Premature stroke and cardiovascular risk in primary Sjögren's syndrome
- 1Department of Rheumatology and Immunology, Hannover Medical School, Hannover, Germany
- 2Department of Neurology, Hannover Medical School, Hannover, Germany
- 3Department of Cardiology, Hannover Medical School, Hannover, Germany
Introduction: Primary Sjögren's syndrome (pSS) is associated with an increased prevalence of traditional risk factors and cardiovascular diseases (CVDs). The study aimed to identify specific risk factors for CVD in pSS patients.
Methods: PSS patients with and without CVD were compared. All patients fulfilled the EULAR/ACR classification criteria. Patients with CVD presented at least one of the following manifestations: myocardial infarction, transient ischemic attacks, ischemic or hemorrhagic stroke, peripheral artery disease, coronary artery disease, and carotid plaques. Data were collected by a standardized protocol and review of medical records.
Results: 61/312 (19.6%) pSS patients presented with CVD. Traditional risk factors such as hypertension, hypercholesterinemia and diabetes (p < 0.05), pSS manifestations, in particular vasculitis (p = 0.033) and Raynaud's phenomenon (p = 0.018) were associated with CVD. Among patients with ischemic events (28/312, 9%), particularly cerebrovascular disease (n = 12/28, 42.9%), correlations with increased EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) (p = 0.039) and EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) (p = 0.048) were observed. Age at first cerebrovascular event was 55.2 [48.9–69.6] years. Multivariate analysis confirmed hypertension [odds ratio (OR) 3.7, 95% confidence interval (CI) 1.87–7.18, p < 0.001], hypercholesterinemia (OR 3.1, 95% CI 1.63–5.72, p < 0.001), male gender (OR 0.4, 95% CI 0.17–0.78, p = 0.009), Raynaud's phenomenon (OR 2.5, 95% CI 1.28–4.82, p = 0.007), and CNS involvement (OR 2.7, 95% CI 1.00–7.15, p = 0.048) as independent CVD predictors.
Conclusion: Raynaud's phenomen as well as vasculitis and high ESSDAI have shown a significant association to CVD. PSS patients with cerebrovascular events were younger than expected. Knowledge about risk factors may help clinicians to identify pSS patients at risk for CVD. After diagnosis of pSS, patients should be screened for risk factors such as hypertension and receive appropriate therapy to prevent or at least reduce sequelae such as infarction. However, further investigations are necessary in order to achieve a reliable risk stratification for these patients.
Introduction
Primary Sjögren syndrome (pSS) is a common connective tissue disease with an estimated prevalence of 1:100 to 1:1,000, predominantly occurring in middle-aged females (10:1) (1). In general, pSS is characterized by lymphocytic infiltration of exocrine glands, typically resulting in ocular and oral dryness. Manifestations can however be diverse, with almost 50% of patients displaying extra-glandular manifestations (2). This appears mainly driven by systemic inflammation of various end-organs, captured within the EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) (3). Recent data revealed that more than a quarter of patients may have manifestations currently not included in the ESSDAI, with cardiovascular (CV) involvement being the most frequent (4).
CVD risk is known to be independently increased in both systemic lupus erythematosus and rheumatoid arthritis (5), and given the immunological similarities to pSS, it remains an area of interest in this cohort. It has previously been shown, that increased rates of atherosclerosis are evident in pSS populations (6). While exact mechanisms remain unknown, various studies have identified endothelial dysfunction, intimal thickening and resulting loss in vessel wall compliance as being evident in pSS cohorts (7).
PSS does however not provide immunity from traditional CVD risk, with recent epidemiological data actually correlating pSS with traditional risk factors, especially hypertension and hypercholesterinemia (8–10). One exception is smoking, with several studies suggesting lower prevalence, likely due to oral discomfort (8, 9). The results for the remaining risk factors are inconclusive. For example, two studies observed a higher frequency of diabetes mellitus (11, 12) while Bartoloni et al. (8) presented opposing results. Nevertheless, a current meta-analysis confirmed a subsequently increased risk for cardiovascular and cerebrovascular events (13).
Aim of this retrospective study is to analyze differences between pSS patients with and without CV involvement, focusing on traditional risk factors, immunological parameters, comorbidities, and extra-glandular pSS manifestations.
Materials and methods
Study population
We retrospectively analyzed clinical data of all confirmed pSS patients attending the outpatient clinics of either the Rheumatology or Neurology Departments at Hannover Medical School between January 2018 and March 2021. Patients were classified according to either the 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria (14) or the 2012 American College of Rheumatology classification criteria (15) depending on time of diagnosis.
pSS diagnosis and evaluation
Clinical data were collected using previously performed, standardized questionnaires. This included age at diagnosis, current symptom profiles and evidence of extra-glandular or systemic involvement based upon ESSDAI, serological tests including antinuclear antibodies, rheumatoid factor, alpha-fodrin, anti-SSA/Ro and anti-SSB/La antibodies, along with differential blood counts and standard biochemistry. Disease activity was scored using ESSDAI (3) and severity of symptoms by EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) (16). Xerostomia and xerophthalmia were confirmed by a positive Saxon test [ < 3.5 g in 2 min (stimulated saliva flow)] and/or positive Schirmer test [ < 5 mm in 5 min (lacrimal flow)]. Sonographic assessment of salivary gland involvement was performed and scored according to the DeVita system (17). When indicated, minor salivary gland biopsy was obtained [scored according to Chisholm and Mason (18)]. The study protocol was approved by the local Ethics committee (8179_BO_S_2018) and declaration of consent was received from all the subjects.
Cardiovascular assessment
Traditional CVD risk factors, manifest CVD events, and objective evidence of asymptomatic atherosclerotic disease were assessed and recorded using standardized criteria, applied when reviewing patient medical records. Traditional CVD risk factors were defined as follows: hypertension (diagnosis by a cardiologist and/or ongoing antihypertensive therapy), hypercholesterinemia (physicians' diagnosis and/or use of lipid-lowering agents), smoking (previous/current or never smoking, pack years), diabetes mellitus (physicians' diagnosis and/or ongoing therapy with oral hypoglycemic agent or insulin), obesity (based on body mass index), and positive family history (CVD event in a first-degree relative ≥65 yrs). Further CV comorbidities like heart failure and atrial fibrillation were assessed. Finally, CVD was recorded as proven myocardial infarct (MI) or catheter-proven coronary artery disease, proven cerebrovascular disease including transient ischemic attacks (TIA) or known carotid artery disease on Duplex sonography and peripheral vascular disease (PVD). PVD was diagnosed by clinical symptoms and duplex sonography findings. All patients in our cohort diagnosed with PVD were symptomatic (Fontaine classification ≥2) (19). CV events only included MI, stroke and proven PVD.
Statistical analysis
Quantitative variables are expressed as mean and SD, whereas categorical variables are reported as frequency (n) and percentage (%). The variables were assured with Pearson's correlation and subsequently with partial correlation, therefore age was excluded as a confounding variable. According to Kolmogorov–Smirnoff, the Gaussian distribution was controlled. Parametric and Non-parametric tests were applied to verify the distribution of each risk factor within the investigated groups. Significant results were proved according to the Chi square test, Mann–Whitney-U- and Kruskal–Wallis-tests. Furthermore, the median values were compared by using the Hodges–Lehmann-test, where the two-sided confidence interval of 95% was given. For categorical variables, missing values have been excluded. The Bonferroni correction to neutralize the type 1 error by multiple statistical tests was performed. Finally, we conducted a multivariate analysis to determine variables that were independently associated with CVDs. A two-sided significance level was set at 0.05 for all test procedures. The statistical data analysis was obtained by using SPSS software (SPSS 27, IBM Corporation and its licensors 1989, 2020).
Results
Characteristics of the study population
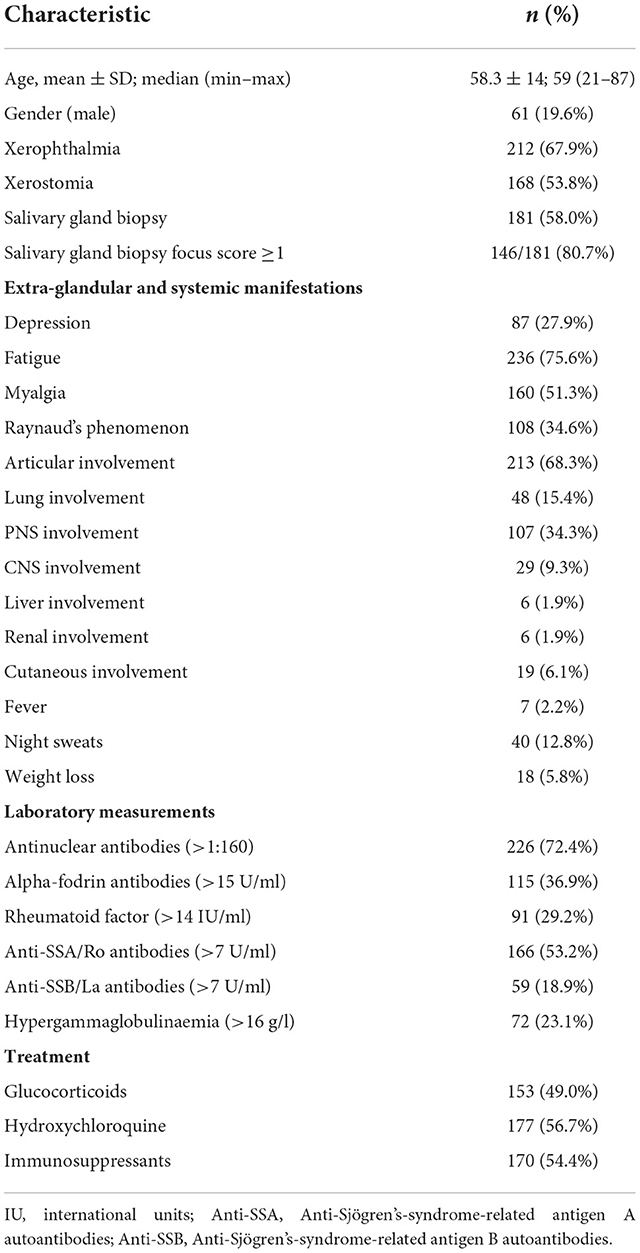
In total 312 patients were included in the cohort. Of these, 251/312 (80.4%) were female. Age at inclusion was 58.3 ± 14 years and mean disease duration was 5.0 ± 6.7 years. Two hundred forty-seven pSS patients were classified according to EULAR/ACR 2016 and 65 patients according to the 2012 ACR classification criteria (14, 15).
More than two-thirds of patients (67.9%) exhibited objective xerophthalmia and 168 (53.8%) patients showed an objective xerostomia. A total of 181 (58.0%) of the cohort underwent salivary gland biopsy and 146 (80.7%) of them had a Chisholm and Mason score ≥3. In addition, we performed ultrasounds of the salivary glands in 311 patients, 96 (30.9%) of these presented a DeVita score ≥ 4. Few patients were detected with scleroderma associated antibodies (n = 21), all of them presenting Raynauds phenomen, but the number in the CVD and control group did not differ significantly (Table 1).
Tables 1, 2 summarizes the general characteristics of our pSS cohort.
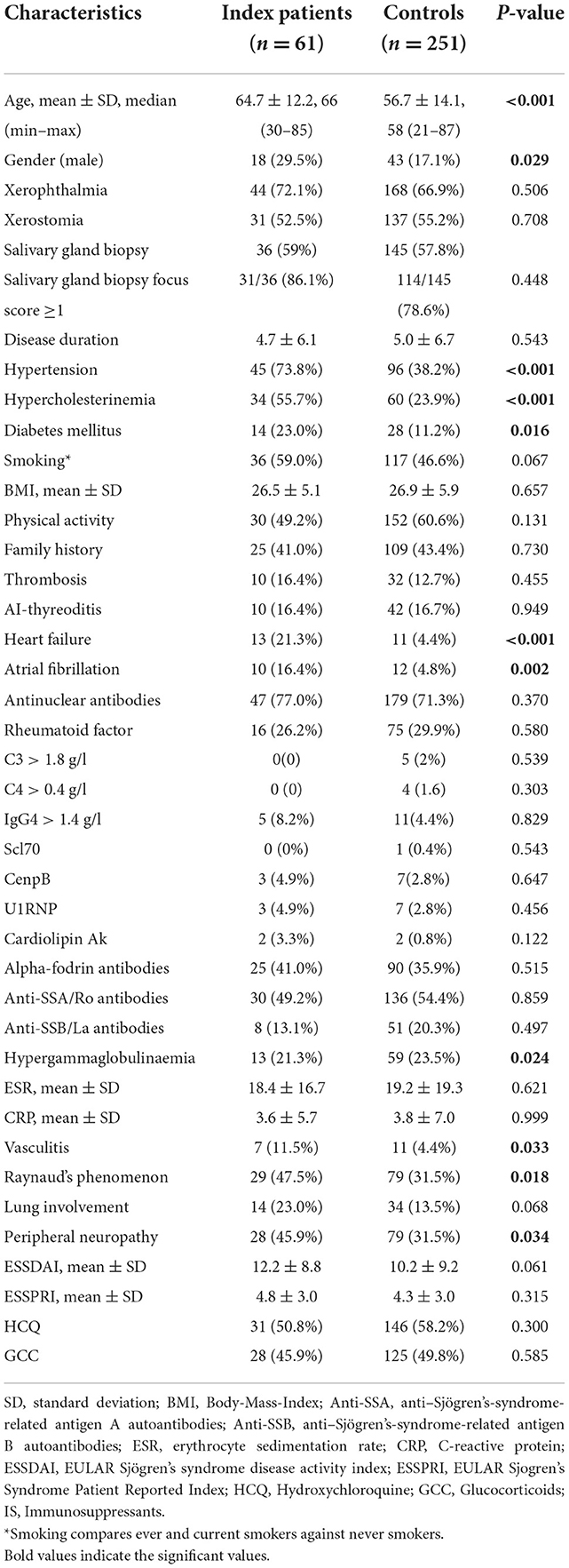
Table 2. Comparison between pSS patients with and without cardiovascular and atherosclerotic endpoints.
Comparison between pSS patients with and without cardiovascular and atherosclerotic endpoints
In total 61/312 (19.6%) patients were identified as having some form of CVD, with the remainder acting as comparative controls (Table 2). CVD patients tended to be older (64.7 ± 12.2, median 66 (30–85) years, p < 0.001) and a greater proportion were male (29.5 vs. 17.1%; p = 0.029). No difference in pSS duration was evident between groups. Established CVD risk factors such as hypertension (73.8 vs. 38.2; p < 0.001), hypercholesterinemia (55.7 vs. 23.9%; p < 0.001) and diabetes mellitus (23.0 vs. 11.2%; p = 0.016) were more prevalent in CVD patients. Smoking prevalence was higher in CVD patients (59.0 vs. 46.6%; p = 0.067), the difference was not significant. Neither family history of CVD nor BMI differed between groups (both p > 0.05). Non surprisingly CVD patients had more CVD-associated comorbidities such as atrial fibrillation (16.4 vs. 4.8%; p = 0.002) and heart failure (21.3 vs. 4.4%; p < 0.001).
CRP, ESR, complement, and autoantibodies were comparable in both groups. CVD patients with manifest atherosclerosis exhibited higher rates for vasculitis (11.5 vs. 4.4%; p = 0.033) and Raynaud's phenomenon (47.5 vs. 31.5%; p = 0.018). Neither glucocorticoid (GC) nor immunosuppressive therapy demonstrated any significant difference between these two groups. There was no association of GCs and hypertension, diabetes mellitus, or hypercholesterinemia in this cohort.
Comparison between pSS patients with and without overt ischemic events
At least one ischemic event, MI, stroke, or PAD, was confirmed in 28/312 (9%) patients (Supplementary Table 1). The remaining (n = 284) were defined as control. As expected, results regarding modifiable and non-modifiable risk factors remained mostly constant. Although absolute numbers are small in addition to the prior results, CNS involvement now correlated with overt ischemic events [6/28 (21.4%) vs. 23/284 (8.1%), p = 0.021].
Age at first cerebrovascular event was 55.2 [48.9–69.6] years. Cerebrovascular events were not associated to arterial fibrillation.
Compared to the control, patients with at least one ischemic manifestation had a higher disease activity (ESSDAI score ≥ 5) (p = 0.039). Consequently, the mean ESSDAI score in these patients was 13.5 ± 7.7 (p = 0.021). With regard to ESSPRI, we found a similar tendency, a high ESSPRI (p = 0.048) was associated with ischemic events.
Despite small numbers, we searched for noticeable differences between pSS patients with manifested MI (n = 8), stroke (n = 12), or PAD (n = 8). All documented strokes were of ischaemic nature. Of interest, anti-SSB/La positivity was more prevalent in pSS patients with MI (p = 0.017), whereas cerebrovascular events were significantly associated with a high CNS domain of the ESSDAI (p = 0.021). Patients with PAD were less likely to be treated with HCQ (p = 0.005).
Predictors of CVDs
Finally, we performed a multivariate analysis, which revealed hypertension, hypercholesterinemia, male gender, Raynaud's phenomenon, and CNS involvement as independent predictors of CVDs in patients with pSS (p < 0.05; Table 3).
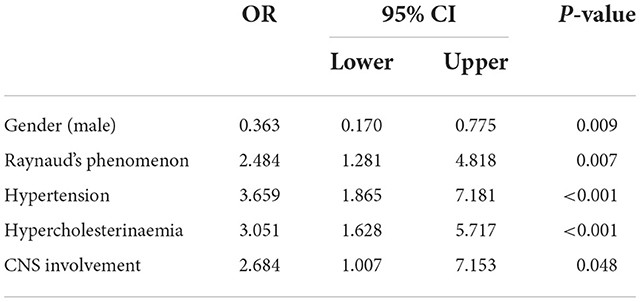
Table 3. Predictors of cardiovascular disease, multivariate analysis of gender, Raynaud's phenomenon, hypertension, hypercholesterolemia, CNS involvement (OR Odds Ratio, CNS central nerve system).
Discussion
This is the first study to our knowledge reporting about premature stroke in Sjögren Syndrome and Raynaud's phenomen as a potential risk factor for CVD in patients with pSS. Whilst vasculitis in various diseases is known to be able to involve coronary arteries, there is no previous data for pSS patients with Raynaud's phenomenon suffering from more CVD (20–22). Although several studies clearly demonstrated, that pSS patients have an increased prevalence of CV risk factors and overt CVDs (8, 13).
In the present study, almost one-fifth of pSS patients were affected by CV involvement. Comparing only presence of MI, other coronary heart disease, heart failure, or stroke in a normal German female age matched cohort you would expect 8–16% suffering of the mentioned diseases. Although these cohorts are not matched, the prevalence of those diseases seems much higher in patients with pSS (23). Next to known cardiac risk factors (cRF), disease-specific manifestations, such as vasculitis and Raynaud's phenomenon were also more common in pSS patients with CVD, suggesting a possible synergistic effect between traditional risk factors and disease-specific factors resulting in a higher atherogenic potential and increased CV burden.
Interestingly in our cohort, patients with stroke were remarkably younger at first cerebral event than expected in an average German cohort (24). As we could not see an association to atrial fibrillation or other obvious risk factors an association of pSS is suggestive.
Due to the inclusion of patients with neurological involvement of Sjögren's syndrome, the proportion of male gender in our cohort is larger than expected. In neurological cohorts, the gender proportion is balanced, unlike in rheumatological cohorts [61/312 (19.6%) were male] (25). In line with other studies, male gender and older age were significantly associated with CVDs (8, 11, 12). On the contrary, disease duration had no impact in our cohort, despite the fact that recent studies were able to establish an association between disease duration and CV events (8, 26). However, our results may be explained by the heterogenous course, some late presenters and the possibility of CVD to occur as one of the first symptoms in patients with pSS (27, 28).
It is undeniable that traditional risk factors have the greatest impact in the development of atherosclerotic manifestations and CV burden (29). In case of pSS, our multivariate analysis confirmed that hypertension and hypercholesterolemia play a pivotal role as predictors of CVDs. Among other classical risk factors, DM was more frequent in pSS patients with CV events, whereas sedentary lifestyle and BMI were similarly distributed between index patients and control. Our results agree with Cai et al. (11), who observed a higher frequency of hypertension, hypercholesterinemia, DM plus an increased BMI in pSS patients with CV involvement. In addition, Bartoloni et al. (26) recently demonstrated a strong association between CV risk factors and CVDs in pSS patients, with hypertension playing a central role. The increased prevalence of risk factors leading to increasing CV events, demands improvement in the prevention and management of pSS. In line with this a recent study showed a far higher prevalence for subclinical atherosclerosis in pSS compared to an age and sex matched control group (30). The authors report an association of an elevated erxthrocyte sedimentation rate and rheumatoid factor with subclinical atherosclerosis in pSS patients. A review from Bartoloni et al. reports about the role of inflammation in the pathogenesis of pSS (31) supporting findings that patients with a higher ESSDAI and presence of SSA antibodies present more often endothelial dysfunction (7, 32).
Of note, despite adjusting for traditional risk factors, Wu et al. (33) found an increased hazard ratio of 1.52 in pSS patients with coronary heart disease, suggesting that the disease itself is a risk factor. Therefore, we investigated in a wide range of disease-specific laboratory and clinical markers. Our results are similar to findings of Italian studies, that demonstrated an increased frequency of immunological abnormalities in pSS patients without traditional risk factors and failed to establish a direct association between autoantibodies and CV events (8, 26). However, a similar amount of data strengthens the opposing hypothesis that immunological and inflammatory abnormalities correlate with atherosclerotic manifestations and thereby also with CVDs. For instance, Mofors et al. (34) have shown a higher risk of cerebral infarction and venous thromboembolism in pSS patients positive for anti-SSA/Ro and anti-SSB/La antibodies. We saw a higher prevalence of anti-SSB/La positivity in pSS patients with MI (p = 0.017), whilst we did not see an association with venous thromboembolism. But numbers are small and therefore these results have to be interpretated with caution.
Interestingly we found a slightly lower presence of hypergammglobulinemia in patients with CVD, similar findings have been shown before by Pérez-De-Lis et al. in a pSS cohort of the same size as ours (n = 312) (12). They detected in the multivariate analysis for patients with more than three cRF a higher frequency of CNS involvement, which fits to our results as well.
Clinical markers including vasculitis and Raynaud's phenomenon can play a crucial role in relation to CVDs. Based on our subanalysis, we identified night sweats as an additional association with ischemic events (Supplementary Table 1). As night sweats can be an indicator for disease activity (3) or systemic inflammation, this finding is easily explained as both have been shown to increase CVD risk (35). On the other hand night sweats can be caused by many other reasons like menopause and autonomic neuropathy (36). Especially early menopause and autonomic neuropathy may increase cardiovascular risk in pSS patients as well (37, 38).
Through multivariate analysis we confirmed that Raynaud's phenomenon is independently associated with CVDs. A correlation between Raynaud's phenomenon and autonomic neuropathy (39) as well as microvascular peripheral endothelial dysfunction, one of the early stages of atherosclerosis, has been shown before (40). Besides microvascular changes, even a correlation between subclinical myocardial fibrosis and Raynaud's phenomenon was discovered, giving additional support for our results, showing Raynaud's phenomenon is an independent predictor (41). Both, Raynaud's phenomenon and vasculitis, are among the most common extra-glandular manifestations in pSS patients, whereas vasculitis is particularly associated with increased mortality (42). Of consequence, special precautions like frequent CV monitoring and tight control of CV risk factors like arterial hypertension, diabetes mellitus, and hyperlipidemia are indispensable. That is why it is so important to recognize and use the EULAR guidelines for the management of cardiovascular comorbidities (43).
A characteristic feature of patients with CVDs was a more pronounced systemic manifestations than glandular involvement. Among the systemic manifestation, polyneuropathy was the most common one, in part due to the inclusion of patients with neurologic involvement from the Department of Neurology. Additionally, our subanalysis revealed CNS involvement as a significant factor for ischemic events, which was also confirmed by the multivariate analysis. Considering that cerebral vasculitis frequently results in stroke, these findings are not surprising (44). Similar results regarding the influence of extra-glandular manifestations on CV events were obtained by studies from China (11) and Italy (8).
In our study, ischemic events were associated with a high to moderate ESSDAI and a high ESSPRI. A closer look at the individual domains of the ESSDAI score revealed four possible trends (constitutional, renal, PNS, CNS), which may be significant in a larger cohort. Agreeing with our finding, lupus patients with carotid intima media thickness and carotid plaques were associated with a severe inflammatory status expressed by the systemic lupus erythematosus disease activity index (SLEDAI) (45), a counterpart of the ESSDAI.
Due to the higher risk of CVD in patients with rheumatic diseases the EULAR recommends an adaption of CVD risk calculators by a 1.5 multiplication factor in patients with rheumatoid arthritis (43). Not only the implementation of altered risk models, also a targeted CV management with routine assessments of traditional risk factors and disease-specific features could benefit pSS patients. Structured CV diagnostic protocols and non-invasive methods (e.g., ultrasound of the carotid artery) should be considered in patients at risk, especially in patients with a high disease activity and any additionally risk factors as well.
Our study is limited by its single-center set up, its retrospective manner and its lack of a healthy matched control group. Specific cardiovascular risk factors for women like age at menopause, hormone replacement therapy, history of pre-eclampsia, or others were not taken into account (37). There may be selection bias by pSS patients suffering from more severe disease courses in our university outpatients pSS clinic as well as the mentioned higher proportion of male patients, both leading to a higher number of patients with CVD. Despite additional assessment of medical records, recall bias in the standardized protocol may have occurred. On the other hand, we present here a very large cohort of patients with pSS who underwent both rheumatologic and neurologic standardized detailed examination. Neurologic examination did only include cerebral magnetic resonance imaging in symptomatic patients, possible asymptomatic functional changes in the brain were not assessed (46). Patients fulfill classification criteria of the time of diagnoses, therefore the cohort is more heterogenic.
In summary, we were able to identify a clinical phenotype presenting traditional cRF and clinical features of pSS that may help clinicians identify patients at risk. After diagnosis of pSS, all patients should be screened for risk factors such as hypertension and receive appropriate therapy to prevent or at least reduce sequelae such as infarction. However, further longitudinal investigations with longer follow up and larger cohorts are warranted to establish valid risk stratifications and thus improving prevention and management of CVDs in pSS patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Hannover Medical School 8179_BO_S_2018. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CZ: data collection, writing of the manuscript, database work, and patient flow. SB: analyses, writing of the manuscript, and database work. EK, SH, and TSe: data collection and critical review. TSk, AJ, FK, KS, and TW: critical review. DE: study design, organization, analyses, and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by Novartis and the Else-Kröner Charity. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We would like to express our gratitude to the staff of the Rheumatology Outpatients Department at Hannover Medical School for their continual help in organization of patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1048684/full#supplementary-material
References
1. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:1983–9. doi: 10.1136/annrheumdis-2014-205375
2. Ienopoli S, Carsons SE. Extraglandular manifestations of primary Sjögren's syndrome. Oral Maxillofac Surg Clin North Am. (2014) 26:91–9. doi: 10.1016/j.coms.2013.09.008
3. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjögren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren's syndrome. Ann Rheum Dis. (2010) 69:1103–9. doi: 10.1136/ard.2009.110619
4. Retamozo S, Acar-Denizli N, Rasmussen A, Horváth IF, Baldini C, Priori R, et al. Systemic manifestations of primary Sjögren's syndrome out of the ESSDAI classification: prevalence and clinical relevance in a large international, multi-ethnic cohort of patients. Clin Exp Rheumatol. (2019) 37:S97–106.
5. Lai CH, Lai CH, Lai CH, Hsieh CY, Barnado A, Huang LC, et al. Outcomes of acute cardiovascular events in rheumatoid arthritis and systemic lupus erythematosus: a population-based study. Rheumatology. (2020) 59:1355–63. doi: 10.1093/rheumatology/kez456
6. Yong WC, Sanguankeo A, Upala S. Association between primary Sjogren's syndrome, arterial stiffness, and subclinical atherosclerosis: a systematic review and meta-analysis. Clin Rheumatol. (2019) 38:447–55. doi: 10.1007/s10067-018-4265-1
7. Łuczak A, Małecki R, Kulus M, Madej M, Szahidewicz-Krupska E, Doroszko A. Cardiovascular risk and endothelial dysfunction in primary sjogren syndrome is related to the disease activity. Nutrients. (2021) 13:2072. doi: 10.3390/nu13062072
8. Bartoloni E, Baldini C, Schillaci G, Quartuccio L, Priori R, Carubbi F, et al. Cardiovascular disease risk burden in primary Sjögren's syndrome: results of a population-based multicentre cohort study. J Intern Med. (2015) 278:185–92. doi: 10.1111/joim.12346
9. Juarez M, Toms TE, De Pablo P, Mitchell S, Bowman S, Nightingale P, et al. Cardiovascular risk factors in women with primary Sjögren's syndrome: United Kingdom primary Sjögren's syndrome registry results. Arthritis Care Res. (2014) 66:757–64. doi: 10.1002/acr.22227
10. Augusto KL, Bonfa E, Pereira RMR, Bueno C, Leon EP, Viana VST, et al. Metabolic syndrome in Sjögren's syndrome patients: a relevant concern for clinical monitoring. Clin Rheumatol. (2016) 35:639–47. doi: 10.1007/s10067-015-3072-1
11. Cai X, Luo J, Wei T, Qin W, Wang X, Li X. Risk of cardiovascular involvement in patients with primary Sjögren's syndrome: a large-scale cross-sectional cohort study. Acta Reumatol Port. (2019) 44:71–7.
12. Pérez-De-Lis M, Akasbi M, Sisó A, Diez-Cascon P, Brito-Zerón P D-LC. Cardiovascular risk factors in primary Sjögren's syndrome: a case – control study in 624 patients. Lupus. (2010) 19:941–8. doi: 10.1177/0961203310367504
13. Beltai A, Barnetche T, Daien C, Lukas C, Gaujoux-Viala C, Combe B, et al. Cardiovascular morbidity and mortality in primary Sjögren's syndrome: a systematic review and meta-analysis. Arthritis Care Res. (2020) 72:131–9. doi: 10.1002/acr.23821
14. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. (2017) 69:35–45. doi: 10.1002/art.39859
15. Shiboski SC, Shiboski CH, Criswell LA, Baer AN, Challacombe S, Lanfranchi H, et al. American college of rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's international collaborative clinical alliance cohort. Arthritis Care Res. (2012) 64:475–87. doi: 10.1002/acr.21591
16. Seror R, Ravaud P, Mariette X, Bootsma H, Theander E, Hansen A, et al. EULAR Sjögren's syndrome patient reported index (ESSPRI): development of a consensus patient index for primary Sjögren's syndrome. Ann Rheum Dis. (2011) 70:968–72. doi: 10.1136/ard.2010.143743
17. De Vita S, Lorenzon G, Rossi G, Sabella M, Fossaluzza V. Salivary gland echography in primary and secondary Sjögren's syndrome. Clin Exp Rheumatol. (1992) 10:351–6.
18. Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. (1968) 21:656–60. doi: 10.1136/jcp.21.5.656
19. Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol. (2014) 31:378–88. doi: 10.1055/s-0034-1393976
20. Naser W, Lishner M. Behçet disease presenting as acute myocardial infarction. Isr Med Assoc J. (2020) 22:458–60.
21. Gandolfo C, Balestrino M, Finocchi C, Viani E. Churg-Strauss syndrome mimicking myocardial infarction with cerebral vascular involvement. J Neurol. (2013) 260:2659–61. doi: 10.1007/s00415-013-7088-7
22. Kastner D, Gaffney M, Tak T. Polyarteritis nodosa and myocardial infarction. Can J Cardiol. (2000) 16:515–8.
23. Dornquast C, Kroll LE, Neuhauser HK, Willich SN, Reinhold T, Busch MA. Regional differences in the prevalence of cardiovascular disease. Dtsch Arztebl Int. (2016) 113:704–11. doi: 10.3238/arztebl.2016.0704
24. Busch MA, Schienkiewitz A, Nowossadeck E, Gößwald A. Prävalenz des schlaganfalls bei erwachsenen im alter von 40 bis 79 jahren in deutschland: ergebnisse der studie zur gesundheit erwachsener in deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsfor Gesundheitssch. (2013) 56:656–60. doi: 10.1007/s00103-012-1659-0
25. Seeliger T, Prenzler NK, Gingele S, Seeliger B, Körner S, Thiele T, et al. Neuro-Sjögren: peripheral neuropathy with limb weakness in Sjögren's syndrome. Front Immunol. (2019) 10:1600. doi: 10.3389/fimmu.2019.01600
26. Bartoloni E, Baldini C, Ferro F, Alunno A, Carubbi F, Cafaro G, et al. Application of artificial neural network analysis in the evaluation of cardiovascular risk in primary Sjögren's syndrome: a novel pathogenetic scenario? Clin Exp Rheumatol. (2019) 37:S133–9.
27. Raja Shariff REF, Kasim S. Recurrent acute ischaemic strokes as the primary presentation of Sjögren's syndrome. Proc Singapore Healthc. (2020) 29:142–4. doi: 10.1177/2010105820911950
28. Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X. Primary Sjögren syndrome. BMJ. (2012) 345:e3821. doi: 10.1136/bmj.e3821
29. Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics. Arch Intern Med. (2001) 161:2657–60. doi: 10.1001/archinte.161.22.2657
30. Novella-Navarro M, Cabrera-Alarcón JL, Rosales-Alexander JL, González-Martín JJ, Carrión O, Peña PG. Primary Sjögren's syndrome as independent risk factor for subclinical atherosclerosis. Eur J Rheumatol. (2022) 9:20–5. doi: 10.5152/eurjrheum.2021.20093
31. Bartoloni E, Alunno A, Cafaro G, Valentini V, Bistoni O, Bonifacio AF, et al. Subclinical atherosclerosis in primary Sjögren's syndrome: does inflammation matter? Front Immunol. (2019) 10:817. doi: 10.3389/fimmu.2019.00817
32. Atzeni F, Sarzi-puttini P, Signorello MC, Gianturco L, Stella D, Boccassini L, et al. New parameters for identifying subclinical atherosclerosis in patients with primary Sjögren's syndrome: a pilot study. Clin Exp Rheumatol. (2014) 32:361–8.
33. Wu XF, Huang JY, Chiou JY, Chen HH, Wei JCC, Dong LL. Increased risk of coronary heart disease among patients with primary Sjögren's syndrome: a nationwide population-based cohort study. Sci Rep. (2018) 8:2209. doi: 10.1038/s41598-018-19580-y
34. Mofors J, Holmqvist M, Westermark L, Björk A, Kvarnström M, Forsblad-d'Elia H, et al. Concomitant Ro/SSA and La/SSB antibodies are biomarkers for the risk of venous thromboembolism and cerebral infarction in primary Sjögren's syndrome. J Intern Med. (2019) 286:458–68. doi: 10.1111/joim.12941
35. Zegkos T, Kitas G, Dimitroulas T. Cardiovascular risk in rheumatoid arthritis: assessment, management and next steps. Ther Adv Musculoskelet Dis. (2016) 8:86–101. doi: 10.1177/1759720X16643340
37. Young L, Cho L. Unique cardiovascular risk factors in women. Heart. (2019) 105:1656–60. doi: 10.1136/heartjnl-2018-314268
38. Andonopoulos AP, Christodoulou J, Ballas C, Bounas A, Alexopoulos D. Autonomic cardiovascular neuropathy in Sjögren's syndrome. A controlled study. J Rheumatol. (1998) 25:2385–8.
39. Gosk-Bierska I, Misterska-Skóra M, Wasilewska M, Bilińska M, Gosk J, Adamiec R, et al. Analysis of peripheral nerve and autonomic nervous system function and the stage of microangiopathy in patients with secondary Raynaud's phenomenon in the course of connective tissue diseases. Adv Clin Exp Med. (2018) 27:1587–92. doi: 10.17219/acem/75618
40. Taher R, Sara JD, Toya T, Shepherd R, Moder K, Lerman LO, et al. Secondary Raynaud's phenomenon is associated with microvascular peripheral endothelial dysfunction. Microvasc Res. (2020) 132:104040. doi: 10.1016/j.mvr.2020.104040
41. Nishiwaki A, Kobayashi H, Ikumi N, Kobayashi Y, Yokoe I, Sugiyama K, et al. Salivary gland focus score is associated with myocardial fibrosis in primary sjögren syndrome assessed by a cardiac magnetic resonance approach. J Rheumatol. (2021) 48:859–866. doi: 10.3899/jrheum.200352
42. Horvath IF, Szanto A, Papp G, Zeher M. Clinical course, prognosis, and cause of death in primary Sjögren's syndrome. J Immunol Res. (2014) 2014:647507. doi: 10.1155/2014/647507
43. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. (2016) 76:17–28. doi: 10.1136/annrheumdis-2016-209775
44. Salvarani C, Brown RD, Hunder GG. Adult primary central nervous system vasculitis. Lancet. (2012) 380:767–77. doi: 10.1016/S0140-6736(12)60069-5
45. Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. (2016) 15:22–37. doi: 10.1016/j.autrev.2015.10.002
Keywords: Sjögren syndrome, Raynaud disease, atherosclerosis, cardiovascular risk factors, cardiovasclar disease, vasculitis, EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI), stroke
Citation: Zippel CL, Beider S, Kramer E, Konen FF, Seeliger T, Skripuletz T, Hirsch S, Jablonka A, Witte T, Sonnenschein K and Ernst D (2022) Premature stroke and cardiovascular risk in primary Sjögren's syndrome. Front. Cardiovasc. Med. 9:1048684. doi: 10.3389/fcvm.2022.1048684
Received: 19 September 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Marta Waliszewska-Prosół, Wroclaw Medical University, PolandClio Mavragani, National and Kapodistrian University of Athens, Greece
Polona Žigon, University Medical Centre Ljubljana, Slovenia
Copyright © 2022 Zippel, Beider, Kramer, Konen, Seeliger, Skripuletz, Hirsch, Jablonka, Witte, Sonnenschein and Ernst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Ernst, ernst.diana@mh-hannover.de
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Clara L. Zippel
Clara L. Zippel Sonja Beider
Sonja Beider Emelie Kramer
Emelie Kramer Franz F. Konen
Franz F. Konen Tabea Seeliger
Tabea Seeliger Thomas Skripuletz
Thomas Skripuletz Stefanie Hirsch
Stefanie Hirsch Alexandra Jablonka
Alexandra Jablonka Torsten Witte
Torsten Witte Kristina Sonnenschein3‡
Kristina Sonnenschein3‡  Diana Ernst
Diana Ernst