Blood Transfusion Predicts Prolonged Mechanical Ventilation in Acute Stanford Type A Aortic Dissection Undergoing Total Aortic Arch Replacement
- Department of Cardiovascular Surgery, Beijing Aortic Disease Center, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
Background: This research aimed to evaluate the impacts of transfusing packed red blood cells (pRBCs), fresh frozen plasma (FFP), or platelet concentrate (PC) on postoperative mechanical ventilation time (MVT) in patients with acute Stanford type A aortic dissection (ATAAD) undergoing after total arch replacement (TAR).
Methods: The clinical data of 384 patients with ATAAD after TAR were retrospectively collected from December 2015 to October 2017 to verify whether pRBCs, FFP, or PC transfusion volumes were associated with postoperative MVT. The logistic regression was used to assess whether blood products were risk factors for prolonged mechanical ventilation (PMV) in all three endpoints (PMV ≥24 h, ≥48 h, and ≥72 h).
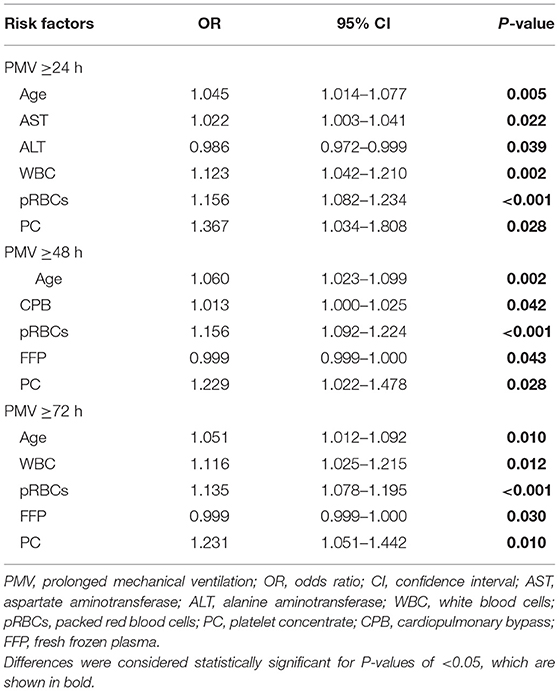
Results: The mean age of 384 patients was 47.6 ± 10.689 years, and 301 (78.39%) patients were men. Median MVT was 29.5 (4–574) h (h), and 213 (55.47%), 136 (35.42%), and 96 (25.00%) patients had PMV ≥24 h, ≥48 h, and ≥72 h, respectively. A total of 36 (9.38%) patients did not have any blood product transfusion, the number of patients with transfusion of pRBCs, FFP, and PC were 334 (86.98%), 286 (74.48%), and 189 (49.22%), respectively. According to the multivariate logistic regression of three PMV time-endpoints, age was a risk factor [PMV ≥ 24 h odds ratio (ORPMV≥24) = 1.045, p = 0.005; ORPMV≥48 = 1.060, p = 0.002; ORPMV≥72 = 1.051, p = 0.011]. pRBC transfusion (ORPMV≥24 = 1.156, p = 0.001; ORPMV≥48 = 1.156, p < 0.001; ORPMV≥72 = 1.135, p ≤ 0.001) and PC transfusion (ORPMV≥24 = 1.366, p = 0.029; ORPMV≥48 = 1.226, p = 0.030; ORPMV≥72 = 1.229, p = 0.011) were independent risk factors for PMV. FFP had no noticeable effect on PMV [ORPMV≥48 = 0.999, 95% confidence interval (CI) 0.998–1.000, p = 0.039; ORPMV≥72 = 0.999, 95% CI: 0.998–1.000, p = 0.025].
Conclusions: In patients with ATAAD after TAR, the incidence of PMV was very high. Blood products transfusion was closely related to postoperative mechanical ventilation time. pRBC and PC transfusions and age increased the incidence of PMV at all three endpoints.
Introduction
Acute Stanford type A aorta dissection (ATAAD) is a fatal cardiovascular disease. Due to the widespread lesion, the mortality with immediate surgical intervention is still as high as 30% and drug treatment is as high as 58% (1, 2). Total arch replacement (TAR) is a common approach for ATAAD. As a complex, traumatic operation, there are complications of multiple organs, such as the lungs and the kidney, after the operation (3, 4). Mechanical ventilation (MV) helps patients after TAR to improve oxygen saturation and to prevent lung atelectasis (5). However, many studies showed that the physiological state, drug use, and postoperative complications lead to a high probability of prolonged mechanical ventilation (PMV), which increases infection and mortality (6, 7). Almost all patients with ATAAD require different blood products to improve circulation and correct anemia and hypoproteinemia intraoperatively and postoperatively (8). It is still unclear whether changes in the internal environment brought by different blood products will affect mechanical ventilation time (MVT). This research attempted to explore whether blood product transfusion is a predictor of PMV in ATAAD patients undergoing TAR.
Materials and Methods
Patients
A total of 384 consecutive patients (47.6 ± 10.689 years) with ATAAD undergoing TAR were enrolled at Beijing Anzhen Hospital of Capital Medical University from December 2015 to October 2017. The inclusion criteria were as follows: patients who had been diagnosed as Stanford type A aortic dissection by aortic enhanced CT and who underwent surgical treatment; those with the onset of disease at ≤14 days; and those aged ≥18 years. Patients with traumatic aortic dissection, those with aortic dissection during pregnancy, those with the onset of disease at more than 14 days, and those aged <18 were excluded. All the clinical data were retrospectively recorded from the medical records; 33 cases had been eliminated with insufficient data. This retrospective study was approved by the Medical Ethical Committee of the Beijing Anzhen Hospital of Capital Medical University (2020100X) and waived the need for individual patient consent.
Mechanical Ventilation Management
The Society of Thoracic Surgeons and most studies define PMV as mechanical ventilation for 24 h (h) or more (9, 10). In our research, by combining preoperative and postoperative clinical parameters in patients with ATAAD undergoing TAR, we assessed different periods of MVT (≥24 h, ≥48 h, and ≥72 h) for outcomes. We adjusted the ventilator parameters appropriately to the patient's respiratory oxygenation by maintaining SaO2 > 90% or PaO2 > 60 mmHg (11, 12). The extubation decision was the consulting anesthetist's independent discretion, usually after the spontaneous breathing or under a low-level pressure support trial. When the tracheal tube was removed, hemodynamic parameters were stable without tachypnea, anxiety, sweating, or descent.
Blood Products and Indications
The respective totality of packed red blood cells (pRBCs), fresh frozen plasma (FFP), or platelet concentrate (PC) that patients transfused intraoperatively and postoperatively were recorded. The total number of pRBCs units (U) did not include autologous blood stored intraoperatively and postoperatively. Indications for pRBCs transfusion included the hemoglobin level of ≤9.0 g/dl on the surgery day or ≤7.0 g/dl postoperatively. The PC transfusion indications included life-threatening active bleeding, the platelet count ≤ 50,000/ml, or the platelet count <100,000/ml with bleeding. The FFP was transfused when the prothrombin time was >1.5 times the upper limit of normal with a prolonged cardiopulmonary bypass (CPB) time or clinical signs of bleeding (13–15).
Total Arch Replacement (TAR)
All procedures were median sternotomy, and total CPB with selective antegrade cerebral perfusion (SCP) was performed in the right axillary artery and the right atrium. The arterial hemoperfusion consisted of right axillary artery perfusion and one branch of a four-branch aortic graft. The ascending aorta was clamped during cooling, and a cold-blood cardioplegic solution was antegrade infused into the coronary ostia by a longitudinal incision in the proximal ascending aorta. An anastomosis of the four-branch aortic graft to the aortic root was finished in the cooling phase; the circulatory anastomosis was arrested after the nasopharyngeal temperature reached 25°C. After the brachiocephalic arteries were clamped, unilateral SCP was started through the right axillary artery. The anastomosis of the stent graft in the aortic arch to the four-branch graft distal end was completed, and the four-branch graft was clamped. The lower body got blood perfusion via the perfusion limb of the four-branch graft. SCP was discontinued after anastomosis of the left common carotid artery, the left subclavian artery, and the innominate artery. Then, CPB flow was gradually resumed normal and began to warm.
Statistical Analysis
Descriptive statistics were expressed as means ± standard deviations or median (interquartile range) for continuous variables and percentages and frequencies for categorical variables. Independent-samples t-test and non-parametric tests were used to compare continuous variables. The categorical variables were compared by the χ2 analysis or Fisher's exact correction. The p-value of <0.05 was considered significant. Then, a stepwise multivariable logistic regression analysis was used to determine factors independently associated with PMV. Statistical Package for the Social Sciences Version 26.0 (IBM Corp, Armonk, NY, USA) was used to analyze data. Prism 9 (GraphPad Software, San Diego, California, USA) was used for drawing figures.
Results
Patients Baseline Characteristics
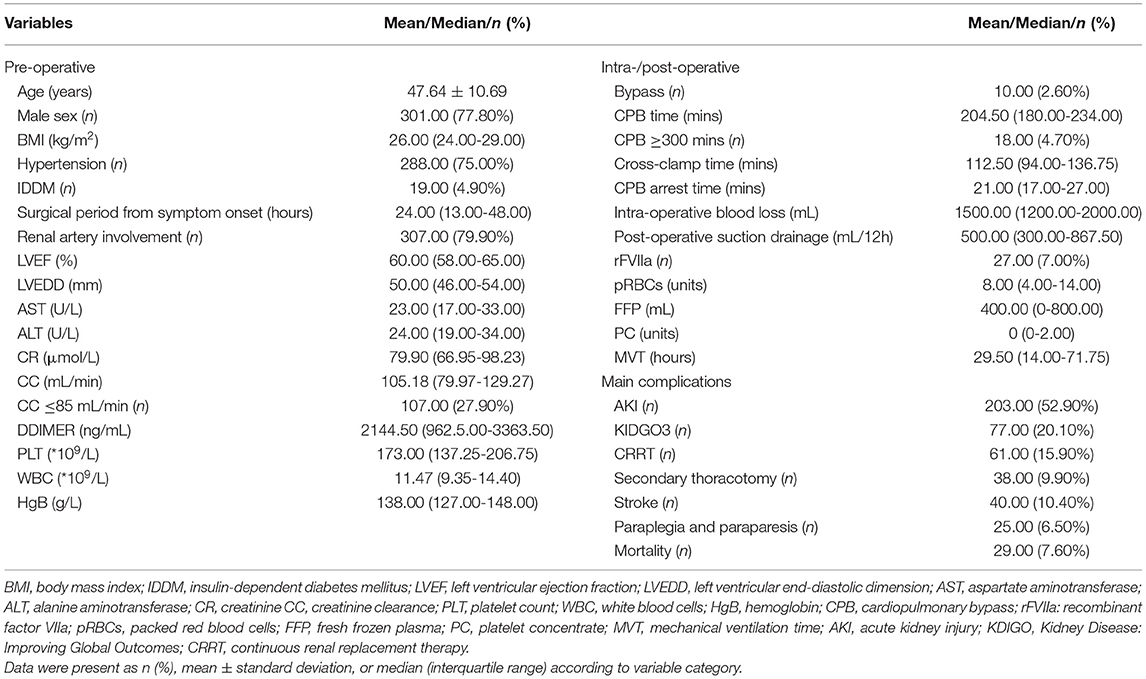
The age (mean ± standard deviation) of all 384 patients was 47.64 ± 10.69 years, 301 (78.39%) patients were men, and time (median, interquartile range) from related symptom onset to TAR was 24 (13.00–48.00) h. The median CPB time was 204.50 (180.00–234.00) min (min), and 18 (4.70%) patients had the median CPB time of over 300 min A total of 27 (7.00%) patients received recombinant factor VIIa (rFVIIa) infusion in-hospital, and the median volume of the total intraoperative and postoperative of pRBCs, FFP, and PC was 8.00 (4.00–14.00) U, 400.00 (0–800.00) ml, and 0 (0–2.00) U, respectively. Median MVT was 29.50 (14.00–71.75) h, the number of cases of PMV ≥24 h, ≥48 h, and ≥72 h was 213 (55.47%), 136 (35.42%), and 96 (25.00%), respectively. More perioperative baseline data and main complications characteristics were shown in Table 1.
Blood Products Transfusion and MVT
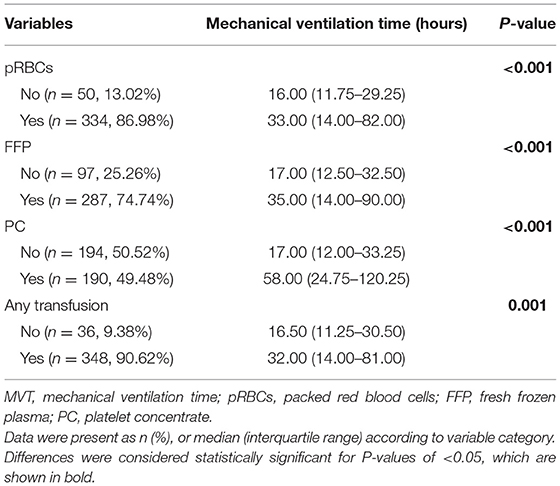
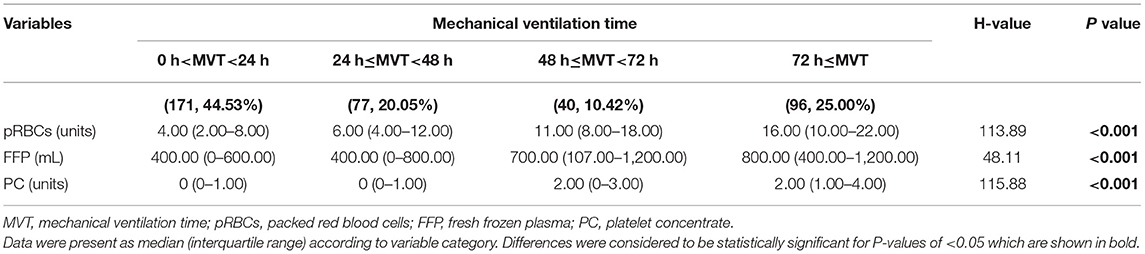
As shown in Table 2 and Figure 1, there were 50 (13.02%) patients without any pRBCs infusion, 97 (25.26%) patients without FFP, and 194 (50.52%) cases without PC. Only 36 (9.38%) cases did not have any transfusion, and the MVT difference in transfusion (16.50, 11.25–30.50 h) or without transfusion (32.00, 14.00–81.00 h) was significant (p = 0.001). In different periods of PMV (≥24 h, ≥48 h, and ≥72 h), the respective transfusion volume of pRBCs, FFP, or PC was statistically different (Table 3; Figure 2).
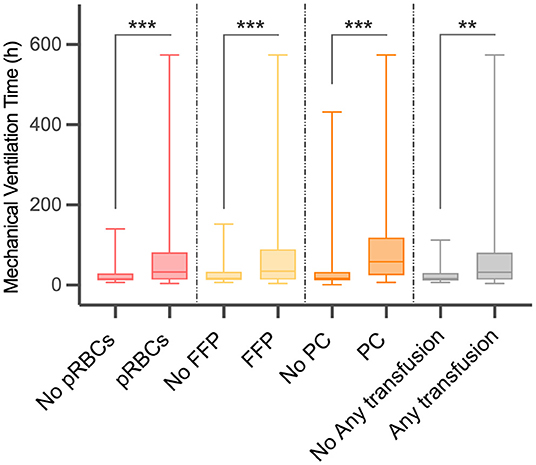
Figure 1. Difference in mechanical ventilation time (MVT) between different blood product transfusion and no transfusion. MVT, mechanical ventilation time; pRBCs, packed red blood cells; FFP, fresh frozen plasma; PC, platelet concentrate. **p < 0.01, ***p < 0.001.
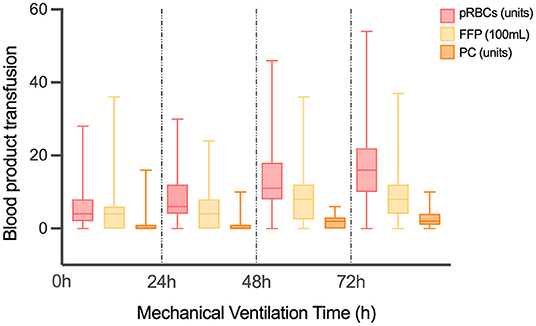
Figure 2. The volume of different transfusion in patients with different MVT. MVT, mechanical ventilation time; pRBCs, packed red blood cells; FFP, fresh frozen plasma; PC, platelet concentrate.
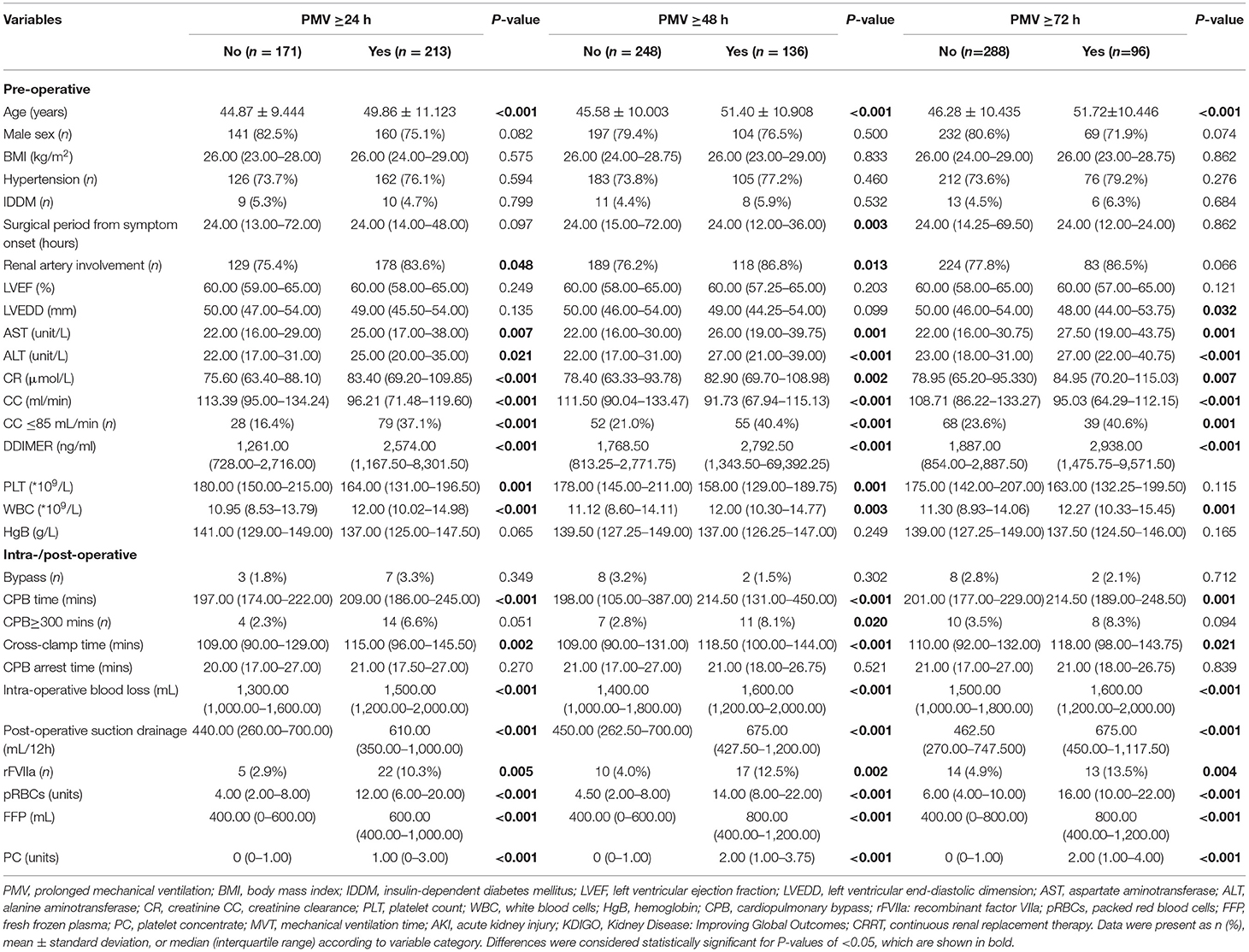
PMV and Clinical Characteristics
In Table 4, the relation of different PMV (≥24 h, ≥48 h, and ≥72 h) and perioperative factors were assessed. Several indicators were noteworthy, such as the surgical period from disease onset showed an effect on PMV ≥ 48 h (p = 0.003), but not on PMV ≥24 h or ≥72 h. PMV ≥24 h and ≥48 h were affected (p = 0.048, p = 0.013, respectively) by renal artery involved dissection, but not PMV ≥72 h. Left ventricular end-diastolic dimension showed a statistical significance (p = 0.032) only in PMV ≥72 h. The platelet (PLT) count was different (p = 0.001, p = 0.001, respectively) both in PMV ≥24 h and ≥48 h. When the CPB was ≥300 min, the difference (p = 0.020) was only shown in PMV ≥48 h. The transfusion of rFVIIa, pRBCs, FFP, and PC, respectively, showed a statistical difference on three PMV.
Risk Factors of PMV
Multivariate logistic regression with three different PMV (≥24 h, ≥48 h, and ≥72 h) endpoints showed that age was a risk factor [PMV ≥ 24 h, odds ratio (ORPMV≥24) = 1.045, p = 0.005; ORPMV≥48 = 1.060, p = 0.002; ORPMV≥72 = 1.051, p = 0.011]. pRBC (ORPMV≥24 = 1.156, p = 0.001; ORPMV≥48 = 1.156, p < 0.001; ORPMV≥72 = 1.135, p ≤0.001) and PC (ORPMV≥24 = 1.366, p = 0.029; ORPMV≥48 = 1.226, p = 0.030; ORPMV≥72 = 1.229, p = 0.011) transfusions were independent risk factors for PMV (Table 5). However, FFP had no significant effect on PMV ≥24 h [OR = 0.999, 95% confidence interval (CI): 0.999–1.000, p = 0.086], on PMV ≥48 h (OR = 0.999, 95% CI: 0.998–1.000, p = 0.039), and PMV ≥72 h (OR = 0.999, 95% CI: 0.998–1.000, p = 0.025). Besides, while considering PMV ≥24 h as the endpoint, AST (OR = 1.022, p = 0.022), ALT (OR = 0.986, p = 0.039), and WBC (OR = 1.123, p = 0.002) were preoperative risk factors. The CPB time (OR = 1.013, p = 0.042) was the predictive factor of PMV ≥48 h. For PMV ≥72 h, WBC (OR = 1.116, p = 0.012) had the predictive effect.
Blood Transfusion and Other Adverse Events
In addition to pRBCs transfusion volume between the paraplegic and non-paraplegic, the difference in blood transfusion volume between with and without different complications was statistically significant (Supplementary Table 1). The difference in the incidence of adverse events such as PMV) between patients with and without blood transfusion was evaluated, with secondary thoracotomy, kidney disease: improving global outcomes criteria stage 3 (KIDGO3), PMV showing statistical significance (Figure 3).
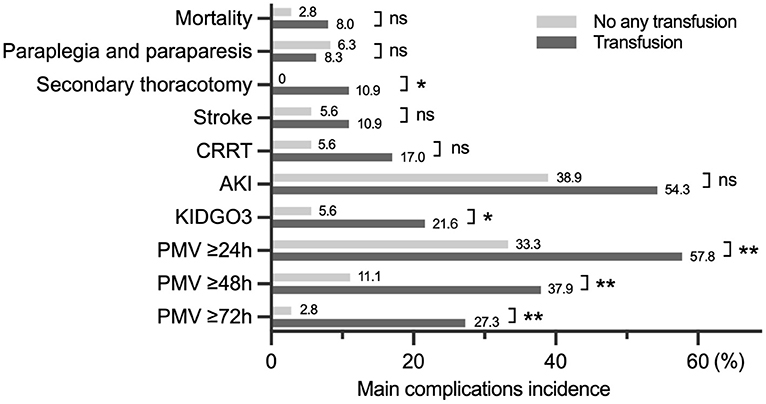
Figure 3. The difference in the incidence of complications between transfusion and no transfusion. CRRT, continuous renal replacement therapy; AKI, acute kidney injury; KDIGO, kidney disease improving global outcomes; PMV, prolonged mechanical ventilation. *p < 0.05, **p < 0.01.
Discussion
Mechanical ventilation can help to recover patients' cardiopulmonary function and circulation quickly after a long, traumatic, and complex operation. However, timely extubation is necessary to avoid mechanical ventilation complications such as infection, trachea damage, and ventilator dependence (6, 7). This study found that MVT exceeded 24 h in 213 (55.47%) cases, 48 h in 136 (35.42%) cases, and 72 h in 96 (25.00%) cases, which is the first time that PMV incidence of different endpoints in ATAAD after TAR were reported. Such PMV incidence was significantly higher than other cardiovascular operations, such as coronary artery bypass grafting and the tetralogy of Fallot (16–18). More studies focused on finding PMV predictors after cardiovascular surgery in blood indicators, CPB process, and cardiac function for patients (16, 19). This study is the first attempt to identify risk factors from three different PMV endpoints for MVT after TAR for severe aortic dissection.
During intraoperative and postoperative aortic surgery, different blood products often need to be infused to improve circulation and prevent multiple organ injuries. Before this, only two studies attempted to explain the relationship between perioperative blood transfusions and PMV during cardiovascular surgery. In one study, PMV ≥ 48 h was correlated with intraoperative blood transfusion in an open-heart surgery (20). However, it neither did provide detailed data on blood products type and transfusion volume nor did it record the transfusion of patients in ICU. Another study showed that the blood products transfusion ≥2,000 ml in ATAAD repair was correlated with PMV ≥48 h, but had limitations such as incomplete blood products data and too few patients involved in comparison (18). These reference values were not evident.
In our study of 384 cases, the MVT was significantly different between the groups with or without transfusion of different blood products (such as pRBCs, FFP, PC, or anyone), and the respective transfusion volume of pRBCs, FFP, or PC in four different MVT groups had statistical significance. Moreover, after summarizing three multivariate logistics regressions of different PMV endpoints, it was found that intraoperative and postoperative pRBC and PC transfusions are effective predictors for PMV (≥24 h, ≥48 h, and ≥72 h). For lack of targeted research, the mechanism by which pRBC and PC transfusions affect PMV remains unclear. According to the existing studies, we speculated that it might be related to the decreased deformability and oxygen transfer of the allogeneic stored red cells, and the hemolysis of the long-stored red cells will lead to electrolyte disorder (the free hemoglobin, iron, and K+ levels, especially) and coagulation dysfunction (21–23). PC contained many different pyrogenic and inflammatory factors that can cause severe fever or even infection in patients with compromised immunity after cardiovascular surgery (24–26). These are all possible causes of delay extubation of mechanical ventilation directly or indirectly.
It was found that some patients showed worse lung function after allogeneic blood transfusion, probably due to the impaired erythrocyte deformability and increased oxygen affinity of stored red blood cells (RBCs) (27, 28). In the rat model, the transfusion of degenerated blood products led to microcirculation disturbance and then blocked the pulmonary capillary (29). Similarly, the microaggregates from degenerated platelets and fibrin led to pulmonary insufficiency (30). The embolism of pulmonary microcirculation from damaged RBCs and microaggregates may be one of the potential causes of PMV in patients with blood transfusion (31–33).
On the other hand, transfusion-related immunomodulation is the direct cause of postoperative infections and multiple organ failure. The plasma from pRBCs induced neutrophils to produce superoxide, enhanced cytotoxicity, and stimulated pulmonary endothelial cells (34, 35). The soluble human leukocyte antigen (HLA) class I molecules circulating in FFP and the proinflammatory cytokines and chemokines (e.g., IL-1, tumor necrosis factor, IL-6, and IL-8) accumulating in PC resulted in diffuse inflammatory and pulmonary dysfunction postoperatively, which then led to PMV (36–39).
It had been proven that older age (≥70 years) means a higher postoperative PMV risk in patients undergoing coronary artery bypass grafting or elective coronary artery surgery, and older age is also an independent risk factor for PMV after total cavopulmanory connection surgery (16, 40, 41). A similar conclusion was found in another medical ICU, where patients with MV extubation failure were older than those with successful extubation (74.0 vs.70.0 years; p = 0.003) (42). When assessing PMV risk factors after corrective surgery for tetralogy of Fallot, the higher risk group was found to be younger (12 months, interquartile range 8–19 months) (17). After surgical repair of congenital heart disease, the younger ones [11.95 months (Min–Max range 0.3–158.7 months)] also had a higher risk (43). Age is an important consideration when defining pediatric PMV because it relates to lung maturity (44). The opposite among older persons may be due to a gradual decline in lung function and the body's capacity to stress. We have not yet found whether age can predict postoperative PMV of ATAAD. In all 384 cases, age followed a normal distribution (47.64 ± 10.69 years), with a median age of 47 years (20–80 years). This study confirmed that age was a significant risk factor for PMV of ATAAD after TAR in all three endpoints (PMV ≥24 h, ≥48 h, and ≥72 h). The mean age of patients with PMV ≥24 h was 49.86 ± 11.123 h, and the mean age was higher in PMV ≥48 h or ≥72 h. The likely cause is a gradual decline in pulmonary function and a tolerance decrease to the lengthy, major traumatic procedure.
Only two studies have confirmed that leukocytosis is a risk factor for PMV after cardiovascular surgery, one had PMV ≥24 h after cardiac surgery with CPB, and the other had PMV ≥72 h after acute DeBakey type I aortic dissection surgery (45, 46). Similar results were obtained; preoperative leukocytosis was an independent risk factor for two endpoints of PMV ≥24 h and ≥72 h in ATAAD after TAR. There is no reference research for why there is no predictive value in PMV ≥48 h. Our subsequent studies will include neutrophil count and proportion, C-reactive protein, and other indicators to find the cause.
Many factors lead to PMV, and our research provided a new idea for ATAAD after TAR to predict PMV. However, for most cardiovascular surgeries requiring blood products transfusion, reducing or restricting the transfusion volume without reference is not a reasonable way to reduce PMV incidence. Restrictive pRBC transfusion failed to show advantages and increased the risk of mortality sometimes (47–49). It may be a more direct way to avoid using the long-stored red cells in a long and traumatic surgery (50). We also considered removing excessive hemolysis products and anticoagulants by the purification system before transfusion to reduce post-transfusion complications (51, 52). More targeted research is needed to facilitate the development of guidelines.
Conclusions
In patients with ATAAD after TAR, PMV incidence was very high. Blood product transfusion was closely related to postoperative mechanical ventilation time. pRBC and PC transfusions and age increased the incidence of PMV at all three endpoints. FFC had no noticeable impact on PMV.
Limitations
This was a single-center study, which is the main limitation of this research. As a retrospective study, there was a lack of data on the transfusion time (intraoperative or postoperative) and how long the blood products were stored, especially pRBCs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethical Committee of the Beijing Anzhen Hospital of Capital Medical University (2020100X). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CLi, YG, LS, and JZhu: conception and design. CLi, YG, LS, YL, JZhe, and JZhu: provision of research materials or patients. QX, YZ, CLu, and RG: data collection and analysis. All authors: manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Beijing Major Science and Technology Projects from the Beijing Municipal Science and Technology Commission (No. Z191100006619093) and the Natural Science Foundation of China (No. 81970393).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.832396/full#supplementary-material
References
1. Wang W, Duan W, Xue Y, Wang L, Liu J, Yu S, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg. (2014) 148:2995–3000. doi: 10.1016/j.jtcvs.2014.07.068
2. Krüger T, Weigang E, Hoffmann I, Blettner M, Aebert H. GERAADA Investigators. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation. (2011) 124:434–43. doi: 10.1161/CIRCULATIONAHA.110.009282
3. Uchida K, Minami T, Cho T, Yasuda S, Kasama K, Suzuki S, et al. Results of ascending aortic and arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg. (2021) 162:1025–31. doi: 10.1016/j.jtcvs.2020.02.087
4. Trivedi D, Navid F, Balzer JR, Joshi R, Lacomis JM, Jovin TG, et al. Aggressive aortic arch and carotid replacement strategy for Type A aortic dissection improves neurologic outcomes. Ann Thorac Surg. (2016) 101:896–903; Discussion 903-5. doi: 10.1016/j.athoracsur.2015.08.073
5. Li CN, Chen L, Ge YP, Zhu JM, Liu YM, Zheng J, et al. Risk factors for prolonged mechanical ventilation after total aortic arch replacement for acute DeBakey type I aortic dissection. Heart Lung Circ. (2014) 23:869–74. doi: 10.1016/j.hlc.2014.03.022
6. Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. (2016) 10:CD008367. doi: 10.1002/14651858.CD008367.pub3
7. Badenes R, Lozano A, Belda FJ. Postoperative pulmonary dysfunction and mechanical ventilation in cardiac surgery. Crit Care Res Pract. (2015) 2015:420513. doi: 10.1155/2015/420513
8. Chen FT, Chou AH, Wu VC, Yang CH, Chu PH, Ting PC, et al. Effect of massive blood transfusion on late outcomes after surgical repair of acute type A aortic dissection. Medicine (Baltimore). (2019) 98:e17816. doi: 10.1097/MD.0000000000017816
9. Hessels L, Coulson TG, Seevanayagam S, Young P, Pilcher D, Marhoon N, et al. Development and validation of a score to identify cardiac surgery patients at high risk of prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. (2019) 33:2709–16. doi: 10.1053/j.jvca.2019.03.009
10. Rose L, McGinlay M, Amin R, Burns KE, Connolly B, Hart N, et al. Variation in definition of prolonged mechanical ventilation. Respir Care. (2017) 62:1324–32. doi: 10.4187/respcare.05485
11. Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. (2014) 18:658. doi: 10.1186/s13054-014-0658-y
12. Bateman RM, Sharpe MD, Jagger JE, Ellis CG, Solé-Violán J, López-Rodríguez M, et al. 36th International Symposium on Intensive Care and Emergency Medicine. Brussels, Belgium. 15–18 March 2016. Crit Care. (2016) 20:94. doi: 10.1186/s13054-016-1208-6
13. Society Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Ferraris SP, Saha SP, Hessel EA 2nd, Haan CK, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. (2007) 83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099
14. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. (2015) 162:205–13. doi: 10.7326/M14-1589
15. Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. (2012) 157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429
16. Faritous ZS, Aghdaie N, Yazdanian F, Azarfarin R, Dabbagh A. Perioperative risk factors for prolonged mechanical ventilation and tracheostomy in women undergoing coronary artery bypass graft with cardiopulmonary bypass. Saudi J Anaesth. (2011) 5:167–9. doi: 10.4103/1658-354X.82786
17. Li S, Zhang Y, Li S, Wang X, Zhang R, Lu Z, et al. Risk factors associated with prolonged mechanical ventilation after corrective surgery for tetralogy of fallot. Congenit Heart Dis. (2015) 10:254–62. doi: 10.1111/chd.12205
18. Jin M, Ma WG, Liu S, Zhu J, Sun L, Lu J, et al. Predictors of prolonged mechanical ventilation in adults after acute type-A aortic dissection repair. J Cardiothorac Vasc Anesth. (2017) 31:1580–7. doi: 10.1053/j.jvca.2017.03.036
19. Ghauri SK, Javaeed A, Mustafa KJ, Khan AS. Predictors of prolonged mechanical ventilation in patients admitted to intensive care units: a systematic review. Int J Health Sci. (2019) 13:31–8.
20. Totonchi Z, Baazm F, Chitsazan M, Seifi S, Chitsazan M. Predictors of prolonged mechanical ventilation after open heart surgery. J Cardiovasc Thorac Res. (2014) 6:211–6. doi: 10.15171/jcvtr.2014.014
21. Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. (2011) 142:1–11. doi: 10.1016/j.jtcvs.2011.02.012
22. Hess JR. Measures of stored red blood cell quality. Vox Sang. (2014) 107:1–9. doi: 10.1111/vox.12130
23. Spitalnik SL. Stored red blood cell transfusions: iron, inflammation, immunity, and infection. Transfusion. (2014) 54:2365–71. doi: 10.1111/trf.12848
24. Muylle L. The role of cytokines in blood transfusion reactions. Blood Rev. (1995) 9:77–83. doi: 10.1016/s0268-960x(95)90028-4
25. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. (2011) 83:719–24.
26. Prodger CF, Rampotas A, Estcourt LJ, Stanworth SJ, Murphy MF. Platelet transfusion: Alloimmunization and refractoriness. Semin Hematol. (2020) 57:92–9. doi: 10.1053/j.seminhematol.2019.10.001
27. Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. (1996) 380:221–6. doi: 10.1038/380221a0
28. Bacalo A, Kivity S, Heno N, Greif Z, Greif J, Topilsky M. Blood transfusion and lung function in children with thalassemia major. Chest. (1992) 101:362–5. doi: 10.1378/chest.101.2.362
29. Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. (1987) 253:H898–903. doi: 10.1152/ajpheart.1987.253.4.H898
30. Solis RT, Goldfinger D, Gibbs MB, Zeller JA. Physical characteristics of microaggregates in stored blood. Transfusion. (1974) 14:538–50. doi: 10.1111/j.1537-2995.1974.tb04575.x
31. Vamvakas EC, Carven JH. Allogeneic blood transfusion and postoperative duration of mechanical ventilation. Transfusion. (2001) 41:885–92. doi: 10.1046/j.1537-2995.2001.41070885.x
32. Kern H, Redlich U, Hotz H, von Heymann C, Grosse J, Konertz W, et al. Risk factors for prolonged ventilation after cardiac surgery using APACHE II, SAPS II, and TISS: comparison of three different models. Intensive Care Med. (2001) 27:407–15. doi: 10.1007/s001340000802
33. van Hout FM, Hogervorst EK, Rosseel PM, van der Bom JG, Bentala M, van Dorp EL, et al. Does a platelet transfusion independently affect bleeding and adverse outcomes in cardiac surgery. Anesthesiology. (2017) 126:441–9. doi: 10.1097/ALN.0000000000001518
34. Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. (1994) 124:684–94.
35. Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. (2000) 13:29–33. doi: 10.1097/00024382-200013010-00006
36. Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. (1994) 331:625–8. doi: 10.1056/NEJM199409083311001
37. Fransen E, Maessen J, Dentener M, Senden N, Buurman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. (1999) 116:1233–9. doi: 10.1378/chest.116.5.1233
38. Muylle L, Joos M, Wouters E, De Bock R, Peetermans ME. Increased tumor necrosis factor alpha (TNF alpha), interleukin 1, and interleukin 6 (IL-6) levels in the plasma of stored platelet concentrates: relationship between TNF alpha and IL-6 levels and febrile transfusion reactions. Transfusion. (1993) 33:195–9. doi: 10.1046/j.1537-2995.1993.33393174443.x
39. Vamvakas EC, Carven JH. Allogeneic blood transfusion and postoperative duration of mechanical ventilation: effects of red cell supernatant, platelet supernatant, plasma components and total transfused fluid. Vox Sang. (2002) 82:141–9. doi: 10.1046/j.1423-0410.2002.00155.x
40. Saleh HZ, Shaw M, Al-Rawi O, Yates J, Pullan DM, Chalmers JA, et al. Outcomes and predictors of prolonged ventilation in patients undergoing elective coronary surgery. Interact Cardiovasc Thorac Surg. (2012) 15:51–6. doi: 10.1093/icvts/ivs076
41. Luo Q, Su Z, Jia Y, Liu Y, Wang H, Zhang L, et al. Risk factors for prolonged mechanical ventilation after total cavopulmonary connection surgery: 8 years of experience at Fuwai Hospital. J Cardiothorac Vasc Anesth. (2020) 34:940–8. doi: 10.1053/j.jvca.2019.10.043
42. Shin HJ, Chang JS, Ahn S, Kim TO, Park CK, Lim JH, et al. Clinical factors associated with weaning failure in patients requiring prolonged mechanical ventilation. J Thorac Dis. (2017) 9:143–50. doi: 10.21037/jtd.2017.01.14
43. Alrddadi SM, Morsy MM, Albakri JK, Mohammed MA, Alnajjar GA, Fawaz MM, et al. Risk factors for prolonged mechanical ventilation after surgical repair of congenital heart disease. Experience from a single cardiac center. Saudi Med J. (2019) 40:367–71. doi: 10.15537/smj.2019.4.23682
44. Sauthier M, Rose L, Jouvet P. Pediatric prolonged mechanical ventilation: considerations for definitional criteria. Respir Care. (2017) 62:49–53. doi: 10.4187/respcare.04881
45. Aksoy R, Karakoc AZ, Cevirme D, Elibol A, Yigit F, Yilmaz Ü, et al. Predictive factors of prolonged ventilation following cardiac surgery with cardiopulmonary bypass. Braz J Cardiovasc Surg. (2021) 36:780–7. doi: 10.21470/1678-9741-2020-0164
46. Ge M, Wang Z, Chen T, Cheng Y, Ye J, Lu L, et al. Risk factors for and outcomes of prolonged mechanical ventilation in patients received DeBakey type I aortic dissection repairment. J Thorac Dis. (2021) 13:735–42. doi: 10.21037/jtd-20-2736
47. Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. (2010) 304:1559–67. doi: 10.1001/jama.2010.1446
48. Mazer CD, Whitlock RP, Fergusson DA, Belley-Cote E, Connolly K, Khanykin B, et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. (2018) 379:1224–33. doi: 10.1056/NEJMoa1808561
49. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. (2015) 372:997–1008. doi: 10.1056/NEJMoa1403612
50. L'Acqua C, Bandyopadhyay S, Francis RO, McMahon DJ, Nellis M, Sheth S, et al. Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children. Am J Hematol. (2015) 90:915–20. doi: 10.1002/ajh.24119
51. Welsby IJ, Norris PJ, Mauermann WJ, Podgoreanu MV, Conn CM, Meade L, et al. Bedside allogeneic erythrocyte washing with a cell saver to remove cytokines, chemokines, and cell-derived microvesicles. Anesthesiology. (2021) 134:395–404. doi: 10.1097/ALN.0000000000003689
52. Warner MA, Welsby IJ, Norris PJ, Silliman CC, Armour S, Wittwer ED, et al. Point-of-care washing of allogeneic red blood cells for the prevention of transfusion-related respiratory complications (WAR-PRC): a protocol for a multicenter randomised clinical trial in patients undergoing cardiac surgery. BMJ Open. (2017) 7:e016398. doi: 10.1136/bmjopen-2017-016398
Keywords: blood transfusion, prolonged mechanical ventilation (PMV), acute Stanford type A aortic dissection (ATAAD), total aortic arch replacement (TAR), risk factor
Citation: Xie Q, Li C, Zhong Y, Luo C, Guo R, Liu Y, Zheng J, Ge Y, Sun L and Zhu J (2022) Blood Transfusion Predicts Prolonged Mechanical Ventilation in Acute Stanford Type A Aortic Dissection Undergoing Total Aortic Arch Replacement. Front. Cardiovasc. Med. 9:832396. doi: 10.3389/fcvm.2022.832396
Received: 10 December 2021; Accepted: 15 March 2022;
Published: 15 April 2022.
Edited by:
Feng Lan, Chinese Academy of Medical Sciences, ChinaReviewed by:
Maruti Haranal, UN Mehta Institute of Cardiology and Research, IndiaJinbao Qin, Shanghai Ninth People's Hospital, China
Cuntao Yu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Xie, Li, Zhong, Luo, Guo, Liu, Zheng, Ge, Sun and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junming Zhu, anzhenzjm@ccmu.edu.cn; Yipeng Ge, yipengge@126.com
†These authors have contributed equally to this work and share first authorship
 Qiang Xie†
Qiang Xie†  Congcong Luo
Congcong Luo Junming Zhu
Junming Zhu