigators ((2012) ) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: , 20–29. |
[10] | Anstey KJ , Sargent-Cox K , Eramudugolla R , Magliano DJ , Shaw JE ((2015) ) Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res Ther 7: , 48. |
[11] | Delis DC , Kramer JH , Kaplan E , Ober BA ((1987) ) California Verbal Learning Test, Psychological Corporation Harcourt Brace Jovanovich, San Antonio. |
[12] | Smith A ((1982) ) Symbol Digit Modalities Test(SDMT) manual, Western Psychological Services, Los Angeles. |
[13] | Baddeley A , Emslie H , Nimmo-Smith I ((1992) ) The Spot-the-Word test, Thames Valley Test Company, Bury St Edmunds. |
[14] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[15] | World Health Organization (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Report of a WHO/IDF consultation. Geneva (Switzerland). |
[16] | Australian Institute of Health and Welfare (2003) The active Australia survey: A guide and manual for implementation, analysis and reporting, AIHW, Canberra. |
[17] | Gorelick PB , Scuteri A , Black SE , Decarli C , Greenberg SM , Iadecola C , Launer LJ , Laurent S , Lopez OL , Nyen-huis D , Petersen RC , Schneider JA , Tzourio C , Arnett DK , Bennett DA , Chui HC , Higashida RT , Lindquist R , Nilsson PM , Roman GC , Sellke FW , Seshadri S , American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia ((2011) ) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: , 2672–2713. |
[18] | Salazar-Pousada D , Arroyo D , Hidalgo L , Perez-Lopez FR , Chedraui P ((2010) ) Depressive symptoms and resilience among pregnant adolescents: A case-control study. Obstet Gynecol Int 2010: , 952493. |
[19] | Etgen T ((2015) ) Kidney disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther 7: , 29. |
[20] | Koye DN , Magliano DJ , Reid CM , Jepson C , Feldman HI , Herman WH , Shaw JE ((2018) ) Risk of progression of nonal-buminuric CKD to end-stage kidney disease in people with diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 72: , 653–661. |
[21] | Ekinci EI , Jerums G , Skene A , Crammer P , Power D , Cheong KY , Panagiotopoulos S , McNeil K , Baker ST , Fioretto P , Macisaac RJ ((2013) ) Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 36: , 3620–3626. |
[22] | Jassal SK , Kritz-Silverstein D , Barrett-Connor E ((2010) ) A prospective study of albuminuria and cognitive function in older adults: The Rancho Bernardo study. Am J Epidemiol 171: , 277–286. |
[23] | Helmer C , Stengel B , Metzger M , Froissart M , Massy ZA , Tzourio C , Berr C , Dartigues JF ((2011) ) Chronic kidney disease, cognitive decline, and incident dementia: The 3C Study. Neurology 77: , 2043–2051. |
[24] | Hsu RK , Powe NR ((2017) ) Recent trends in the prevalence of chronic kidney disease: Not the same old song. Curr Opin Nephrol Hypertens 26: , 187–196. |
[25] | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group ((2013) ) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: , 1–150. |



