Risk Factors for Anthracycline-Induced Cardiotoxicity
- 1Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xi'an, China
- 2Department of General Surgery, Tangdu Hospital, Air Force Medical University, Xi'an, China
- 3School of Nursing, Air Force Medical University, Xi'an, China
- 4Department of Hematology, Tangdu Hospital, Air Force Medical University, Xi'an, China
- 5Department of Biochemistry and Molecular Biology, Air Force Medical University, Xi'an, China
Background: Several cardiovascular risk factors have been suggested to be associated with anthracycline-induced cardiotoxicity, but their quantitative effects have not reached a consensus.
Methods: We searched PubMed, EMBASE, and Cochrane Library databases for manuscripts published from inception to February 2021, which reported the results of cardiotoxicity due to anthracycline chemotherapy without trastuzumab. Cardiotoxicity defined by any reduction of left ventricular eject fraction (LVEF) to below 50% or a >10% reduction from baseline was defined as the primary endpoint. Odd ratios (OR) with 95% confidence intervals (CI) were calculated using a random-effects model meta-analysis.
Results: A total of 7,488 patients receiving anthracycline chemotherapy without trastuzumab were included, who had at least one risk factor at baseline. Hypertension (OR: 1.99; 95% CI: 1.43–2.76), diabetes mellitus (OR: 1.74; 95% CI: 1.11–2.74), and obesity (OR: 1.72; 95% CI: 1.13–2.61) were associated with increased risk of cardiotoxicity. In addition, the relative reduction of global longitudinal strain (GLS) from baseline after anthracycline treatment could significantly improve the detection ability of cardiotoxicity (28.5%, 95% CI: 22.1–35.8% vs. 16.4%, 95% CI: 13.4–19.9%) compared with LVEF. The early detection rate of anthracycline-induced cardiotoxicity (3 months after chemotherapy) by GLS was 30.2% (95% CI: 24.9–36.1%), which is similar with the overall result of GLS.
Conclusions: Hypertension, diabetes mellitus, and obesity are associated with increased risk of anthracycline-induced cardiotoxicity, which indicates that corresponding protective strategies should be used during and after anthracycline treatment. The findings of higher detection rate and better early detection ability for cardiotoxicity than LVEF added new proofs for the advantages of GLS in detection of AIC.
Introduction
In recent years, due to advances in cancer treatment and early detection strategies, the mortality rates of cancer have significantly declined (1). Studies have shown that in the next 10 years, long-term cancer survivors are expected to increase by about 30% (2). However, the improvement in survival rate comes with an increased risk of cardiotoxicity (3). Anthracyclines, a vital component of numerous cytotoxic regimens, is a broadly used chemotherapy for many malignancies including breast cancer, sarcoma, lymphoma, bladder cancer, and some leukemias (4). For anthracyclines, such as doxorubicin, when the cumulative dose is 250 to 400 mg/m2, there will be 6 to 33% of patients with reduced left ventricular ejection fractions (LVEF) (5). Cardiotoxicity and subsequent heart failure caused by anthracyclines are the most common clinical symptoms in patients with breast cancer, lymphoma, and hematopoietic cancers (6).
Pre-existing cardiovascular risk factors have been suggested to be associated with increased incidences of anthracycline-induced cardiotoxicity (AIC). It was reported that the incidence of cardiotoxicity in patients with hypertension, diabetes mellitus, or obesity is remarkably higher than that in normal patients (7). However, the results of the quantitative risk effects of those cardiovascular factors vary between different studies. It was mainly attributed to the confounding treatment of other chemotherapy and/or cardioprotective drugs and different definitions of cardiotoxicity (8–11).
Echocardiography is the cornerstone for cardiac imaging in patients with chemotherapy, with advantages of wide availability, easy repeatability, and free from radiation exposure. The new onset of heart failure symptoms, a 10% asymptomatic drop in LVEF or a drop in LVEF from baseline to <55% determined by two-dimensional (2D) echocardiography after cancer treatment, are considered as cardiotoxicity (12). However, the low sensitivity of LVEF for the detection of subclinical changes in cardiac function may cause delayed diagnosis of AIC (13). With the development of echocardiography technology, speckle tracking echocardiography (STE) has been widely used in early and sensitive detection of the subclinical myocardial impairment, among which the most commonly used method is global longitudinal strain (GLS). A recent meta-analysis showed that GLS has a good prognostic performance for the development of cardiotoxicity in patients receiving anthracyclines (14). The early detection value of GLS in AIC is of great interest, which may change the current LVEF-guided AIC treatment.
We conducted a meta-analysis to assess the risk factors associated with AIC and explore the early detection ability of GLS for AIC.
Methods
Search Strategy
A systematic review was performed, adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15) (Supplementary Table 1). We searched PubMed, EMBASE, and Cochrane Library databases for manuscripts published from inception to February 2021. Key words included in the search were anthracycline, doxorubicin, chemotherapy, cancer therapy, cardiotoxicity, cardiomyopathy, risk factors, hypertension, diabetes mellitus, obesity, overweight, hypercholesterinemia, and smoking. In addition, we have expanded the scope of our search to manually search the list of references from original studies, gray literature, and records, regardless of language and publication years.
Study Selection and Quality Assessment
Two reviewers (SQ and TZ) independently searched the titles and abstracts of the above databases by using a standardized form. Any discrepancies between the two reviewers were resolved by consensus. Eligible studies were identified if they met the following inclusion criteria: (1) adult participants ≥18 years of age; (2) research types, both retrospective studies, case–control study, and clinical randomized controlled trials; (3) LVEF or GLS was assessed at baseline and at the end of treatment; and 4) follow-up duration ≥3 months. Studies were mainly excluded for the following reasons: (1) the type of study is not appropriate, including reviews, editorials, and case report; (2) published in a format other than English; (3) LVEF data were incomplete; and (4) animal studies. For multiple manuscripts published from the same cohort, the meta-analysis included the most recent analysis and the highest number of results. In addition, for studies including patients with trastuzumab, we only extract the data from the group of patients without trastuzumab. The methodological quality of included studies was assessed by the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability (16).
Data Extraction and Clinical End Points
The literature search, study selection, study appraisal, and data extraction were pre-defined in the protocol and were independently conducted by two investigators (SQ and TZ). Data were extracted and included study setting, cohort descriptors, prevalence of cardiotoxicity, baseline and follow-up data for LVEF, and diagnostic criteria. Any discrepancies were resolved by consensus.
The primary clinical endpoint was defined as any reduction of LVEF to below 50% or a >10% reduction from baseline. Another endpoint is GLS, which is currently used as an indicator in 2D STE parameters to predict cardiotoxicity (13, 17–19). In previous studies, patients with cardiotoxicity were defined as GLS with an average reduction of 10–20% (20). This study defines cardiotoxicity as relative percentage reduction of ≥10% in GLS from baseline.
Statistical Analysis
The summary incidence of AIC was calculated from the number of patients receiving chemotherapy based on anthracycline in retrospective cohort studies, case–control study, and clinical randomized controlled trials. In order to calculate the odds ratio (OR) and 95% confidence interval (CI) of each risk factor, data on the number of patients with various risk factors in the cardiotoxicity group and the non-cardiotoxicity group were extracted.
The pooled estimates were derived using the DerSimonian and Laird random-effect model. Heterogeneity among studies was assessed using Cochran's Q test and by measuring consistency (I2). To assess the potential impact of publication bias, we checked the symmetry of the funnel chart. Sensitivity analyses were performed by comparing the conclusion after removing the study with the largest proportion with the original one. Statistical analyses were performed with Review Manager 5.3 (version 5.3.5, Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration) and Comprehensive Meta-Analysis Version 3.0 software (Biostat, Englewood, NJ), and P < 0.05 was considered statistically significant.
Results
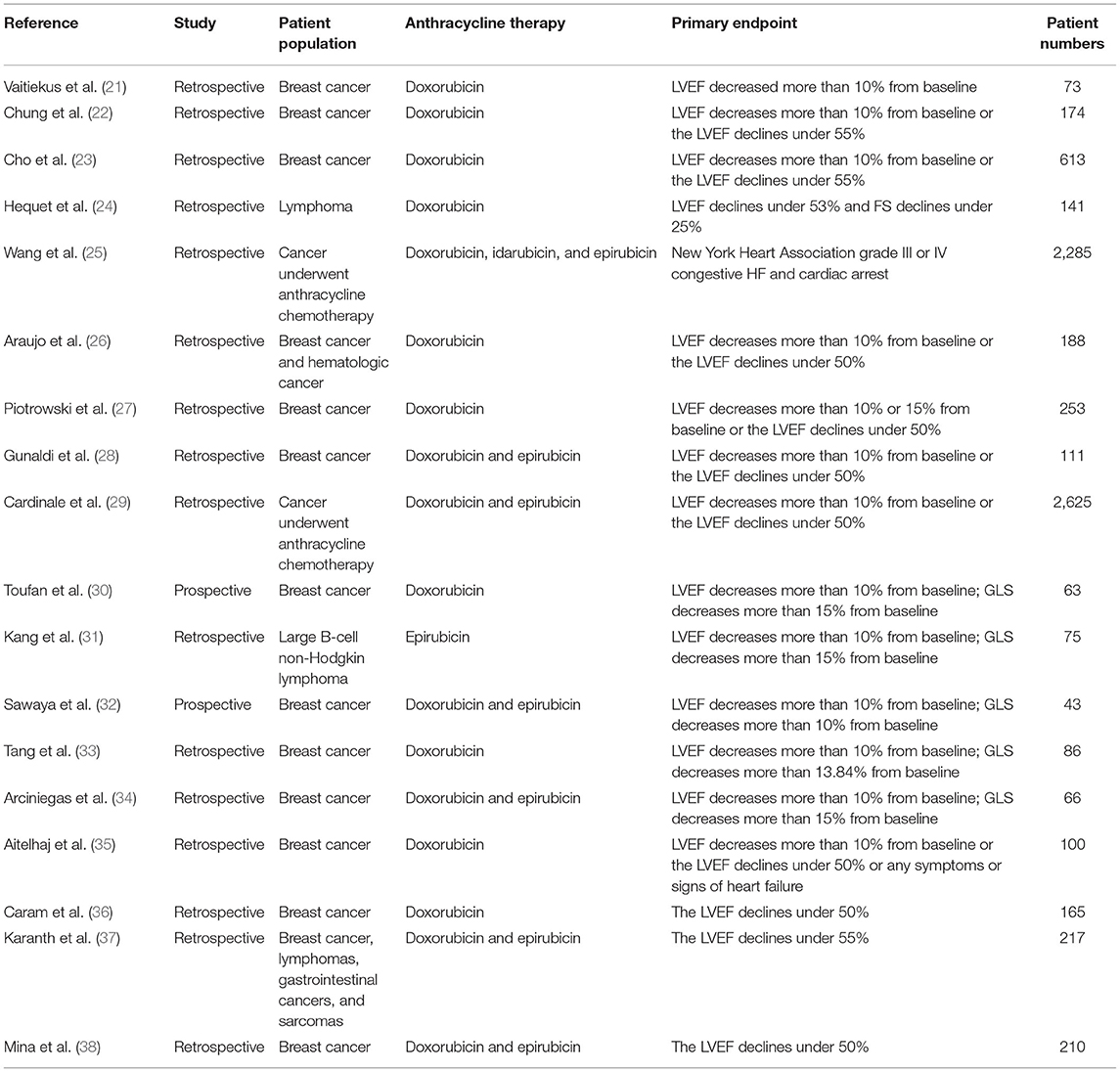
Study Selection and Baseline Characteristics
The process of study selection is presented in a PRISMA flow diagram (Figure 1). According to the inclusion and exclusion criteria, a total of 18 studies (21–38) were included for analysis. The detailed characteristics of these studies, including treatment regimens, patient population, primary endpoint, and number of patients are shown in Table 1. The total number of various types of cancer patients treated with anthracycline in the 18 studies was 7,488. Furthermore, 66.7% (12 studies) of the included studies were breast cancer. In terms of various risk factors analyses, 12 studies were included for hypertension, 8 studies for diabetes mellitus, 5 studies for obesity, 3 studies for overweight, 5 studies for hypercholesterinemia, and 9 studies for smoking. Moreover, the detection ability of LVEF and GLS for AIC with corresponding endpoints was analyzed in six studies.
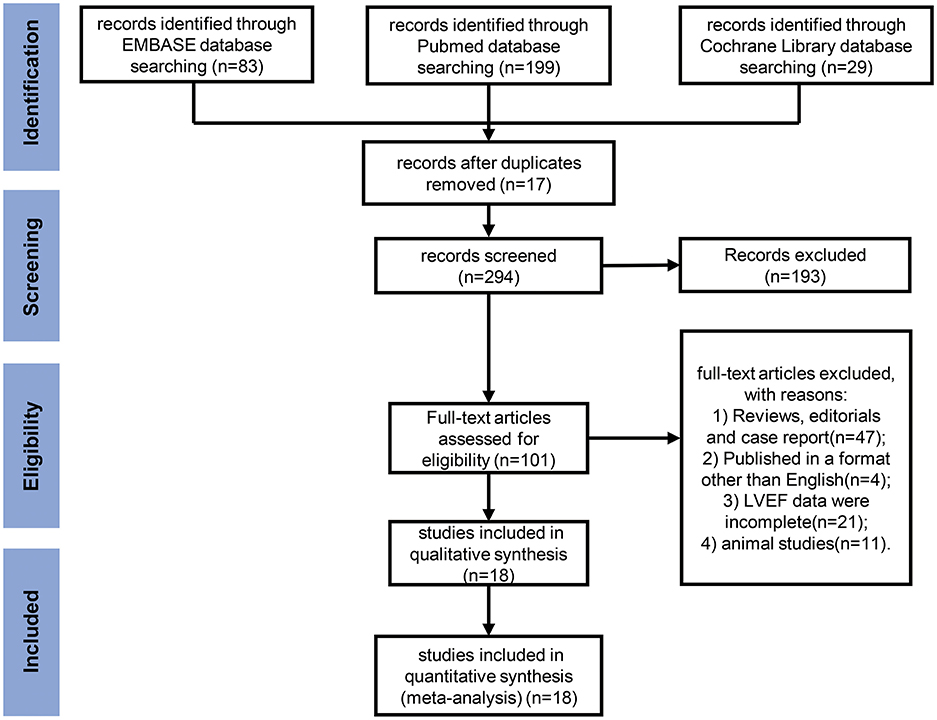
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for meta-analysis. A flow diagram of the studies screened, assessed for eligibility, and included in the review, with reasons for exclusions.
Quality Assessment
As assessed by QUADAS-2, the bias risk mainly came from reference standard and flow and timing domains (Figure 2, Supplementary Table 2, and Supplementary Figure 1). Nine studies (50%) did not explain whether blinding was used in the interpretation of the results, for which only “unclear” or “high risk” was given for reference standard domain. Cases of five studies (27.8%) are not judged by a gold standard and thus evaluated as “high risk” for flow and timing domain. Additionally, two studies (11.1%) did not explain whether patients were included in a continuous or random way and are thus “high risk” for patient selection domain. Overall, the included studies were able to meet the appropriate quality standards.
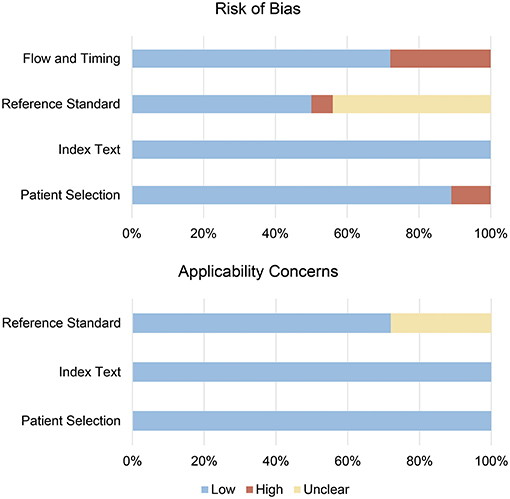
Figure 2. Risk of bias summary for included studies. The methodological quality of included studies was assessed with the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability.
Incidence of AIC
According to the primary clinical endpoint of LVEF, 690 out of the 7,488 patients were assessed as cardiotoxicity, resulting in an overall incidence of 14.0% (95% CI: 10.2 to 18.9%) for cardiotoxicity with high heterogeneity (I2 = 94%) (Figure 3). The result was still similar when the study contributing the largest number of patients was excluded (14.4%; 95% CI: 10.0–20.4%; P < 0.001).
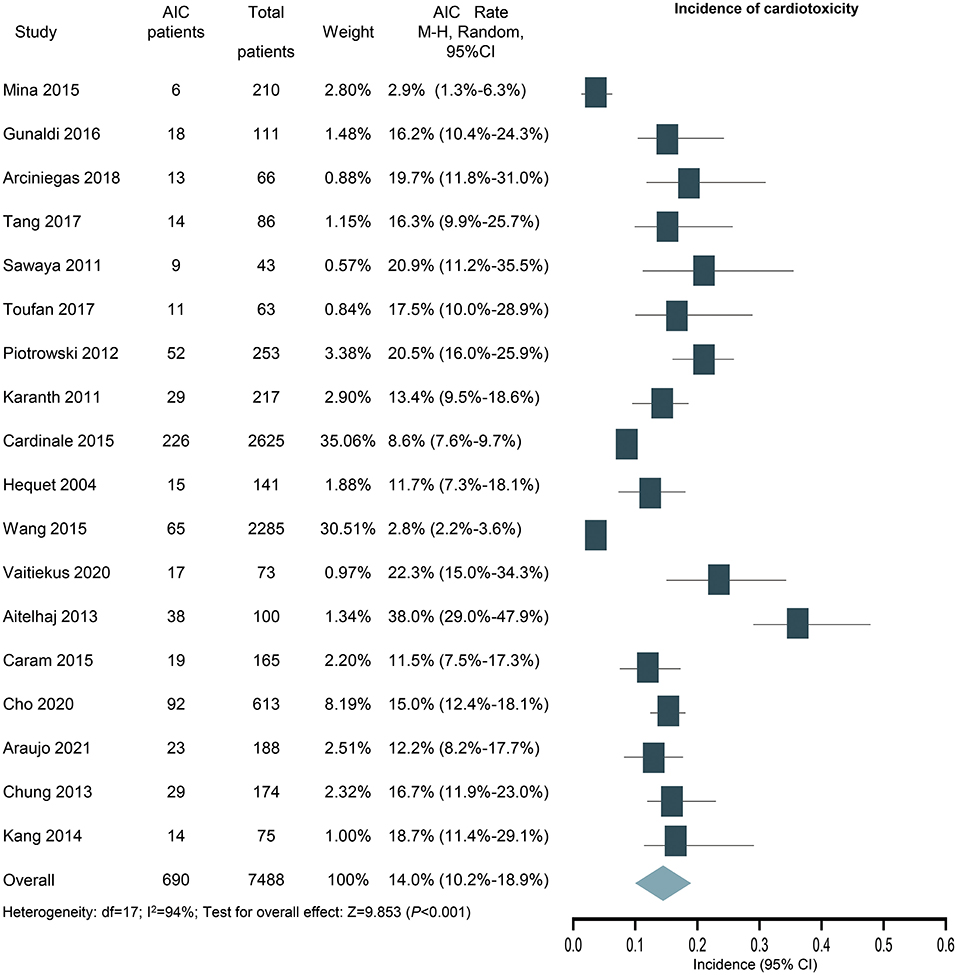
Figure 3. Forest plot of the incidence of anthracycline-induced cardiotoxicity. CI, confidence intervals; OR, odds ratio.
Risk Factors of AIC
Hypertension
For the assessment of hypertension as a potential risk factor, 12 studies with 6,902 patients were included. Hypertension is identified as a risk factor for cardiotoxicity (OR: 1.99; 95% CI: 1.43–2.76; P < 0.001; Figure 4) with medium heterogeneity (I2 = 48%). In addition, the result was still similar when the study with the largest number of patients was excluded (OR: 2.18; 95% CI: 1.50–3.15; P < 0.001).
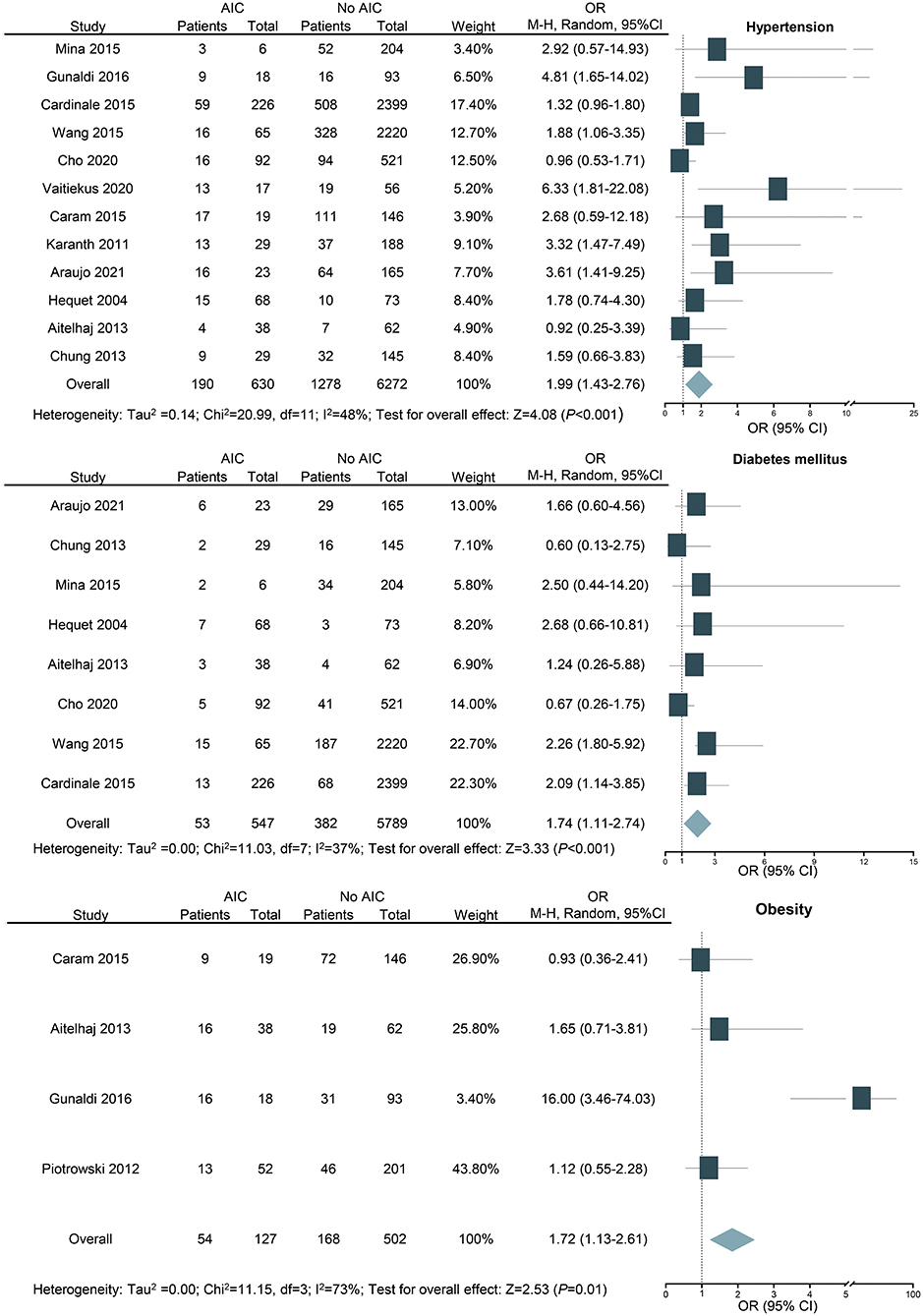
Figure 4. Forest plot for the effects of hypertension, diabetes mellitus, and obesity on the risk of anthracycline-induced cardiotoxicity. CI, confidence intervals; OR, odd ratios.
Diabetes Mellitus
For the assessment of diabetes mellitus as a potential risk factor, eight studies with 6,336 patients were included. Diabetes mellitus is revealed as a risk factor for AIC (OR: 1.74; 95% CI: 1.11–2.74; P < 0.001; Figure 4) with medium heterogeneity (I2 = 37%). Among the studies we included, there are three studies with fewer than five people with diabetes mellitus, which may be one of the sources of heterogeneity. As seven out of the eight included studies were based on previous medical history and the other one study was included based on random blood glucose (≥11.1 mmol/l), there is a potential reporting bias. The result of the recalculation for the seven studies that were based on previous medical history of diabetes mellitus was still similar (OR: 1.68; 95% CI: 1.02–2.75; P < 0.05; I2 = 45%).
Obesity
In our study, we performed subgroup analyses according to WHO-recommended cutoff points for BMI and defined overweight as BMI 25 to 29.9 kg/m2 and obesity as BMI ≥ 30 kg/m2 (39). For the assessment of obesity as a potential risk factor, four studies with 629 patients were included. The result shows that obesity is a risk factor for cardiotoxicity (OR: 1.72; 95% CI: 1.13–2.61; P = 0.010; I2 = 73%; Figure 4). Overweight was not associated with cardiotoxicity (OR: 1.08; 95% CI: 0.65–1.80; P = 0.770; Supplementary Figure 1).
Hypercholesterinemia
For the assessment of hypercholesterinemia as a potential risk factor, five studies with 5,789 patients were included. No significant association was found between hypercholesterinemia and AIC events (OR: 1.48; 95% CI: 0.99–2.20; P > 0.05; I2 = 29%; Supplementary Figure 2).
Smoking
For the assessment of smoking as a potential risk factor, nine studies with 5,862 patients were included. No significant association was found between smoking and AIC events (OR: 1.62; 95% CI: 0.94–2.77; P > 0.05; I2 = 69%; Supplementary Figure 2).
GLS in the Detection of AIC
In the six studies examining GLS, 155 out of the 521 patients were identified as cardiotoxicity. The total incidence was 28.5, and 95% CI was 22.1 to 35.8% (I2 = 63%, P < 0.001, Figure 5). In sensitivity analysis, the result was still similar when the study with the largest number of patients was excluded (26.5%; 95% CI: 19.3–35.2%; P < 0.001). Furthermore, the above studies were also analyzed using LVEF, and the incidence of cardiotoxicity was 16.4% (84 out of the 521 patients), and 95% CI ranged from 13.4 to 19.9% (I2 = 0%, P < 0.001, Figure 5). For further detailed analysis, we consulted a review and meta-analysis of the literature published in 2019, and it showed that the inclusion of strain parameters in echocardiographic evaluation during cancer treatment may help to detect cardiac change earlier (40). Therefore, the detection time was taken as the grouping condition for subgroup analysis. Four studies examined the cardiotoxicity just after 3 months of chemotherapy; the incidence of AIC according to GLS was 30.2% (81 out of the 270 patients), and 95% CI was 24.9% to 36.1% (I2 = 7.70%, P < 0.001, Figure 5).
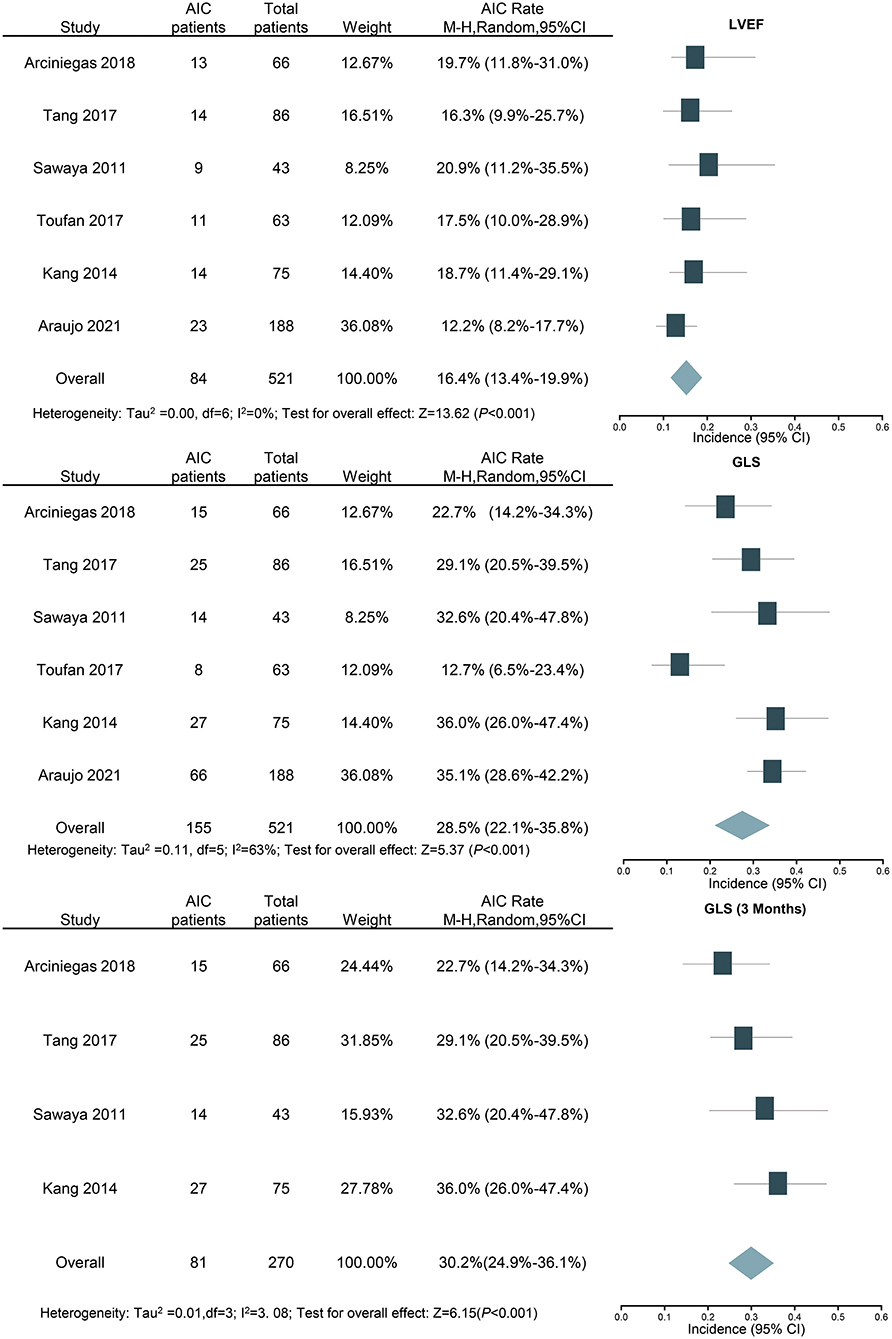
Figure 5. Forest plot for the incidence of anthracycline-induced cardiotoxicity by LVEF and GLS (overall and 3 months). CI, confidence intervals; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
Publication Bias
The funnel plots for all studies are combined in Figure 6. No evidence of publication bias was identified.
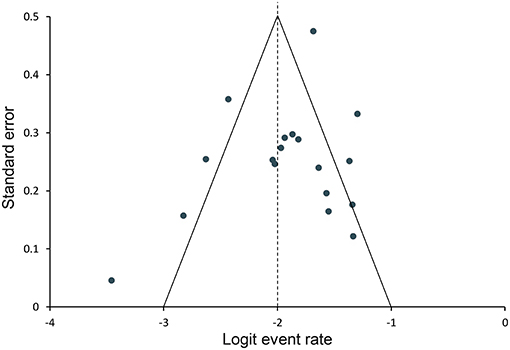
Figure 6. Funnel plot for assessing publication bias. Data points represent individual studies. The y-axis represents the measurement of study precision (plotted as standard error of effect size), and the x-axis represents point estimates for each study. Dashed triangular lines represent the region in which 95% of studies are expected to lie in the absence of bias and heterogeneity.
Discussion
In this large-scale meta-analysis, we calculated the total incidence of cardiotoxicity in cancer patients receiving anthracycline chemotherapy and assessed the relationship between potential risk factors and cardiotoxicity. Among the survivors of cancer chemotherapy, hypertension (OR: 1.99; 95% CI: 1.43–2.76), diabetes mellitus (OR: 1.74; 95% CI: 1.11–2.74), and obesity (OR: 1.72; 95% CI: 1.13–2.61) were revealed to be risk factors for AIC (Figure 7). GLS showed better detection ability than LVEF, which can increase the detection rate of cardiotoxicity in the early stage (3 months after chemotherapy).
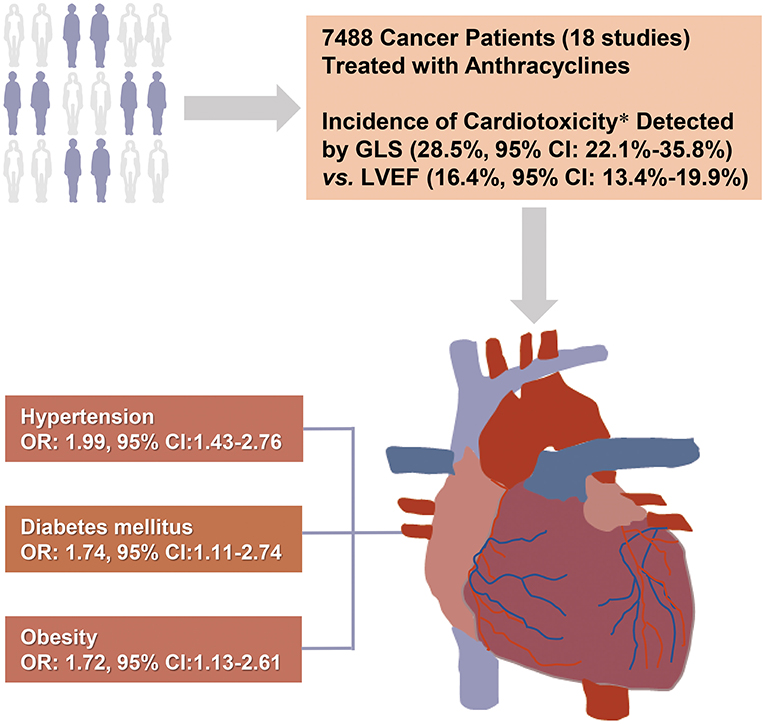
Figure 7. Central illustration: Risk factors for anthracycline-induced cardiotoxicity. The present systematic review included 18 studies of 7,488 cancer patients receiving anthracycline chemotherapy. Hypertension, diabetes, and obesity were identified as risk factors for anthracycline-induced cardiotoxicity. GLS showed higher detection rate and better early detection ability for cardiotoxicity than LVEF. *6 of the 18 studies used both LVEF and GLS. CI, confidential interval; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain.
Incidence of AIC
In a recent review, it was shown that 9% of people receiving anthracycline chemotherapy developed cardiotoxicity, and cardiotoxicity occurred within the first year after the completion of chemotherapy in 98% of cases (41). Our results were largely in agreement with those data, but with obvious heterogeneity, which was mainly attributed to the different cancer types and corresponding treatment options. There are 12 studies that only study breast cancer, and the remaining six studies include breast cancer, sarcoma, lymphoma, bladder cancer, and some leukemias. Another source of the heterogeneity is the different follow-up time. It has been reported that the cumulative incidence of cardiotoxicity increases with time (42). The follow-up time in the included studies ranged from 1 to 10 years. Twelve (66.7%) studies had a follow-up period less than 2 years.
The Risk Factors With AIC
The link between hypertension and AIC was first proposed in 1979. Current cellular and molecular research suggests that the pathophysiological mechanism of hypertension on myocardial cell damage in AIC patients may be related to oxidative stress and inflammatory response of cardiomyocyte fibrosis (43). Although hypertension has been suggested as a risk factor for cardiotoxicity, the existing evidence has not yet reached agreement (44, 45). The limitations of previous studies included small sample size and the confounding cardioprotective drugs for hypertension. Therefore, we conducted this meta-analysis with 7,815 patients in 16 retrospective studies. Furthermore, the latest review confirmed that various anti-hypertension drugs, especially β-blockers (carvedilol and nebivolol), have shown a preventive effect for AIC (46). Thus, we excluded the studies reporting any history of these anti-hypertension drugs, which improved the quality of our report.
The exposure of the heart to the environment of diabetes and high blood sugar (including fatty acids and triglycerides) will increase fatty acids and cytokines, leading to the continuous accumulation of fat droplets in myocardial cells and ultimately mediating cardiotoxicity (47). In our research, it is further confirmed that diabetes is a risk factor for cardiotoxicity, which is consistent with previous reports (48). However, previous researches rarely classified diabetes types, which further ignored the influence of different diabetes treatment regimens (49). Therefore, the present results still need confirmation by multi-center large-scale randomized controlled studies.
Several animal experiments have shown that high-fat-diet-induced obese or overweight rats demonstrated increased sensitivity to AIC (50, 51). It may be related to increased cardiac oxidative stress and metabolic changes, such as increased leptin and decreased adiponectin levels (52, 53). A recent meta-analysis showed that obesity and overweight in breast cancer patients receiving anthracycline chemotherapy increase the risk of cardiotoxicity (39). Our results only agreed that obesity is a risk factor for AIC. Being overweight seems not a risk factor for AIC. The main reason for the discrepancy was that previous studies did not separate obesity from overweight, leading to an overlap in the analysis. However, the potential negative effect of overweight on the cardiovascular system in cancer patients should still be alerted.
Hypercholesterolemia and other metabolic stresses lead to endothelial dysfunction and vascular complications (54), which can cause cardiotoxicity in patients undergoing chemotherapy. Although it seems that hypercholesterolemia might be related to increased risk of cardiotoxicity as reported in some studies (27, 29, 55), our analysis found no obvious association between hypercholesterolemia and AIC. The various definitions of hypercholesterolemia and limited number of related studies might account for the indeterminate results.
Smoking is known to induce thrombosis and cardiovascular toxicity through inflammation and oxidative stress (56, 57). However, current data cannot confirm the relationship between smoking and subsequent cardiotoxicity in patients with anthracycline chemotherapy. Smoking habit can be very different among patients, regarding the numbers of cigarettes, frequency of smoking, number of years smoking, and other detailed items. Therefore, it is hard to draw the conclusion without a specialized and comprehensive study design, as smoking is always reported as yes or no in AIC studies.
Advantages of GLS in the Detection of AIC
AIC mainly leads to cardiomyocyte apoptosis and myofilament degradation and thus to damage of myocardial deformation (46). Many studies have shown that GLS was superior to LVEF in the detection of early and subclinical myocardial dysfunction, with good repeatability (32, 58, 59). The diagnostic and prognostic values of GLS for cardiotoxicity have been validated by previous original researches and meta-analysis (14, 60, 61), with LVEF as the reference method. GLS has also been recommended as an early diagnostic parameter for cardiotoxicity in ESC Cardio-Oncology position statements (18, 19). In the present study, a pathologically reduced GLS was found when LVEF was still preserved in a significant fraction of patients. On the other hand, the incidence of AIC diagnosed by GLS just after 3 months of chemotherapy was quite similar with the overall detection rate during the follow-up, while the detection rate by LVEF increased with the extension of follow-up period until 5 to 10 years. All these findings provided additional proofs for the advantages of GLS in detection of AIC.
Study Limitations
The current analysis has some limitations worth discussing. First, the number of studies included in each risk factor group was limited. Nevertheless, the publication bias assessment and sensitivity analysis showed that our results are reassuring. Second, the median age of patients in 13 of 18 studies was over 50 years old, causing a bias with lack of the young adult patients. The effects of these risk factors identified in the present studies on young adult cancer patients merit further investigation. Third, the current study did not propose a dose-dependent relationship between the risk factors and the incidence of cardiotoxicity. It was because the data we extracted are dichotomous rather than continuous variables. In addition, although most of the included studies (13 out of 18) followed the definitions of cardiotoxicity by ESC Cardio-Oncology position statements (reduction of LVEF to below 50% or a >10% reduction from baseline) (13, 18, 19), some of the early literatures did not meet the cut-offs identically. The major reason is that those studies were performed before the uniform cut-off was proposed. We removed the five studies using different cut-offs and got similar overall incidence of cardiotoxicity (14.1%, 95% CI: 10.1 to 19.4%).
Conclusions
Our research has determined hypertension, diabetes mellitus, and obesity as risk factors for AIC, causing nearly double morbidity of cardiotoxicity. Careful attention should be paid to patients with these risks, and corresponding protective strategies should be used during and after anthracycline treatment. The higher detection rate and better early detection ability of cardiotoxicity than LVEF added new proofs for the advantages of GLS in detection of AIC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to th corresponding author/s.
Author Contributions
SQ and TZ developed the search strategy and wrote the manuscript. BQ, YZha, YZho, and HY checked the search and reviewed the manuscript. JZ and LL performed literature screening and data extraction and conducted the quality assessment of the included studies. LY and GY carried out the data analysis. YD and CX designed the project, reviewed the manuscript, and finally approved the version to be published. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81901751). CX was also supported by the Eyas Program of the Air Force Medical University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.736854/full#supplementary-material
Abbreviations
AIC, anthracycline-induced cardiotoxicity; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; STE, speckle tracking echocardiography; 2D, two-dimensional; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies.
References
1. Sulaiman L, Hesham D, Abdel Hamid M, Youssef G. The combined role of NT-proBNP and LV-GLS in the detection of early subtle chemotherapy-induced cardiotoxicity in breast cancer female patients. Egyptian heart J: (EHJ). (2021) 73:20. doi: 10.1186/s43044-021-00142-z
2. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. (2016) 66:309–25. doi: 10.3322/caac.21341
3. Shelburne N, Adhikari B, Brell J, Davis M, Desvigne-Nickens P, Freedman A, et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J Nat Cancer Inst. (2014) 106:dju232. doi: 10.1093/jnci/dju232
4. Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analys is of randomised controlled trials. BMC Cancer. (2010) 10:337. doi: 10.1186/1471-2407-10-337
5. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three tria ls. Cancer. (2003) 97:2869–79. doi: 10.1002/cncr.11407
6. Sawyer DB. Anthracyclines and heart failure. N Engl J Med. (2013) 368:1154–6. doi: 10.1056/NEJMcibr1214975
7. Cai F, Luis MAF, Lin X, Wang M, Cai L, Cen C, et al. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: preventive strat egies and treatment. Molec Clin Oncol. (2019) 11:15–23. doi: 10.3892/mco.2019.1854
8. Kabor? EG, Guenancia C, Vaz-Luis I, Di Meglio A, Pistilli B, Coutant C, et al. Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study. PLoS Med. (2019) 16:e1002989. doi: 10.1371/journal.pmed.1002989
9. Pearson EJ, Nair A, Daoud Y, Blum JL. The incidence of cardiomyopathy in BRCA1 and BRCA2 mutation carriers after anthracycline-based adjuva nt chemotherapy. Breast Cancer Res Treat. (2017) 162:59–67. doi: 10.1007/s10549-016-4101-8
10. Ali MT, Yucel E, Bouras S, Wang L, Fei HW, Halpern EF, et al. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr. (2016) 29:522–7.e3. doi: 10.1016/j.echo.2016.02.018
11. Fanous I, Dillon P. Cancer treatment-related cardiac toxicity: prevention, assessment and management. Med Oncol. (2016) 33:84. doi: 10.1007/s12032-016-0801-5
12. Kongbundansuk S, Hundley WG. Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovasc Imag. (2014) 7:824–38. doi: 10.1016/j.jcmg.2014.06.007
13. Zamorano JL, Lancellotti P, Rodriguez Muoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines? The Task Force for cancer treatments and cardiovascu lar toxicity of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
14. Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. (2019) 4:1007–18. doi: 10.1001/jamacardio.2019.2952
15. Liberati A, Altman DG, Tetzlaff J, Mulrow C. G?tzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate heal thcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
16. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
17. Santoro C, Arpino G, Esposito R, Lembo M, Paciolla I, Cardalesi C, et al. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: a balance with feasibility. Eur Heart J Cardiovasc Imaging. (2017) 18:930–6. doi: 10.1093/ehjci/jex033
18. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2014) 15:1063–93. doi: 10.1093/ehjci/jeu192
19. Celutkiene J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. (2020) 22:1504–24. doi: 10.1002/ejhf.1957
20. Negishi K, Negishi T, Agler DA, Plana JC, Marwick TH. Role of temporal resolution in selection of the appropriate strain technique for evaluation of subcli nical myocardial dysfunction. Echocardiography. (2012) 29:334–9. doi: 10.1111/j.1540-8175.2011.01586.x
21. Vaitiekus D, Muckiene G, Vaitiekiene A, Maciuliene D, Vaiciuliene D, Ambrazeviciute G, et al. Impact of arterial hypertension on doxorubicin-based chemotherapy-induced subclinical cardiac damage in breast cancer patients. Cardiovasc Toxicol. (2020) 20:321–7. doi: 10.1007/s12012-019-09556-3
22. Chung WB, Yi JE, Jin JY, Choi YS, Park CS, Park WC, et al. Early cardiac function monitoring for detection of subclinical Doxorubicin cardiotoxicity in young ad ult patients with breast cancer. J Breast Cancer. (2013) 16:178–83. doi: 10.4048/jbc.2013.16.2.178
23. Cho H, Lee S, Sim SH, Park IH, Lee KS, Kwak MH, et al. Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breas t cancer patients. Breast Cancer Res Treat. (2020) 182:333–43. doi: 10.1007/s10549-020-05703-5
24. Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. (2004) 22:1864–71. doi: 10.1200/JCO.2004.06.033
25. Wang L, Tan TC, Halpern EF, Neilan TG, Francis SA, Picard MH, et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracyclin e-based chemotherapy. Am J Cardiol. (2015) 116:442–6. doi: 10.1016/j.amjcard.2015.04.064
26. Araujo-Gutierrez R, Chitturi KR, Xu J, Wang Y, Kinder E, Senapati A, et al. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardio-Oncol. (2021) 7:4. doi: 10.1186/s40959-021-00090-2
27. Piotrowski G, Gawor R, Stasiak A, Gawor Z, Potemski P, Banach M. Cardiac complications associated with trastuzumab in the setting of adjuvant chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2 - a prospective study. Arch Med Sci. (2012) 8:227–35. doi: 10.5114/aoms.2012.28549
28. Gunaldi M, Duman BB, Afsar CU, Paydas S, Erkisi M, Kara IO, et al. Risk factors for developing cardiotoxicity of trastuzumab in breast cancer patients: An observational single-centre study. J Oncol Phar Prac. (2016) 22:242–7. doi: 10.1177/1078155214567162
29. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777
30. Toufan M, Pourafkari L, Ghahremani Nasab L, Esfahani A, Sanaat Z, Nikanfar A, et al. Two-dimensional strain echocardiography for detection of cardiotoxicity in breast cancer patients und ergoing chemotherapy. J Cardiovasc Thorac Res. (2017) 9:29–34. doi: 10.15171/jcvtr.2017.04
31. Kang Y, Xu X, Cheng L, Li L, Sun M, Chen H, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. (2014) 16:300–8. doi: 10.1002/ejhf.8
32. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. (2011) 107:1375–80. doi: 10.1016/j.amjcard.2011.01.006
33. Tang Q, Jiang Y, Xu Y, Xia H. Speckle tracking echocardiography predicts early subclinical anthracycline cardiotoxicity in patients with breast cancer. J Clin Ultrasound. (2017) 45:222–30. doi: 10.1002/jcu.22434
34. Arciniegas Calle MC, Sandhu NP, Xia H, Cha SS, Pellikka PA, Ye Z, et al. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associate d with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. (2018) 18:1037. doi: 10.1186/s12885-018-4935-z
35. Aitelhaj M, Lkhouyaali S, Rais G, Mohtaram A, Raissouni S, Ghissassi B, et al. Cardiac safety of the adjuvant Trastuzumab in a Moroccan population: observational monocentric study of about 100 patients. BMC Res Notes. (2013) 6:339. doi: 10.1186/1756-0500-6-339
36. Caram MEV, Guo C, Leja M, Smerage J, Henry NL, Giacherio D, et al. Doxorubicin-induced cardiac dysfunction in unselected patients with a history of early-stage breast c ancer. Breast Cancer Res Treat. (2015) 152:163–72. doi: 10.1007/s10549-015-3454-8
37. Karanth NV, Roy A, Joseph M, de Pasquale C, Karapetis C, Koczwara B. Utility of prechemotherapy echocardiographical assessment of cardiac abnormalities. Suppor Care Cancer. (2011) 19:2021–6. doi: 10.1007/s00520-010-1054-z
38. Mina A, Rafei H, Khalil M, Hassoun Y, Nasser Z, Tfayli A. Role of baseline echocardiography prior to initiation of anthracycline-based chemotherapy in breast c ancer patients. BMC Cancer. (2015) 15:10. doi: 10.1186/s12885-014-1004-0
39. Guenancia C, Lefebvre A, Cardinale D, Yu AF, Ladoire S, Ghiringhelli F, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a system atic review and meta-analysis. J Clin Oncol. (2016) 34:3157–65. doi: 10.1200/JCO.2016.67.4846
40. Bergamini C, Dolci G, Truong S, Zanolla L, Benfari G, Fiorio E, et al. Role of speckle tracking echocardiography in the evaluation of breast cancer patients undergoing chem otherapy: review and meta-analysis of the literature. Cardiovasc Toxicol. (2019) 19:485–92. doi: 10.1007/s12012-019-09523-y
41. Sawicki KT, Sala V, Prever L, Hirsch E, Ardehali H, Ghigo A. Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol. (2021) 61:309–32. doi: 10.1146/annurev-pharmtox-030620-104842
42. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retr ospective cohort study. J Natl Cancer Inst. (2012) 104:1293–305. doi: 10.1093/jnci/djs317
43. Kuriakose RK, Kukreja RC Xi L. Potential therapeutic strategies for hypertension-exacerbated cardiotoxicity of anticancer drugs. Oxid Med Cell Longev. (2016) 2016:8139861. doi: 10.1155/2016/8139861
44. Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a mult icenter cohort analysis. Ann Oncol. (2012) 23:3058–63. doi: 10.1093/annonc/mds127
45. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxor ubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant thera py for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2012) 30:3792–9. doi: 10.1200/JCO.2011.40.0010
46. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. (2020) 17:474–502. doi: 10.1038/s41569-020-0348-1
47. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. (2020) 17:585–607. doi: 10.1038/s41569-020-0339-2
48. Valcovici M, Andrica F, Serban C, Dragan S. Cardiotoxicity of anthracycline therapy: current perspectives. Arch Med Sci. (2016) 12:428–35. doi: 10.5114/aoms.2016.59270
49. Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D, et al. Metformin use is associated with better survival of breast cancer patients with diabetes: a meta-analysis. Oncologist. (2015) 20:1236–44. doi: 10.1634/theoncologist.2015-0096
50. Mitra MS, Donthamsetty S, White B, Mehendale HM. High fat diet-fed obese rats are highly sensitive to doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol. (2008) 231:413–22. doi: 10.1016/j.taap.2008.05.006
51. Guenancia C, Hachet O, Aboutabl M, Li N, Rigal E, Cottin Y, et al. Overweight in mice, induced by perinatal programming, exacerbates doxorubicin and trastuzumab cardiot oxicity. Cancer Chemother Pharmacol. (2016) 77:777–85. doi: 10.1007/s00280-016-2995-9
52. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. (2014) 63:250–9. doi: 10.1016/j.jjcc.2013.11.006
53. Habbout A, Delemasure S, Goirand F, Guilland JC, Chabod F, Sediki M, et al. Postnatal overfeeding in rats leads to moderate overweight and to cardiometabolic and oxidative alter ations in adulthood. Biochimie. (2012) 94:117–24. doi: 10.1016/j.biochi.2011.09.023
54. Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. (2018) 123:477–94. doi: 10.1161/CIRCRESAHA.118.313237
55. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
56. Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. Gum arabic ameliorates impaired coagulation and cardiotoxicity induced by water-pipe smoke exposure in mice. Front Physiol. (2019) 10:53. doi: 10.3389/fphys.2019.00053
57. Clark RA, Marin TS, Berry NM, Atherton JJ, Foote JW, Koczwara B. Cardiotoxicity and cardiovascular disease risk assessment for patients receiving breast cancer treatment. Cardio-Oncol. (2017) 3:6. doi: 10.1186/s40959-017-0025-7
58. Motoki H, Koyama J, Nakazawa H, Aizawa K, Kasai H, Izawa A, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2012) 13:95–103. doi: 10.1093/ejechocard/jer172
59. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. (2014) 63:2751–68. doi: 10.1016/j.jacc.2014.01.073
60. Charbonnel C, Convers-Domart R, Rigaudeau S, Taksin AL, Baron N, Lambert J, et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. (2017) 18:392–401. doi: 10.1093/ehjci/jew223
Keywords: anthracycline, hypertension, diabetes mellitus, obesity, chemotherapy, cardiotoxicity, global longitudinal strain
Citation: Qiu S, Zhou T, Qiu B, Zhang Y, Zhou Y, Yu H, Zhang J, Liu L, Yuan L, Yang G, Duan Y and Xing C (2021) Risk Factors for Anthracycline-Induced Cardiotoxicity. Front. Cardiovasc. Med. 8:736854. doi: 10.3389/fcvm.2021.736854
Received: 06 July 2021; Accepted: 30 August 2021;
Published: 29 September 2021.
Edited by:
Alberto Milan, Università di Torino, ItalyReviewed by:
Lars Michel, Essen University Hospital, GermanyKonstantinos Charitakis, University of Texas Health Science Center at Houston, United States
Copyright © 2021 Qiu, Zhou, Qiu, Zhang, Zhou, Yu, Zhang, Liu, Yuan, Yang, Duan and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changyang Xing, xingcy712@163.com; Yunyou Duan, duanyy@fmmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Shuo Qiu
Shuo Qiu Tian Zhou1†
Tian Zhou1†  Lijun Yuan
Lijun Yuan Guodong Yang
Guodong Yang Changyang Xing
Changyang Xing