Clock genes and the role of melatonin in cancer cells: an overview
Clock genes, melatonin, and cancer
Abstract
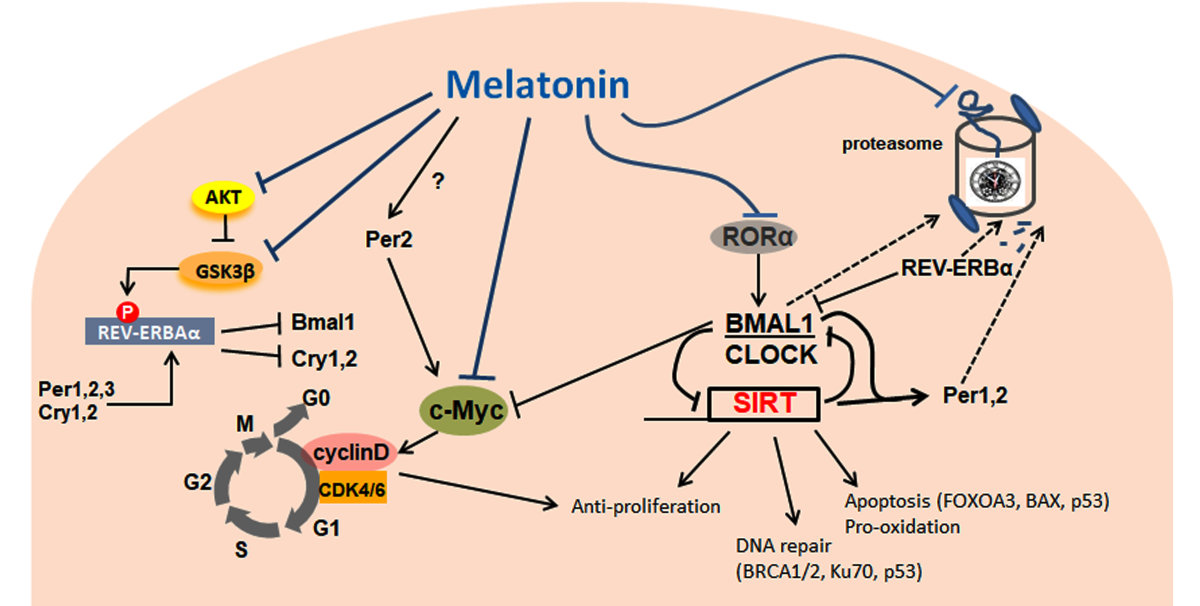
Circadian rhythms control most biological processes in every organism and their disruption or an aberrant function in the expression of clock genes are associated with a number of cancers including some hormone-dependent and independent cancers. The processes involved in carcinogenesis and tumor progression are complex, but understanding the daily profiles of the core clock genes and their clock-controlled genes is essential to evaluate specifically the molecular program of the cancer phenotype; this may be helpful in providing a more realistic strategy for both diagnosis and treatment during the course of the disease. Because melatonin production and secretion oscillates rhythmically through the light:dark cycle and is related to the circadian machinery genes (Clock, Bmal1, Periods, and Cryptochromes), its regulatory role on clock genes in cancer cells may bring additional evidence regarding the mechanism(s) by which melatonin is involved. Mechanistically, melatonin acts via proteasome inhibition and sirtuins to indirectly modulate clock genes in cancer; however, melatonin seems to be capable of directly altering the expression of clock genes to affect cancer development. Depending on cancer cell type, melatonin might up or downregulate specific clock genes to control cell cycle, survival, repair mechanisms, etc. In parallel, melatonin exerts pro-apoptotic, anti-proliferative and pro-oxidative effects, metabolic shifting, reduction in neovasculogenesis and inflammation, and restores chemosensitivity of cancer cells. Finally, melatonin improves the life quality of patients. This review focuses on the main functions of melatonin on clock genes, and reviews, from a clinical and experimental standpoint, how melatonin regulates the expression of clock genes in some prevalent cancer types such as breast, prostate, liver, and colon cancers, leukemia and melanoma. We further emphasized possible signaling mechanisms whereby melatonin interferes with clockwork genes and circadian-controlled genes within cancer cells.
References
2. Albrecht U (2004) The mammalian circadian clock: a network of gene expression. Front. Biosci. 9: 48–55.
3. Sharma VK and Chandrashekaran MK (2005) Zeitgebers (time cues) for biological clocks. Curr. Sci. 89: 1136–1146.
4. Hardeland R (2017) Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 62: e12377.
5. Doi M, Hirayama J, Sassone-Corsi P (2006) Circadian regulator CLOCK is a histone acetyltransferase. Cell 125: 497–508.
6. Ko CH and Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 15: R271–R277.
7. Akhtar RA, Reddy AB, Maywood ES, et al.(2002) Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 12: 540–550.
8. Alenghat T, Meyers K, Mullican SE, et al. (2008) Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456: 997–1000.
9. Sahar S and Sassone-Corsi P (2012) Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol. Metab. 23: 1-8.
10. Robles MS, Humphrey SJ, Mann M (2017) Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 25: 118–127.
11. Wang J, Mauvoisin D, Martin E, et al. (2017) Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab. 25: 102–117.
12. Davis K, Roden LC, Leaner VD, van der Watt PJ (2019) The tumour suppressing role of the circadian clock. IUBMB Life 23: doi: 10.1002/iub.2005.
13. Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12: 151–180.
14. Reiter RJ, Tan DX, Korkmaz A, et al. (2007) Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit. Rev. Oncog. 13: 303–328.
15. Erren TC, Reiter RJ (2008) A generalized theory of carcinogenesis due to chronodisruption. Neuro. Endocrinol. Lett. 29: 815–821.
16. Jung-Hynes B, Reiter RJ, Ahmad N (2010) Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J. Pineal Res. 48: 9-19.
17. Erren TC, Reiter RJ, Piekarski C (2003) Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften 90: 485–494.
18. Stevens RG (2009) Electric light causes cancer? Surely you're joking, Mr. Stevens. Mutat. Res. 682: 1-6.
19. Pandi-Perumal SR, Srinivasan V, Maestroni GJ, et al. (2006) Melatonin: nature’s most versatile biological signal? FEBS J. 273: 2813–2838.
20. Andrade-Silva J, Cipolla-Neto J, Peliciari-Garcia RA (2014) The in vitro maintenance of clock genes expression within the rat pineal gland under standard and norepinephrine-synchronized stimulation. Neurosci. Res. 81-82: 1-10.
21. Jung B, Ahmad N (2006) Melatonin in cancer management: progress and promise. Cancer Res. 66: 9789–9793.
22. Hardeland R (2008) Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol. Life. Sci. 65: 2001–2018.
23. Peyrot F, Ducrocq C (2008) Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 45: 235–246.
24. Vriend J, Reiter RJ (2014) Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 115: 8–14.
25. Korf HW, von Gall C. (2006) Mice, melatonin and the circadian system. Mol. Cell Endocrinol. 252: 57-68.
26. Pévet P (2016) Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus. Expert Opin. Ther. Targets 20: 1209-1218.
27. Liu C, Weaver D, Jin X, et al. (1997) Molecular dissection of two distinct actions of melatonin in the suprachiasmatic circadian clock. Neuron 19: 91–102.
28. Jin X, von Gall C, Pieschl RL, et al. (2003) Targeted disruption of the mouse Mel (1b) melatonin receptor. Mol. Cell Biol. 3: 1054-1060.
29. Wagner GC, Johnston JD, Tournier BB, et al. (2007) Melatonin induces gene-specific effects on rhythmic mRNA expression in pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur. J. Neurosci. 25: 485–490.
30. Dardente H, Menet JS, Poirel VJ, et al. (2003) Melatonin induces Cry1 expression in the pars tuberalis of the rat. Brain Res. Mol. Brain Res. 114: 101–106.
31. Johnston JD, Tournier BB, Andersson H, et al. (2006) Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology 147: 959–965.
32. Lincoln G, Messager S, Andersson H, Hazlerigg D (2002) Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc. Natl. Acad. Sci. USA 99: 13890–13895.
33. Ross AW, Morgan PJ (2002) The pars tuberalis as a target of the central clock. Cell Tissue Res. 1: 163-171.
34. von Gall C, Weaver DR, Moek J, Jilg A, et al. (2005) Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann. NY. Acad. Sci. 1040: 508-511.
35. Vriend J, Reiter RJ (2015) Melatonin feedback on clock genes: a theory involving the proteasome. J. Pineal Res. 58: 1-11.
36. Gatfield D, Schibler U (2007) Proteasomes keep the circadian clock ticking. Science 316: 1135–1136
37. Stojkovic K, Wing SS, Cermakian N (2014) A central role for ubiquitination within a circadian clock protein modification code. Front. Mol. Neurosci. 7: 69.
38. Kodadek T, Sikder D, Nalley K (2006) Keeping transcriptional activators under control. Cell 127: 261–264.
39. Park EJ, Woo SM, Min KJ, Know TK (2014) Transcriptional and post-translational regulation of Bim controls apoptosis in melatonin-treated human renal cancer Caki cells. J. Pineal Res. 56: 97–106.
40. Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid M J, et al. (2014) Protecting the melatonin rhythm through circadian healthy light exposure. Int. J. Mol. Sci. 15: 23448-500.
41. Poirel VJ, Boggio V, Dardente H, et al. (2003) Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120: 745-55.
42. Mattam U, Jagota A (2014) Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerontology 15: 257-68.
43. Agez L, Laurent V, Pevet P, et al. (2007) Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 144: 522–530.
44. Torres-Farfan C, Seron-Ferre M, Dinet V, Korf HW (2006) Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J. Pineal Res. 40: 64–70.
45. Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW (2003) Melatonin and activity rhythm responses to light pulses in mice with the Clock mutation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284: R1231-1240.
46. Zeman M, Szantoova K, Stebelova K, et al. (2009) Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive TGR (mRen2)27 rats. J. Hypertens. 27: S21– S26.
47. Imbesi M, Arslan AD, Yildiz S, et al. (2009) The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. J. Pineal Res. 46: 87-94.
48. Weissová K, Škrabalová J, Skálová K, et al. (2018) Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 52: 1-6.
49. Satyanarayanan SK, Chien YC, Chang JP, et al. (2019) Melatonergic agonist regulates circadian clock genes and peripheral inflammatory and neuroplasticity markers in patients with depression and anxiety. Brain Behav. Immun. pii: S0889-1591(19)30099-6 doi: 10.1016/j.bbi.2019.03.003.
50. Weissová K, Bartoš A, Sládek M, et al. (2016) Moderate changes in the circadian system of Alzheimer's disease patients detected in their home environment. PLoS One 11: e0146200.
51. Nagy AD, Iwamoto A, Kawai M, et al. (2015) Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol. Int. 32: 447-57.
52. Hardeland R (2016) Melatonin and synthetic melatoninergic agonists in psychiatric and age-associated disorders: successful and unsuccessful approaches. Curr. Pharm. Des. 22: 1086-101.
53. Meneses-Santos D, Buonfiglio DDC, Peliciari-Garcia RA, et al. (2018) Chronic treatment with dexamethasone alters clock gene expression. and melatonin synthesis in rat pineal gland at night. Nat. Sci. Sleep 10: 203-215.
54. Viswanathan AN, Hankinson SE, Schernhammer ES (2007) Night shift work and the risk of endometrial cancer. Cancer Res. 67: 10618-10622.
55. Stevens RG, Brainard GC, Blask DE, et al. (2014) Breast cancer and circadian disruption from electric lighting in the modern world. CA. Cancer J. Clin. 64: 207-218.
56. Huisman SA, Oklejewicz M, Ahmadi AR, et al. (2015) Colorectal liver metastases with a disrupted circadian rhythm phase shift the peripheral clock in liver and kidney. Int. J. Cance. 136: 1024-1032.
57. Wendeu‐Foyet MG and Menegaux F (2017) Circadian disruption and prostate cancer risk: an updated review of epidemiological evidences. Cancer Epidemiol. Biomarkers Prev. 26: 985-991.
58. Liu Z, Yu K, Zheng J, et al. (2019) Dysregulation, functional implications, and prognostic ability of the circadian clock across cancers. Cancer Med. doi: 10.1002/cam4.2035.
59. Stevens RG (2009) Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int. J. Epidemiol. 38: 963–970.
60. Viswanathan AN and Schernhammer ES (2009) Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 281: 1–7.
61. Giudice A, Crispo A, Grimaldi M, et al. The effect of light exposure at night (LAN) on carcinogenesis via decreased nocturnal melatonin synthesis. Molecules 2018; 23: E1308.
62. Tosini G, Owino S, Guillaume JL, et al. (2014) Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays 36: 778-787
63. Ma TJ, Zhang ZW, Lu YL, et al. (2018) CLOCK and BMAL1 stabilize and activate RHOA to promote F-actin formation in cancer cells. Exp. Mol. Med. 50: 130.
64. Savvidis C and Koutsilieris M. (2012) Circadian rhythm disruption in cancer biology. Mol. Med. 18: 1249–1260.
65. Stevens G. (1987) Review and commentary: electric power use and breast cancer: a hypothesis. Am. J. Epidemiol. 125: 556–561.
66. Baan R, Grosse Y, Straif K, et al. (2009) A review of human carcinogens. Part F: chemical agents and related occupations. Lancet Oncol. 10: 1143–1144.
67. Kuo SJ, Chen ST, Yeh KT, et al. (2009) Disturbance of circadian gene expression in breast cancer. Virchows. Arch. 454: 467-474.
68. Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL (2007) Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia 9: 797-800.
69. Hua H, Wang Y, Wan C, et al. (2007) Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer Gene Ther. 14: 815-818.
70. Akira O, Yu K, Shinichi Y, et al. (2009) Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res. 29: 1201-1210.
71. Zheng B, Larkin DW, Albrecht U, et al. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169–173.
72. Fu L, Pelicano H, Liu J, et al. (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111: 41–50.
73. You S, Wood PA, Xiong Y, et al. (2005) Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res. Treat. 91: 47–60
74. Oda A, Katayose Y, Yabuuchi S, et al. Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res. 29: 1201–1209.
75. Le Romancer M, Poulard C, Cohen P, et al. (2011) Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr. Rev. 32: 597–622.
76. Lin HH and Farkas ME (2018) Altered circadian rhythms and breast cancer: from the human to the molecular level. Front. Endocrinol. (Lausanne) 9: 219.
77. Gery S, Virk RK, Chumakov K, et al. (2007) The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene 26: 7916–7920.
78. Xiang S, Coffelt SB, Mao L, et al. (2008) Period-2: a tumor suppressor gene in breast cancer. J. Circadian Rhythms. 6: 4.
79. Cadenas C, van de Sandt L, Edlund K, et al. (2014) Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 13: 3282–3291.
80. De Mei C, Ercolani L, Parodi C, et al. (2014) Dual inhibition of REV-ERBβ and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene 34: 2597–2608.
81. Hoffman AE, Yi CH, Zheng T, et al. (2010) CLOCK in breast tumorigenesis: evidence from genetic,epigenetic, and transcriptional profiling analyses. Cancer Res. 70: 1459–1468.
82. Xiao L, Chang AK, Zang MX, et al. (2014) Induction of the CLOCK gene by E2-ERα signaling promotes the proliferation of breast cancer cells. PLoS One 9: e95878.
83. Reszka E, Przybek M, Muurlink O, Pepłonska B (2017) Circadian gene variants and breast cancer. Cancer Lett. 390: 137-145.
84. Xiang S, Coffelt SB, Mao L, et al. (2008) Period-2: a tumor suppressor gene in breast cancer. J. Circadian Rhythms 6: 4.
85. Xiang S, Mao L, Duplessis T, et al. (2012) Oscillation of clock and clock controlled genes induced by serum shock in human breast epithelial and breast cancer cells: regulation by melatonin. Breast Cancer (Auckl). 6: 137-150.
86. Hill SM, Blask DE, Xiang S, et al. (2011) Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J. Mammary Gland Biol. Neoplasia 16: 235–245.
87. Proietti S, Cucina A, Dobrowoly G, et al. (2014) Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J. Pineal Res. 57: 120–129.
88. Morales-Santana S, Morell S, Leon J, et al. (2019) An overview of the polymorphisms of circadian genes associated with endocrine cancer. Front. Endocrinol. (Lausanne). 10: 104.
89. Tokunaga H, Takebayashi Y, Utsunomiya H, et al. (2008) Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet. Gynecol. Scand. 87: 1060-1070.
90. Yeh CM, Shay J, Zeng TC, et al. (2014) Epigenetic silencing of ARNTL, a circadian gene and potential tumor suppressor in ovarian cancer. Int. J. Oncol. 45: 2101-2107.
91. Sun Y, Jin L, Sui YX, et al. (2017) Circadian gene CLOCK affects drug-resistant gene expression and cell proliferation in ovarian cancer SKOV3/DDP cell lines through autophagy. Cancer Biother. Radiopharm. 32: 139-146.
92. Carter BD, Diver WR, Hildebrand JS, et al. (2014) Circadian disruption and fatal ovarian cancer. Am. J. Prev. Med. 46: 34-41.
93. Zhao M, Wan J, Zeng K, et al. (2016) The reduction in circulating melatonin level may contribute to the pathogenesis of ovarian cancer: a retrospective study. J. Cancer 7: 831-836.
94. Chuffa LG, Fioruci-Fontanelli BA, Mendes LO, et al. (2015) Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 15: 34.
95. Chuffa LG, Alves MS, Martinez M, et al. (2016) Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr. Relat. Cancer 23: 65-76.
96. Zonta YR, Martinez M, Camargo IC, et al. (2017) Melatonin reduces angiogenesis in serous papillary ovarian carcinoma of ethanol-preferring rats. Int. J. Mol. Sci. 18: E763.
97. Chuffa LGA, Reiter RJ, Lupi LA. (2017) Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis 38: 945-952.
98. de Almeida Chuffa LG, Seiva FRF, Cucielo MS, et al. (2019) Mitochondrial functions and melatonin: a tour of the reproductive cancers. Cell Mol. Life Sci. 76: 837-863.
99. Litlekalsoy J, Rostad K, Kalland KH, et al. (2016) Expression of circadian clock genes and proteins in urothelial cancer is related to cancer-associated genes. BMC Cancer 16: 549.
100. Wendeu-Foyet MG, Koudou Y, Cénée S, et al. (2019) Circadian genes and risk of prostate cancer: Findings from the EPICAP study. Int. J. Cancer doi: 10.1002/ijc.32149.
101. Kiss Z and Ghosh PM. (2016) Women in cancer thematic review: circadian rhythmicity and the influence of 'clock' genes on prostate cancer. Endocr. Relat. Cancer 23: T123-T134.
102. Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. (2010) Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res. 49: 60-68.
103. Markt SC, Valdimarsdottir UA, Shui IM, et al. (2015) Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control 26: 25-33.
104. Fekry B, Ribas-Latre A, Baumgartner C, et al. (2018) Incompatibility of the circadian protein BMAL1 and HNF4α in hepatocellular carcinoma. Nat. Commun. 9: 4349.
105. Polo A, Singh S, Crispo A, et al. (2017) Evaluating the associations between human circadian rhythms and dysregulated genes in liver cancer cells. Oncol. Lett. 14: 7353-7359.
106. Li H, Lu YF, Chen H, et al. (2017) Dysregulation of metallothionein and circadian genes in human hepatocellular carcinoma. Chronobiol. Int. 34: 192-202.
107. Mteyrek A, Filipski E, Guettier C, et al. (2017) Critical cholangiocarcinogenesis control by cryptochrome clock genes. Int. J. Cancer 140: 2473-2483.
108. Kettner NM, Voicu H, Finegold MJ, et al. (2016) Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30: 909-924.
109. Chen L, Zhou T, Wu N, et al. (2019) Pinealectomy or light exposure exacerbates biliary damage and liver fibrosis in cholestatic rats through decreased melatonin synthesis. Biochim. Biophys. Acta Mol. Basis Dis. pii: S0925-4439(19)30076-6. doi:10.1016/j.bbadis.2019.03.002.
110. González-Fernández B, Sánchez DI, Crespo I, et al. (2018) Melatonin attenuates dysregulation of the circadian clock pathway in mice with CCl(4)-induced fibrosis and human hepatic stellate cells. Front. Pharmacol. 9: 556.
111. Sánchez DI, González-Fernández B, San-Miguel B, et al. (2017) Melatonin prevents deregulation of the sphingosine kinase/sphingosine 1-phosphate signaling pathway in a mouse model of diethylnitrosamine-induced hepatocellular carcinoma. J. Pineal Res. 62: doi: 10.1111/jpi.12369
112. Sánchez DI, González-Fernández B, Crespo I, et al. (2018) Melatonin modulates dysregulated circadian clocksin mice with diethylnitrosamine-induced hepatocellular carcinoma. J. Pineal Res. 65: e12506
113. Yoshida K, Sato M, Hase T, et al. (2013) TIMELESS is overexpressed in lung cancer and its expression correlates with poor patient survival. Cancer Sci. 104: 171–177.
114. Chi L, Zou Y, Qin L, et al. (2017) TIMELESS contributes to the progression of breast cancer through activation of MYC. Breast Cancer Res. 19: 53
115. Zhang W, He W, Shi Y, et al. (2017) Aberrant TIMELESS expression is associated with poor clinical survival and lymph node metastasis in early-stage cervical carcinoma. Int. J. Oncol. 50: 173-184.
116. Neilsen BK, Frodyma DE, McCall JL, et al. (2019) ERK-mediated TIMELESS expression suppresses G2/M arrest in colon cancer cells. PLoS One 14: e0209224
117. Krugluger W, Brandstaetter A, Kallay E, et al. (2007) Regulation of genes of the circadian clock in human colon cancer: reduced period‐1 and dihydropyrimidine dehydrogenase transcription correlates in high‐grade tumors. Cancer Res. 67: 7917-7922.
118. Zeng ZL, Wu MW, Sun J, et al. (2010) Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. J. Biochem. 148: 319-326
119. Soták M, Polidarová L, Ergang P, et al. (2013) An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int. J. Cancer 132: 1032-1041
120. Korkmaz T, Aygenli F, Emisoglu H, et al. (2018) Opposite carcinogenic effects of circadian clock gene BMAL1. Sci. Rep. 8: 16023.
121. Zeng ZL, Luo HY, Yang J, et al. (2014) Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin. Cancer Res. 20: 1042-1052.
122. Mazzoccoli G, Colangelo T, Panza A, et al. (2016) Deregulated expression of cryptochrome genes in human colorectal cancer. Mol. Cancer 15: 6.
123. Nemeth C, Humpeler S, Kallay E, et al. (2011) Decreased expression of the melatonin receptor 1 in human colorectal adenocarcinomas. J. Biol. Regul. Homeost. Agents 25: 531-542
124. Taniguchi H, Fernández AF, Setién F, et al. (2009). Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 69: 8447-8454.
125. Yang MY, Yang WC, Lin PM, et al. (2011) Altered expression of circadian clock genes in human chronic myeloid leukemia. J. Biol. Rhythms. 26: 136-148.
126. Puram RV, Kowalczyk MS, de Boer CG, et al. (2016) Core circadian clock genes regulate leukemia stem cells in AML. Cell 165: 303-316
127. Rana S, Munawar M, Shahid A, et al. (2014) Deregulated expression of circadian clock and clock-controlled cell cycle genesin chronic lymphocytic leukemia. Mol. Biol. Rep. 41: 95-103.
128. Sandu C, Dumas M, Malan A, et al. (2012) Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cell Mol. Life Sci. 69: 3329–3339.
129. Rigel DS (2010) Epidemiology of melanoma. Semin. Cutan. Med. Surg. 29: 204–209.
130. Kvaskoff M, Weinstein P (2010) Are some melanomas caused by artificial light? Med. Hypotheses. 75: 305-311
131. Lengyel Z, Lovig C, Kommedal S, et al. (2013) Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. 34: 811–819.
132. de Assis LVM, Kinker GS, Moraes MN, et al. (2018) Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Front. Oncol. 8: 185.
133. Markova-Car EP, Jurišić D, Ilić N, Kraljević-Pavelić S (2014) Running for time: circadian rhythms and melanoma. Tumour Biol. 35: 8359-8368.
134. Otálora BB, Madrid JA, Alvarez N, et al. (2008) Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J. Pineal Res. 43: 307-315.
135. Slominski AT, Hardeland R, Zmijewski MA, et al. (2018) A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 138: 490-499.
136. Sulli G, Rommel A, Wang X, et al. (2018). Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553: 351-355.
137. Sancar A, Lindsey-Boltz LA, Kang TH, et al. (2010) Circadian clock control of the cellular response to DNA damage. FEBS Lett. 584: 2618-2625.
138. Mayo JC, Sainz RM, González Menéndez P, et al. (2017) Melatonin and sirtuins: A "not-so unexpected" relationship. J. Pineal Res. 62: e12391. doi: 10.1111/jpi.12391.
139. Grimaldi B, Nakahata Y, Kaluzova M, et al. (2009) Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int. J. Biochem. Cell Biol. 41: 81–86.
140. Chang H-C, Guarente L (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153: 1448–1460.
141. Perdomo J, Cabrera J, Estevez F, et al. (2013) Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species independent mechanism in human leukemia Molt-3 cells. J. Pineal Res. 55: 195–206
142. Quintana C, Cabrera J, Perdomo J, et al. (2016) Melatonin enhances hyperthermia induced apoptotic cell death in human leukemia cells. J. Pineal Res. 61: 381-395.
143. Liu J, Zhou B, Yan M, et al. (2016) CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology 157: 2259–2269.
144. Jung-Hynes B, Schmit TL, Reagan-Shaw SR, et al. (2011) Melatonin, a novel Sirt1 inhibitor, imparts proliferative effects against prostate cancer cells in vitro culture and in vivo in TRAMP model. J. Pineal Res. 50: 140–149.
145. Hill SM, Frasch T, Xiang S, et al. (2009) Molecular mechanisms of melatonin anticancer effects. Integr. Cancer Ther. 8: 337-346.
146. Borgs L, Beukelaers P, Vandenbosch R, et al. (2009) Cell "circadian" cycle: new role for mammalian core clock genes. Cell Cycle 8: 832-837
147. Kelleher FC, Rao A, Maguire A (2014) Circadian molecular clocks and cancer. Cancer Lett. 342: 9-18.
148. Hunt T, Sassone-Corsi P (2007) Riding tandem: circadian clocks and the cell cycle. Cell 129: 461-464.
149. Mediavilla MD, Cos S, Sanchez-Barcelo EJ (1999) Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci. 65: 415–420.
150. Smith A, Simanski S, Fallahi M et al. (2007) Redundant ubiquitin ligase activities regulate wee1 degradation and mitotic entry. Cell Cycle 6: 2795–2799.
151. Hasanali Z, Sharma K, Epner E (2012) Flipping the cyclin D1 switch in mantle cell lymphoma. Best. Pract. Res. Clin. Haematol. 25: 143–152.
152. Shi Z, Li Z, Li ZJ, et al. (2015) Cables1 controls p21/Cip1protein stability by antagonizing proteasome subunit alpha type 3. Oncogene 34: 2538-2545.
153. Rogelsperger O, Wlcek K, Ekmekcioglu C et al. (2011) Melatonin receptors, melatonin metabolizing enzymes and cyclin D1 in human breast cancer. J. Recept. Signal. Transduct. Res. 31: 180–187.
154. Lee H, Lee HJ, Jung JH, et al. (2018) Melatonin disturbs SUMOylation-mediated crosstalk between c-Myc and nestin via MT1 activation and promotes the sensitivity of paclitaxel in brain cancer stem cells. J. Pineal Res. 65: e12496.
155. Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat. Rev. Cancer 3: 350-361.
156. Blask DE, Hill SM, Dauchy RT, et al. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J. Pineal Res. 51: 259–269.
157. Dauchy RT, Xiang S, Mao L, et al. (2014) Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 74: 4099-4110.
158. Wang J, Xiao X, Zhang Y, et al. (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J. Pineal Res. 53: 77-90.
159. Jung CH, Kim EM, Park JK, et al. (2013) Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2signaling pathway. Oncol. Rep. 29: 2109-2113.
160. Chen W, Baler R (2000) The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res. Mol. Brain Res. 81:43-50.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


