Coronary Artery Calcification Under Statin Therapy and Its Effect on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis
- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Changping District Hospital of Integrated Traditional Chinese and Western Medicine, Beijing, China
Objective: To compare Agatston scores between patients without statin therapy and those under standard and intensive statin therapy and to systematically review the relationship between coronary artery calcification (CAC) progression under statin therapy and cardiovascular outcomes.
Methods: Literature search was conducted across databases. Randomized controlled trials and observational studies that reported Agatston scores at baseline and follow-up from patients with and without statin therapy were included. A systematic review and meta-analysis was conducted.
Results: Seven studies were subjected to qualitative and quantitative analyses. Agatston scores in all groups were increased at follow-up. Meta-analysis of data from the included studies revealed an insignificantly lower CAC score at follow-up in the experimental groups. Subgroup analysis showed that statins slowed down CAC progression mildly but with statistical significance in population with baseline CAC score >400 in the experimental groups (P = 0.009). Despite that calcification progressors had worse cardiovascular outcome than did non-progressors, it appeared that baseline CAC score had more decisive effects on cardiovascular outcomes. CAC progression under statin therapy did not increase cardiovascular risk, although more supportive data are needed.
Conclusion: Statins do not reduce or enhance CAC as measured by Agatston score in asymptomatic populations at high risk of cardiovascular diseases, but seem to slow down CAC progression. Although our result was robust, it was restricted by small sample size and relatively short follow-up period. Further studies on the relationship between CAC progression under statin therapy and cardiovascular outcomes are needed.
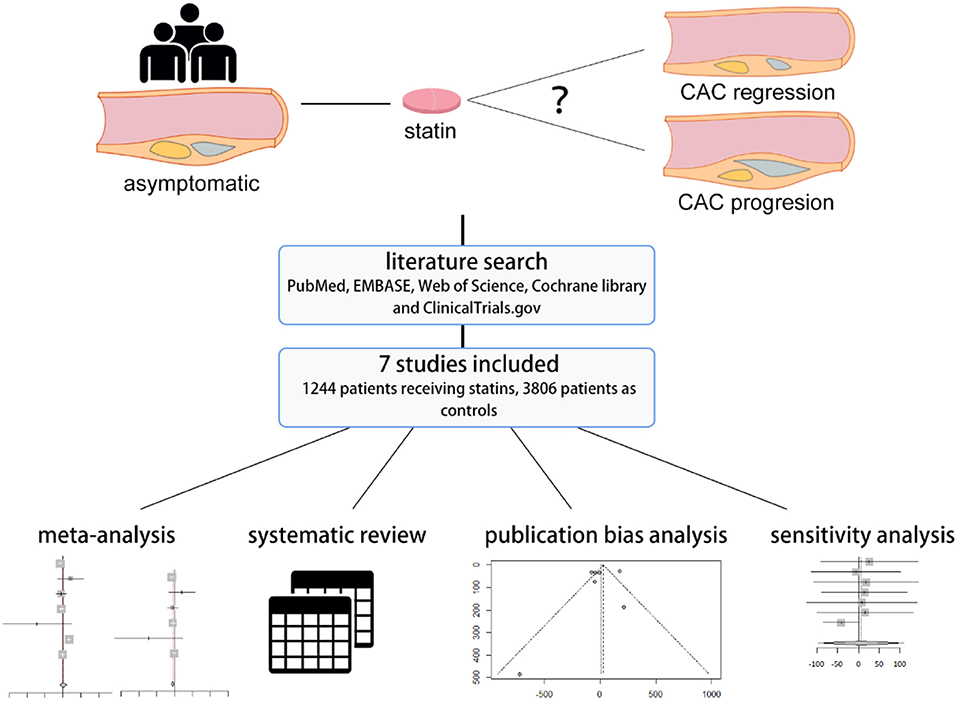
Graphical Abstract. A systematic review and meta-analysis was performed to investigate coronary artery calcification change in asymptomatic population under statin therapy.
Introduction
Calcium in coronary arteries has been used as a surrogate marker of coronary atherosclerosis since the 1940s, and its existence and progression have been correlated with higher cardiovascular risks (1). Development of various imaging techniques allows us to detect coronary calcium more accurately for cardiovascular risk assessment; nevertheless, computed tomography (CT) remains the most established and sensitive non-invasive tool to detect coronary artery calcium and to quantify it using a clinical coronary artery calcification (CAC) score (2). The Agatston scoring method of CAC remains the gold standard; it has been adopted by some major guidelines in cardiovascular risk assessment (3) and encouraged on the basis of large cohort data to be used in risk stratification for statin therapy (4).
Statins, while being effective in improving cardiovascular outcome, have also been found associated with CAC progression (5, 6). Previous meta-analyses included studies measuring coronary calcification with intravascular ultrasound (IVUS) (5) and coronary computed tomographic angiography (CCTA) (6), while the former tends to overestimate calcification due to echo shadow (1), and the latter may have difficulty in identifying and quantifying calcium in the presence of iodine in contrast media (2). To our knowledge, no previous meta-analysis has investigated the correlation between statin therapy and coronary calcification measured by Agatston score in asymptomatic populations at high risk of cardiovascular disease. Furthermore, calcification exists in the natural progression of atherosclerosis; whether statins accelerate this process or simply fail to attenuate it remains to be determined. Although it is suggested that coronary calcification enhanced by statin therapy is a marker for plaque stabilization (7), no study has systematically determined the relationship between cardiovascular outcome and coronary calcification progression under statin therapy. Therefore, we performed a systematic review and meta-analysis of CAC measured by Agatston score under statin therapy, as well as its relationship with cardiovascular outcomes.
Methods
Protocol and Registration
This systematic review and meta-analysis was registered on PROSPERO (CRD42020142911) and was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) (8). No patient and public are involved in the design, or conduct, or reporting of the research. All analyses were based on previous published studies; thus, no ethical approval and patient consent were required.
Search Strategy
Literature searches were carried out in PubMed, EMBASE, Web of Science, Cochrane Library, and ClinicalTrials.gov (last search data, May 15, 2020). The following keywords in different combination were used: statin, coronary artery, calcification or calcified or calcium. Search categories were restricted to reviews, meta-analyses, clinical trials, letters, and conference or meeting papers. Reference lists of articles relevant to this topic were also screened. The following search strategy was used for Web of Science and modified to suit other databases:
#1 statin
#2 coronary artery
#3 calcification OR calcified OR calcium
#4 #1 AND #2 AND #3.
Eligibility Criteria
Our study PICOS were as follows: (1) P: asymptomatic individuals at moderate to high risk of cardiovascular disease; (2) I: statin therapy of various intensity; (3) C: placebo or no use of statins (or in the case of intensive statin therapy as intervention, standard statin therapy); (4) O: CAC score as measured by Agatston method; and (5) S: both randomized controlled trials (RCTs) and observational studies. Study exclusion criteria were as follows: (1) full text not published in English; (2) results of Agatston scores arranged in quantiles; (3) CAC scores measured by other methods; (4) patients with diseases or under conditions that might seriously affect results, e.g., end-stage renal disease; (5) no discussion on the relationship between statins and CAC scores, or no comparison between experimental and control group or various statin dosage groups; and (6) study period <6 months. For different studies published under the same data source, the one with the most complete information was included.
Data Extraction
Two authors (RM Lai, JQ Ju) independently conducted literature screens and data extractions for baseline demographic data and clinical outcomes. Any discrepancies were resolved by consensus or by a third reviewer (H Xu). Especially, Agatston scores were extracted as means with standard deviations (SDs), and those reported as medians with interquartile ranges were calculated to their means and SDs using previously described methods (9).
Risk-of-Bias Analysis
Risk-of-bias analyses of RCTs were conducted using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2), whereas observational studies were assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS).
Statistical Analysis
The meta-analysis was performed using the meta package (10) in R (3.6.2). Outcomes were analyzed using a random-effects model to obtain more conservative results as we included both randomized and non-randomized studies, and heterogeneity was inevitable. Summary estimates were reported as weighted mean difference (WMD) with 95% confidence intervals (CIs). Heterogeneity among studies was tested with Q test and I2 statistic. A P-value of 0.1 was considered significant for Q-test, and thresholds for the interpretation of the I2 statistic were defined according to the Cochrane Handbook (11) as low heterogeneity for values from 0 to 40%, moderate heterogeneity for values from 30 to 60%, substantial heterogeneity for values from 50 to 90%, and considerable heterogeneity for values from 75 to 100%. Risk of publication bias was analyzed and presented with funnel plot. Sensitivity analysis was performed by omitting studies one at a time from the overall analysis and presented with forest plot.
Results
Study Selection
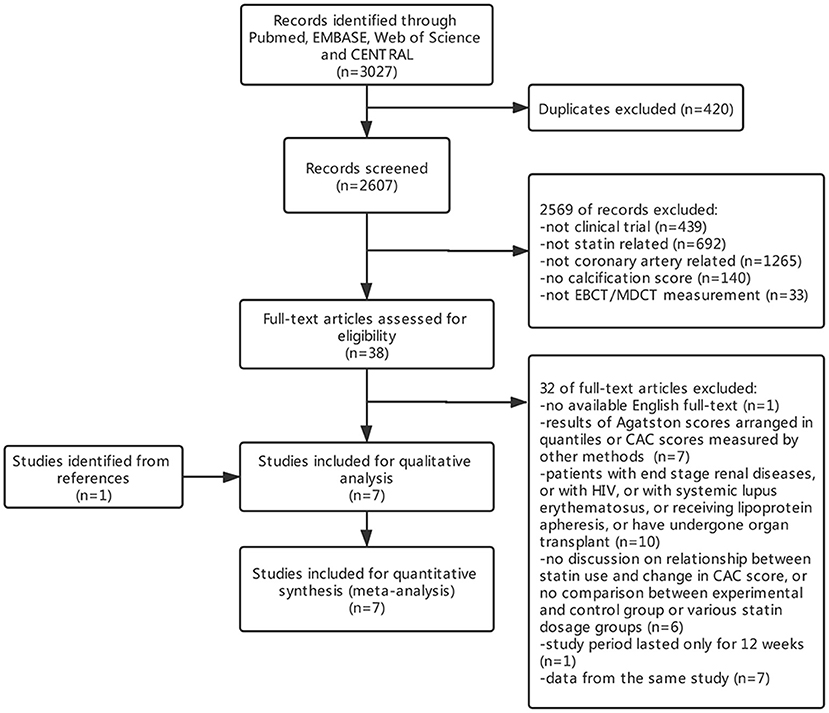
A total of 3,027 initial citations were returned by searching these databases, and 2,607 items were screened by titles and abstracts after removing 420 duplicates. Thirty-eight studies were subjected to full-test review, and 32 were excluded (Figure 1). By screening reference lists of relevant articles, one study was additionally included. In all, seven studies were subjected to qualitative and quantitative analyses. During quantitative analysis, one study was excluded for its skewed distribution between experimental and control group sizes and baseline CAC scores, which exerted too much heterogeneity to the results.
Study Characteristics
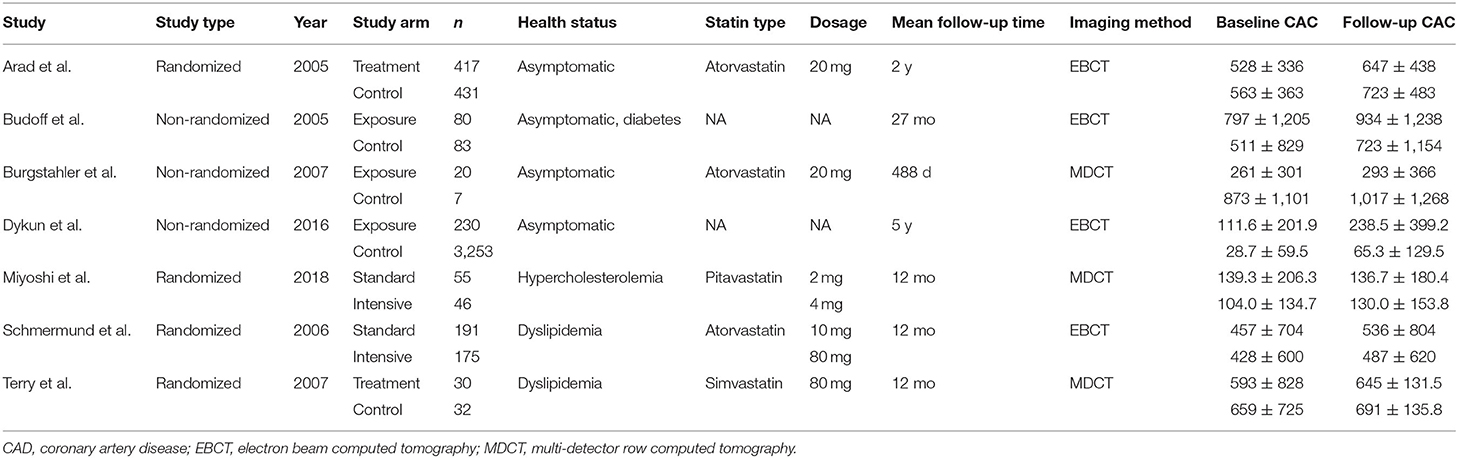
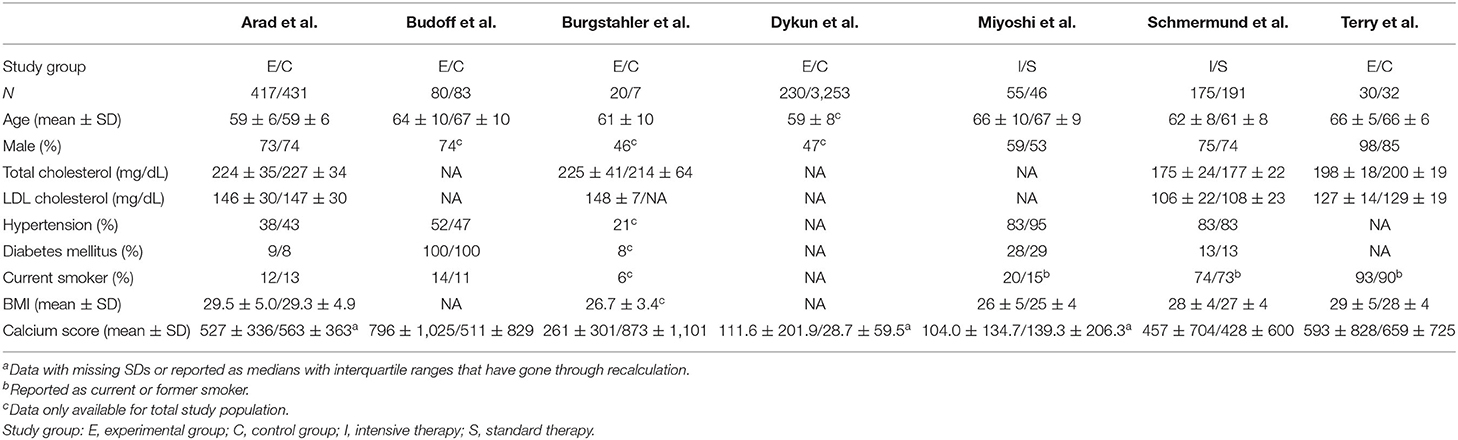
Of the seven included studies (12–18), four were RCTs (12, 16–18), and three were non-randomized observational studies (13–15). Baseline demographic and clinical characteristics of these studies are shown in Tables 1, 2. These seven studies included 1,014 patients receiving standard statin therapy, 230 patients receiving intensive statin therapy, and 3,806 patients as controls. The average age of patients ranged from 59 to 67 years. Between 46 and 98% of patients were male. The length of follow-up period ranged from 344 days to 5 years.
Risk of Bias
Among the four RCTs included, three showed a low risk of bias, one (18) showed a high risk of bias due to a high lost-to-follow-up rate, which caused CAC scores between experimental and control groups to be quite unbalanced (Supplementary Table 1). According to the NOS, all non-randomized studies were rated as high quality (Supplementary Table 2).
CAC Assessment
Imaging processes were performed using electron beam computed tomography or multi-detector row computed tomography, with synchronized electrocardiographic triggering at 80% of the R-R interval in all but one study (13), which obtained images corresponding to 40% of the R-R interval. All studies obtained slices at 3-mm intervals but one study (18), which obtained slices at 2.5 mm intervals. Four studies made it clear that imaging interpretations were performed by blinded examiners. A calcified lesion threshold of >130 Hounsfield units was used, and the lesion score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit within this area, as described by Agatston et al. (19).
The Effect of Statins on CAC
Agatston scores in both experimental groups and control groups in all studies increased at follow-up compared with that at baseline. Compared with the control groups, meta-analysis of all seven studies showed higher CAC scores in the experimental groups by a WMD of 6.31 but with no significance (95% CI = 97.88–110.50; P = 0.91) (Figure 2A). However, including all seven studies showed substantial heterogeneity among studies (I2 = 88%), which could be largely attributed to the study of Dykun et al. (15). Because of its greatly skewed distribution of baseline CAC scores and population sizes between the experimental group and the control group, and the omission of considerable amounts of baseline demographic information, we decided to omit this study, leaving 547 patients receiving standard therapy, 221 patients receiving intensive therapy, and 799 patients in the control group in the final quantitative analysis. Heterogeneity was lowered to 18% after we omitted this study. Meta-analysis of the remaining six studies showed that CAC scores in the experimental groups were lower than that in the control groups by a WMD of 41.99 (95% CI = −85.05–1.07; P = 0.06) also with no statistical significance (Figure 2B), and the insignificance remained when stratifying studies according to statin therapy intensity.
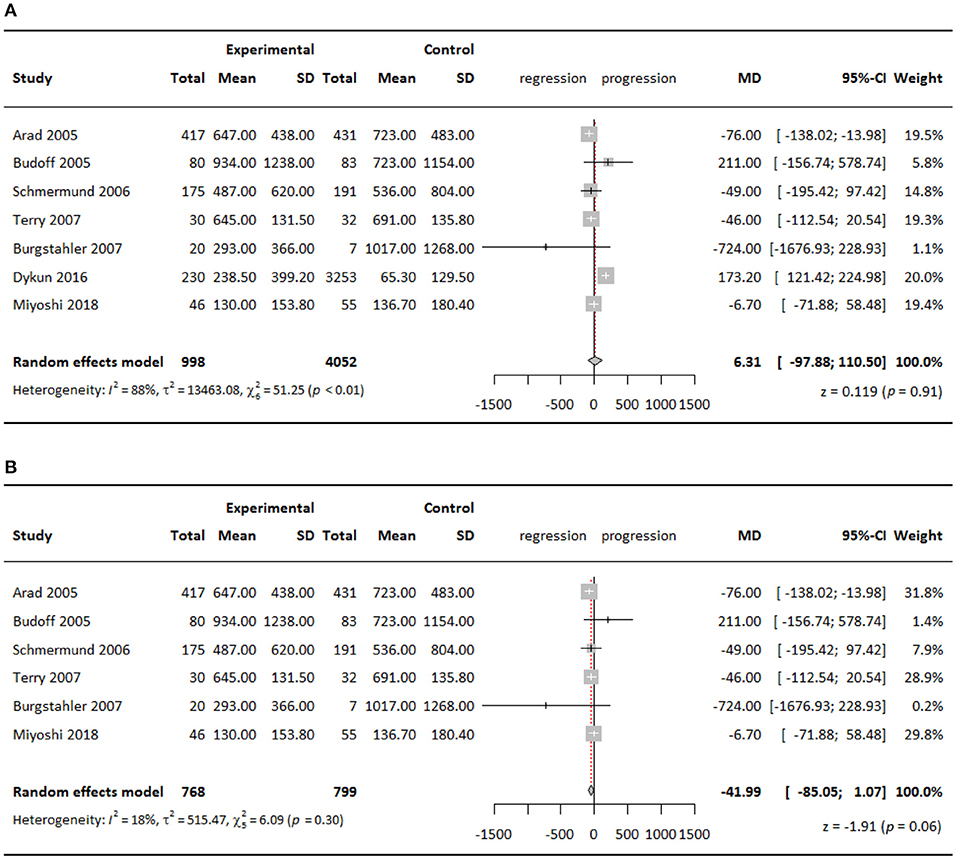
Figure 2. Meta-analysis of effect of statins on CAC Agatston scores comparing experimental groups to control groups, (A) all seven studies included; (B) six studies included.
When stratifying studies according to baseline CAC score with a cutoff point of 400, we found that in the subgroup with baseline CAC score >400, statins slowed down CAC progression by a WMD of 57.19 (95% CI = −100.23 to −14.15; P = 0.009) in the experimental groups compared with the control groups (Figure 3). The effect was statistically significant, although minor.
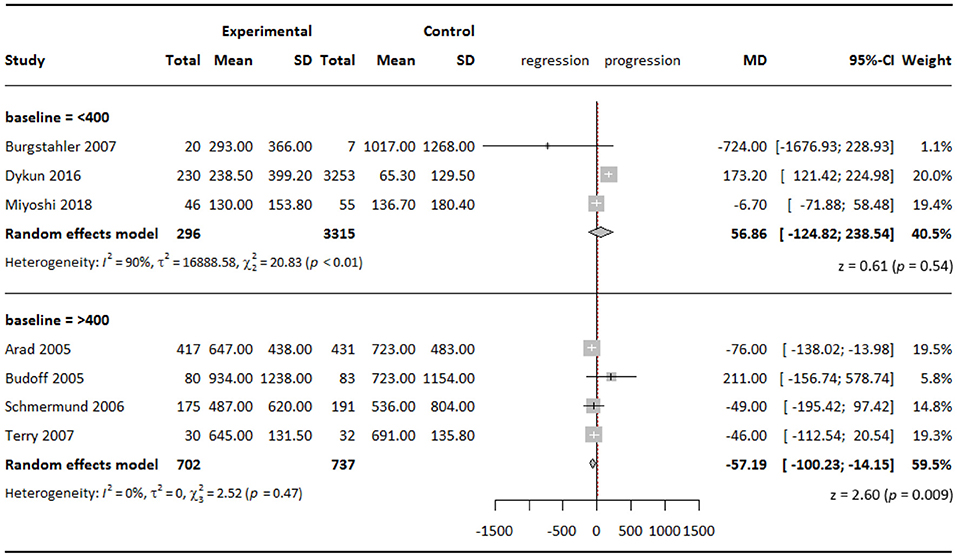
Figure 3. Subgroup analysis of effect of statins on CAC Agatston scores when stratified by baseline CAC score.
Publication Bias and Sensitivity Analysis
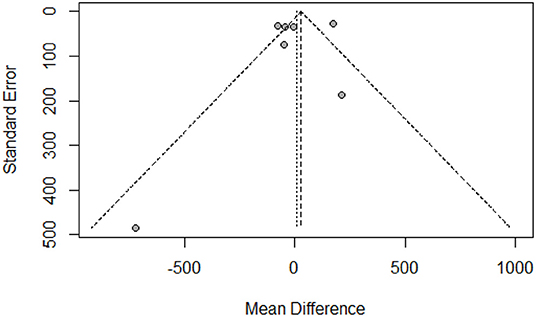
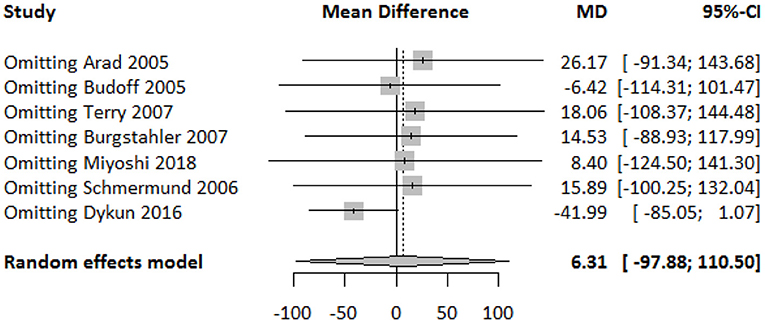
The funnel plot showed a largely symmetrical distribution of included studies, indicating no serious publication bias (Figure 4). Sensitivity analysis showed that our result was robust. Excluding any one of the studies did not greatly affect the result (Figure 5).
Cardiovascular Outcomes
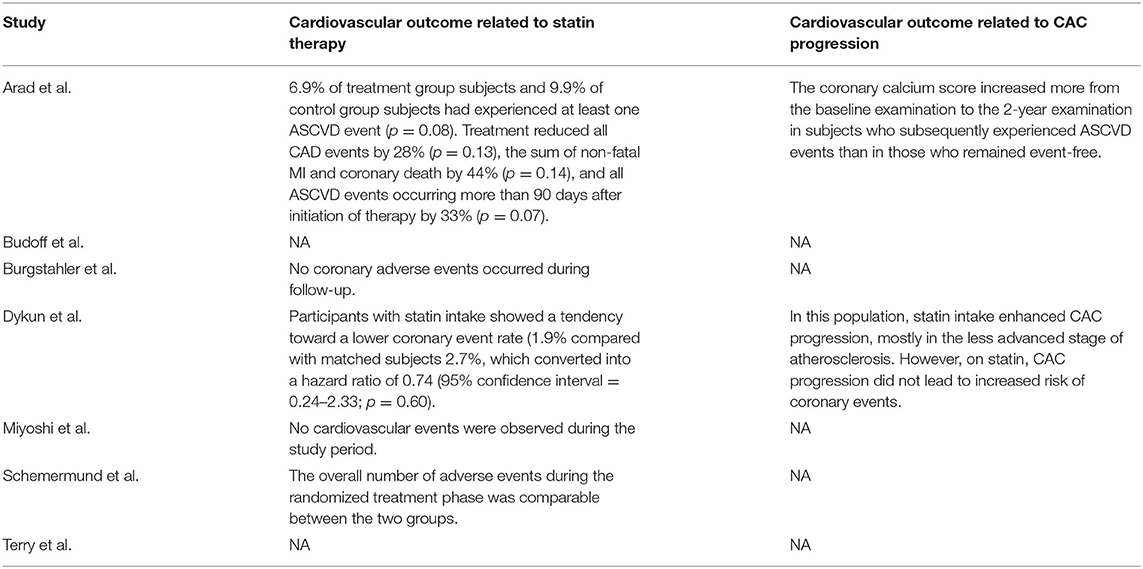
While it is widely recognized that statin therapy improves cardiovascular outcomes, most included studies did not mention or only briefly mentioned the relationship between CAC progression under statin therapy and cardiovascular outcome. We therefore systematically reviewed and displayed cardiovascular outcomes reported by the included studies (Table 3). The overall cardiovascular outcomes between experimental group and control group were largely comparable. Dykun et al. (15) showed that statin intake enhanced CAC progression while resulting in a lower coronary event rate after performing a matched case-control analysis. On the other hand, Arad et al. (12) compared patients with and without cardiovascular events during follow-up and found out that subjects who subsequently experienced atherosclerotic cardiovascular disease events had greater absolute progression in CAC score than those who did not. However, they did not investigate cardiovascular outcomes difference between calcification progressors and non-progressors under statin therapy. In their later multivariate analysis, baseline CAC score instead of absolute change in CAC score was found to be the only significant predictor for cardiovascular events after various adjustment, while these two factors were highly positively correlated. It appeared that, compared to the progression level and rate, a lower baseline CAC score was more decisive for better cardiovascular outcomes under statin therapy.
Lipid Parameters
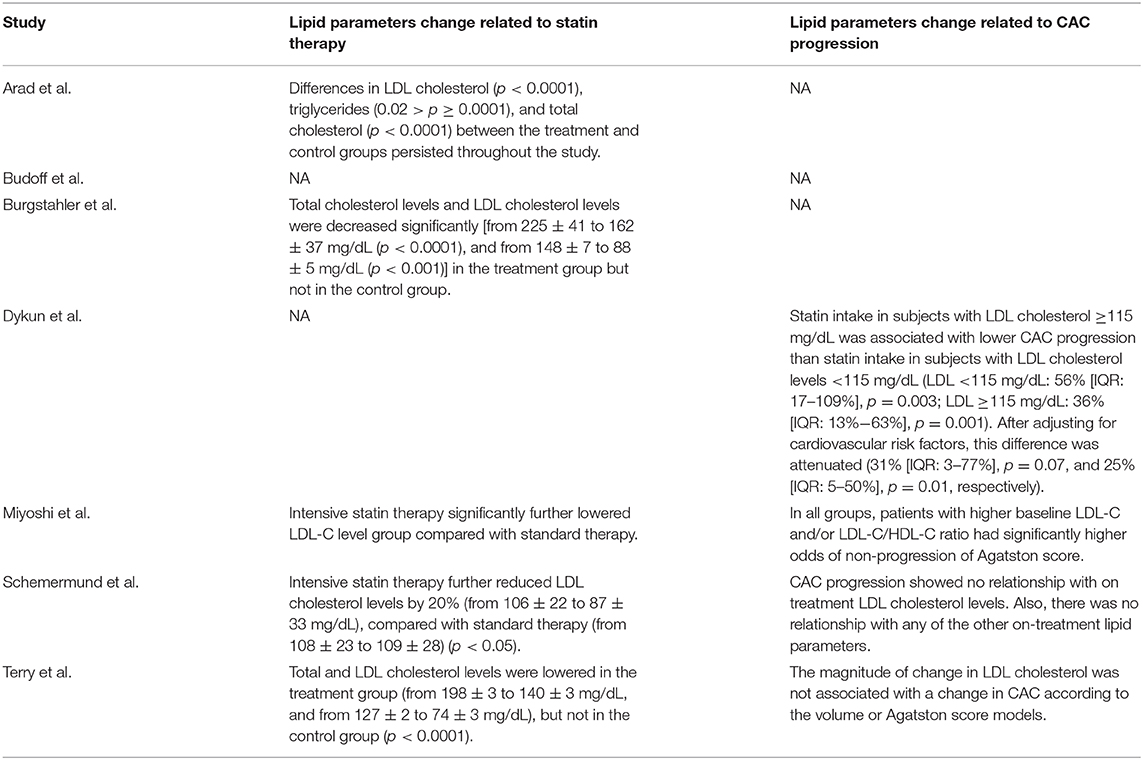
Some of the included studies reported lipid parameters change related to statin therapy and CAC progression (Table 4). Under statin therapy, total and low-density lipoprotein (LDL) cholesterol levels in experiment groups were lowered significantly than those in the control groups in most studies. However, relationship between LDL cholesterol level and CAC progression was inconsistent among the included studies. Two (15, 16) reported that a higher LDL cholesterol level was associated with less progression in CAC, whereas the other two (17, 18) reported no significant association.
Discussion
In this systematic review and meta-analysis of statin therapy on CAC as measured by Agatston score, we demonstrated that statins failed to reduce CAC but still slowed down CAC progression in some of the asymptomatic population, instead of promoting CAC progression. Our results were partly similar to those of previous meta-analysis and studies on coronary plaque change under statin therapy measured by CT (20) and optical coherence tomography (21, 22); however, our results were inconsistent with those that measured plaque change using IVUS (5) and CCTA (6). Discrepancies between studies using different imaging methods have been mentioned previously (7). Although IVUS gives a higher resolution on plaque volume and other compositional features such as necrotic core and fibrous cap, its accuracy in measuring calcium does not necessarily exceed that of CT; more studies concerning this area are needed. While CCTA uses similar imaging techniques as CT, it introduces iodine contrast media for more morphology and composition information of plaques, which could make it difficult to precisely identify and quantify CAC (2).
The Agatston score was reported by Agatston et al. in the 1990s and has become the most commonly used method for quantifying CAC measured by various CT scanners, including electron beam, multi-detector row, and dual-source CT and CCTA (23, 24). It considers both calcium volume and density and requires only simple calculation, thereby suiting the needs of clinical practice. Despite wide application, the Agatston score has been questioned regarding its interscan reproducibility. The score could vary with higher heart rates, motion artifacts, equipment from different vendors, and software platforms (25). Other methods for calcium scoring have been developed, including the volume score and the mass score; however, none were found to be preferable to another in terms of reproducibility of results from consecutive scans in a patient (26).
We chose not to use change in CAC score from baseline to follow-up as did other meta-analyses (5, 6, 20); rather, we used specific CAC score at follow-up in statistical analysis because analyzing change scores does not control for baseline imbalance due to regression to the mean (27). Furthermore, it has been suggested that a comparable result using either absolute difference or relative difference between CAC score at follow-up and CAC score at baseline might not be achieved because of non-linear progression pattern of CAC score (23, 28). We noted that Puri et al. (5) in their meta-analysis managed to obtain a more comparable result between groups by applying a propensity score weighting method and adjusting for various covariates. However, the patients included in their meta-analysis had angiographically confirmed coronary artery disease and therefore had clinical indications for IVUS examination. A more active atherosclerotic status and later stage of plaque progression (7) could be one reason for the significant coronary calcification progression in their treatment group but not in ours. Calcification during atherosclerosis is closely associated with both progression stage and healing stage of inflammation (29), and previous studies have shown that the effect of statins on reducing inflammation was more pronounced within advanced coronary lesions as measured using 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic imaging (30). Another reason that we did not see an enhanced CAC score due to statin therapy could be the relatively short follow-up periods of the included studies. In asymptomatic populations who have milder atherosclerosis than those with confirmed CAD, it could take longer for calcification to progress.
Interestingly, we even observed a slowdown effect from statin therapy on CAC progression in the subgroup with baseline CAC score >400, which could be a revelation that statins affect CAC differently in a less atherosclerotic but still high-risk plaque environment. Mitchell et al. (31) have previously proven that there existed a threshold of CAC score above which asymptomatic patients would benefit more from statin therapy, and they determined the threshold as 100 through a large-scale cohort analysis. In our case, the cutoff point was chosen according to distribution of baseline CAC scores across studies. More differences between various baseline CAC score intervals might be observed within a larger sample.
The relationship between CAC progression under statin therapy and cardiovascular outcome could be confusing and conflicting. Some suggested that calcification progression is a sign of micro, fragmented calcium evolving into macro, sheet calcium, which stabilizes plaques (7); other studies indicated that, in patients receiving statins and other lipid-lowering agents, those with subsequent cardiovascular events had greater CAC progression (12, 32). Previous research from the Multi-Ethnic Study of Atherosclerosis showed that, while CAC volume was positively and independently associated with cardiovascular risks, CAC density was significantly inversely associated with cardiovascular risks (33). A more recent study found out that CAC > 0 compared with CAC = 0 was associated with a significantly higher risk of atherosclerotic cardiovascular disease events regardless of baseline or incident statin use or when accounting for time-varying statin use (34). Despite the tendency of plaques becoming more calcific and stable over time under statin therapy, at an early stage of atherosclerosis, CAC progression could be both a marker of plaque stability and an indication for more intensive primary prevention therapy given different backgrounds. More studies into the mechanisms and risk factors for different cardiovascular outcomes among calcification progressors are needed.
With cholesterol, especially LDL cholesterol being an important initiator and component of atherosclerotic plaques and target of statin therapy, it is reasonable to assume an association between LDL cholesterol and CAC under statin therapy. Two included studies reported an inverse association, which is in tune with recent theory that statins stabilize plaques by promoting calcification while lowering LDL cholesterol (1); another two reported no association, which is consistent to previous findings from clinical data (5, 35, 36). Healy et al. (37) in a recent article demonstrated that statins, by inhibiting mevalonate synthesis, inhibited downstream cholesterol synthesis and activated downstream Rac1-IL-1β signaling axis. This activation led to a procalcific effect in animal models. However, considering the pleiotropic effects of statins, they could have promoted CAC through other more pathways.
Limitations
Our study has some limitations, first being the limited number of included studies and small overall sample size, which could have restricted our conclusion from being applicable to a larger population; thus, our results should be interpreted with caution. Despite wide application of CT and Agatston score in clinical practice, few relevant studies have been done in recent years because of development of other more advanced imaging techniques. Some studies chose to publish data in the form of quantiles, progression percentiles, or calcium scores by other calculating methods, resulting in many being excluded from our analysis. Therefore, efforts to pool larger cohort data are needed.
Second, information concerning cardiovascular outcomes, especially cardiovascular outcomes related to CAC progression, is limited, partly due to the study population who were asymptomatic and atherosclerotic and relatively short follow-up period. In fact, not many studies to date have systematically evaluated CAC progression's implication on cardiovascular outcomes. Radford et al. (38) and Lehmann et al. (39) both reported based on large cohort data that CAC progression is associated with coronary and cardiovascular event rates, but adds only weakly to risk prediction. However, in both studies, participants under statin therapy took up only a small proportion of the cohorts.
Third, differences in baseline characteristics, length of follow-up period, study types, CT vendors, software platforms, and variability of Agatston scores also brought heterogeneity and some uncertainty to our result. However, our final result is robust with low to moderate heterogeneity. With CT remaining an important early screening method for cardiovascular risk, we believe that our study is of value for asymptomatic populations at high risk of cardiovascular diseases in general clinical practice.
Conclusion and Perspectives
Statins do not reduce or enhance CAC as measured by Agatston scores in asymptomatic populations at high risk of cardiovascular diseases, but seem to slow down their CAC progression. Our results reveal the value of CT scan and CAC quantification in early screening and importance of early initiation of statins for this population. The relationship between calcification progression under statin therapy and different cardiovascular outcomes calls for further investigation, and effort to pool large cohorts with longer follow-up period and acquire individual patient data is needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author Contributions
The research question was developed by RL. The study protocol was written and registered by RL and QL. The literature search and assessment of articles were conducted by RL and JJ, with HX acting as supervisor and a third assessor in case of disagreement authors. The manuscript was drafted by RL, polished by JJ, and revised by QL and HX. RL and HX would be responsible for the overall content in this article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from National Key R&D Program of China (no. 2018YFC2002502), Guiding Projects for Major achievements of TCM science and technology, China Academy of Chinese Medical Science (ZZ13-ZD-03), and Capital's Funds for Health Improvement and Research (No. 2018-1-4171).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.600497/full#supplementary-material
Abbreviations
CAC, coronary artery calcification; CAD, coronary heart disease; CCTA, coronary computed tomographic angiography; CT, computed tomography; IVUS, intravascular ultrasound; LDL, low-density lipoprotein; MDCT, multi-detector row computed tomography; OCT, optical coherence tomography; RCT, randomized controlled trials.
References
1. Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. (2017) 10:582–93. doi: 10.1016/j.jcmg.2017.03.005
2. Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc. (2018) 7:e008564. doi: 10.1161/JAHA.118.008564
3. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. (2018) 72:434–47. doi: 10.1016/j.jacc.2018.05.027
4. Mortensen MB, Falk E, Li D, Nasir K, Blaha MJ, Sandfort V, et al. Statin trials, cardiovascular events, and coronary artery calcification: implications for a trial-based approach to statin therapy in MESA. JACC Cardiovasc Imaging. (2018) 11(2 Pt 1):221–30. doi: 10.1016/j.jcmg.2017.01.029
5. Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. (2015) 65:1273–82. doi: 10.1016/j.jacc.2015.01.036
6. Andelius L, Mortensen MB, Norgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. (2018) 19:850–58. doi: 10.1093/ehjci/jey012
7. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. (2018) 11:127–42. doi: 10.1016/j.jcmg.2017.10.012
8. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
9. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
10. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
11. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
12. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. (2005) 46:166–72. doi: 10.1016/j.jacc.2005.02.089
13. Budoff MJ, Yu D, Nasir K, Mehrotra R, Chen L, Takasu J, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J. (2005) 149:695–700. doi: 10.1016/j.ahj.2004.07.034
14. Burgstahler C, Reimann A, Beck T, Kuettner A, Baumann D, Heuschmid M, et al. Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol. (2007) 42:189–95. doi: 10.1097/01.rli.0000254408.96355.85
15. Dykun I, Lehmann N, Kalsch H, Mohlenkamp S, Moebus S, Budde T, et al. Statin medication enhances progression of coronary artery calcification: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. (2016) 68:2123–25. doi: 10.1016/j.jacc.2016.08.040
16. Miyoshi T, Kohno K, Asonuma H, Sakuragi S, Nakahama M, Kawai Y, et al. Effect of intensive and standard pitavastatin treatment with or without eicosapentaenoic acid on progression of coronary artery calcification over 12 months- prospective multicenter study. Circ J. (2018) 82:532–40. doi: 10.1253/circj.CJ-17-0419
17. Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation. (2006) 113:427–37. doi: 10.1161/CIRCULATIONAHA.105.568147
18. Terry JG, Carr JJ, Kouba EO, Davis DH, Menon L, Bender K, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am J Cardiol. (2007) 99:1714–7. doi: 10.1016/j.amjcard.2007.01.060
19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-T
20. Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int J Cardiol. (2011) 153:31–5. doi: 10.1016/j.ijcard.2010.08.031
21. Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, et al. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol. (2014) 114:549–54. doi: 10.1016/j.amjcard.2014.05.035
22. Hou J, Xing L, Jia H, Vergallo R, Soeda T, Minami Y, et al. Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and intravascular ultrasound imaging. Am J Cardiol. (2016) 117:800–6. doi: 10.1016/j.amjcard.2015.11.062
23. Mahabadi AA, Lehmann N, Dykun I, Muller T, Kalsch H, Erbel R. Progression of coronary artery calcification by cardiac computed tomography. Herz. (2015) 40:863–8. doi: 10.1007/s00059-015-4342-z
24. Ahmed W, de Graaf MA, Broersen A, Kitslaar PH, Oost E, Dijkstra J, et al. Automatic detection and quantification of the Agatston coronary artery calcium score on contrast computed tomography angiography. Int J Cardiovasc Imaging. (2015) 31:151–61. doi: 10.1007/s10554-014-0519-4
25. Malguria N, Zimmerman S, Fishman EK. Coronary artery calcium scoring: current status and review of literature. J Comput Assist Tomogr. (2018) 42:887–97. doi: 10.1097/RCT.0000000000000825
26. Andrews J, Psaltis PJ, Bartolo BAD, Nicholls SJ, Puri R. Coronary arterial calcification: a review of mechanisms, promoters and imaging. Trends Cardiovasc Med. (2018) 28:491–501. doi: 10.1016/j.tcm.2018.04.007
27. Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. (2001) 323:1123–4. doi: 10.1136/bmj.323.7321.1123
28. Schmermund A, Baumgart D, Mohlenkamp S, Kriener P, Pump H, Gronemeyer D, et al. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: an electron-beam CT study. Arterioscler Thromb Vasc Biol. (2001) 21:421–6. doi: 10.1161/01.ATV.21.3.421
29. Shioi A, Ikari Y. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb. (2018) 25:294–303. doi: 10.5551/jat.RV17020
30. Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, et al. Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic study. Circ Cardiovasc Imaging. (2016) 9:e004195. doi: 10.1161/CIRCIMAGING.115.004195
31. Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. (2018) 72:3233–42. doi: 10.1016/j.jacc.2018.09.051
32. Raggi P, Cooil B, Shaw LJ, Aboulhson J, Takasu J, Budoff M, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol. (2003) 92:827–29. doi: 10.1016/S0002-9149(03)00892-0
33. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. (2014) 311:271–8. doi: 10.1001/jama.2013.282535
34. Rifai MA, Blaha MJ, Patel J, Xiaoming J, Cainzos-Achirica M, Greenland P, et al. Coronary artery calcification, statin use and long-term risk of atherosclerotic cardiovascular disease events (from the multi-ethnic study of atherosclerosis). Am J Cardiol. (2020) 125:835–39. doi: 10.1016/j.amjcard.2019.12.031
35. Lee D, Joo HJ, Jung HW, Lim DS. Investigating potential mediator between statin and coronary artery calcification. PLoS ONE. (2018) 13:e0203702. doi: 10.1371/journal.pone.0203702
36. Tenenbaum A, Shemesh J, Koren-Morag N, Fisman EZ, Adler Y, Goldenberg I, et al. Long-term changes in serum cholesterol level does not influence the progression of coronary calcification. Int J Cardiol. (2011) 150:130–4. doi: 10.1016/j.ijcard.2010.03.001
37. Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, et al. Statins disrupt macrophage rac1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler Thromb Vasc Biol. (2020) 40:714–32. doi: 10.1161/ATVBAHA.119.313832
38. Radford NB, DeFina LF, Barlow CE, Lakoski SG, Leonard D, Paixao AR, et al. Progression of CAC score and risk of incident CVD. JACC Cardiovasc Imaging. (2016) 9:1420–9. doi: 10.1016/j.jcmg.2016.03.010
Keywords: statins, Agatston score, coronary artery calcification, atherosclerosis cardiovascular disease, computed tomography
Citation: Lai R, Ju J, Lin Q and Xu H (2020) Coronary Artery Calcification Under Statin Therapy and Its Effect on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 7:600497. doi: 10.3389/fcvm.2020.600497
Received: 30 August 2020; Accepted: 17 November 2020;
Published: 18 December 2020.
Edited by:
Ronny Ralf Buechel, University Hospital Zürich, SwitzerlandReviewed by:
Carmen Chan, Queen Mary Hospital, Hong KongÖzge Özden Tok, Memorial Hospital, United States
Copyright © 2020 Lai, Ju, Lin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xu, xuhaotcm@hotmail.com
 Runmin Lai
Runmin Lai Jianqing Ju2
Jianqing Ju2  Hao Xu
Hao Xu