Graft preservation confers myocardial protection during coronary artery bypass grafting
- 1Karl Landsteiner Institute for Cardiac and Vascular Surgical Research, Vienna, Austria
- 2Deutsches Herzzentrum Berlin, Berlin, Germany
- 3Clinic for Cardiovascular Surgery, Charité University Medicine Berlin, Berlin, Germany
- 4German Heart Centre Munich, Technical University Munich, Munich, Germany
- 5Vienna Health Association, Vienna, Austria
- 6Medical University of Vienna, Vienna, Austria
- 7Clinical Department of Cardiac Surgery, University Department of Surgery, Medical University of Vienna, Vienna, Austria
- 8Sigmund Freud University Vienna, Vienna, Austria
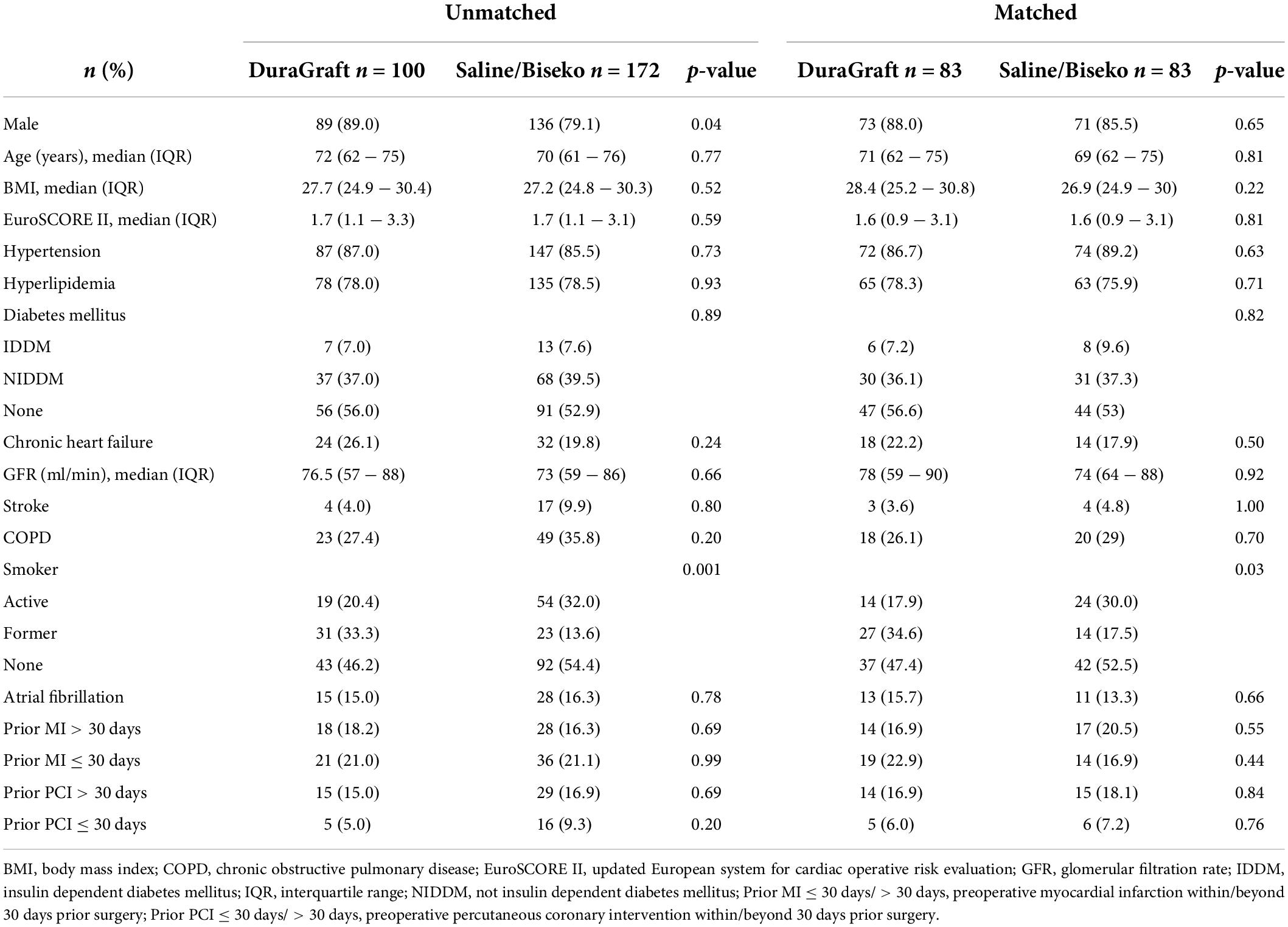
Background: During on-pump coronary artery bypass grafting (ONCAB), graft flushing for distal anastomoses testing also perfuses the downstream myocardium. This single-center retrospective study evaluated the impact of specific preservation solutions on myocardial protection during ONCAB.
Materials and methods: Between July 2019 and March 2020 either DuraGraft (DG) or 0.9% Saline/Biseko (SB) was applied to 272 ONCAB. Overall, 166 patients were propensity-matched into two groups. Cardiac enzymes [high-sensitive Troponin I (hs-TnI) and creatine kinase (CK)] were evaluated 7 days post-surgery.
Results: Post-surgery, hs-TnI values were significantly lower from 3 to 6 h (h) up to 4 days in the DG group: 3–6 h: 4,034 ng/L [IQR 1,853–8,654] vs. 5,532 ng/L [IQR 3,633—8,862], p = 0.05; 12–24 h: 2,420 ng/L [IQR 1,408–5,782] vs. 4,166 [IQR 2,052–8,624], p < 0.01; 2 days: 1,095 ng/L [IQR 479–2,311] vs. 1,564 ng/L [IQR 659–5,057], p = 0.02 and at 4 days: 488 ng/L [IQR 232–1,061] vs. 745 ng/L [IQR 319–1,820], p = 0.03. The maximum value: 4,151 ng/L [IQR 2,056–8,621] vs. 6,349 ng/L [IQR 4,061–12,664], p < 0.01 and the median area under the curve (AUC): 6,146 ng/L/24 h [IQR 3,121–13,248] vs. 10,735 ng/L/24 h [IQR 4,859–21,484], p = 0.02 were lower in the DG group. CK values were not significantly different between groups: maximum value 690 [IQR 417–947] vs. 631 [464–979], p = 0.61 and AUC 1,986 [1,226–2,899] vs. 2,081 [1,311–3,063], p = 0.37.
Conclusion: Repeated graft flushing with DG resulted in lower Troponin values post-surgery suggesting enhanced myocardial protection compared to SB. Additional studies are warranted to further assess the myocardial protection properties of DG.
Introduction
Although graft storage solutions (GSS) are known to influence graft failure rates following coronary artery bypass grafting (CABG), their impact on myocardial protection in the context of ischemia reperfusion injury (IRI) during cardiopulmonary bypass remains unknown. Saphenous vein grafts (SVG) remain the most commonly used conduits in CABG procedures (1). However, SVGs are prone to vein graft disease and failure. While graft failure and the documented early pathohistological or functional changes in SVG are initiated by various means including underlying harvesting techniques (2), inadequate tissue protection during graft storage appears to be the main trigger (3). Although saline and autologous blood are frequently used as GSS, their use is inadequate to maintain graft patency compared to subsequent GSS (3–5). DuraGraft (DG; Marizyme, Jupiter FL, United States) is an endothelial damage inhibitor, formulated into a preventive solution to protect the integrity and function of the graft when used in this manner. Several in vitro studies have shown the protective potential of DG (6–9). Recent research demonstrated reduced graft intimal hyperplasia in SVGs treated with DG (10), and a large-scale retrospective analysis from the Boston West Roxbury Veterans Hospital suggested that treatment of SVGs is associated with significantly lower rates of repeat revascularization and major adverse cardiac events compared to saline (11). While DG has demonstrated its capacity for vascular graft preservation, its potential for myocardial protection in on-pump coronary artery bypass grafting (ONCAB) after intracoronary perfusion by graft flushing for distal anastomosis leak testing during cardiopulmonary bypass remains unclear. In this study, we examined the impact of DG on myocardial protection. This was evaluated by assessing the post-operative biomarker release profile following SVG storage in DG and its downstream intracoronary infusion (via the distal anastomosis) in patients undergoing ONCAB procedures and compared to 0.9% Saline/Biseko (SB; control).
Patients and methods
Study design
This study was a retrospective single-center analysis of cardiac biomarker release in patients undergoing ONCAB whose SVGs were treated with DG or SB at the Vienna Heart Center, Floridsdorf Nord, Austria between July 2019 and March 2020. Both GSS were applied separately in different subsequent time intervals. Every patient underwent ONCAB with the treatment of at least one SVG with one of the indicated GSS. Follow-up was conducted by outpatient management and telephone follow-up to obtain information on post-operative myocardial infarction (MI), repeat revascularization, and death. Mortality data were obtained by request from the mortality registry of Statistic Austria, the Austrian statistical office. Ethics approval for the study protocol was received by the local ethics committee.
Surgical technique
In all ONCAB procedures, aortic cross-clamping was implemented. Cardioplegia was applied in standard antegrade and/or retrograde fashion using cold blood cardioplegia. SVGs were harvested by either the open or endoscopic technique. After harvesting and preparation, grafts were stored and flushed either in DG or SB until distal anastomosis was carried out. If arterial grafts were used, only the radial artery was stored in either GSS. After completion of the distal anastomosis in sequential, graft-to-graft, or direct single anastomosis fashion, a careful repeated graft flushing with the respective GSS was performed for leak testing.
Laboratory evaluation of cardiac markers
Cardiac enzymes high-sensitive Troponin I (hs-TnI) and creatine kinase (CK) were measured pre-surgery, 1–3, 3–6, and 12–24 h after CABG, and once daily up to 7 days post-CABG. Measurement of both cardiac markers was conducted each by the Abbott Alinity assay. Hs-TnI-related detection limits are 1 ng/L, with sex-specific normal upper reference limit of 34.2 ng/L for men and 15.6 ng/L for women. CK-related detection limits are 7 U/L, with sex-specific normal upper reference limit of 200 U/L for men and 168 U/L for women.
Graft storage solutions
DuraGraft (Marizyme, Jupiter FL, United States) is a GSS which preserves endothelial function and structure during CABG procedures. DG contains L-glutathione, L-ascorbic acid, L-arginine, glucose, and balanced salts for minimizing ischemic and metabolic damage to the conduits during graft preservation, graft handling, and IRI. Moreover, the pH is buffered in the physiologic range. Biseko (Biotest Pharma GmbH, Dreieich, Germany), which is known to be used off-label for graft storage, is an ionized plasma derivate containing human serum protein, albumin, and human immunoglobulin. Saline 0.9% was added due to the lower volume of Biseko. Additionally, heparin was added to both DG and SB during their preparation for surgical application.
Statistical analysis
Categorical variables, shown as numbers and percentage, were compared by Chi-Square test or Fisher-Exact test for small sample size correction. Continuous variables, expressed as median and interquartile range (IQR), were compared by Mann–Whitney–U-test. Propensity score matching (PSM) was performed to minimize potentially responsible bias for the evaluation of biomarker values and cardiac adverse events between both study groups. Matching was conducted by a multivariate logistic model, which consisted of 1-to-1 patient pair formation, nearest-neighbor matching, and no case-replacement. The corresponding caliper of 0.15 was chosen. The model incorporated the following variables: additional cardiac procedure, age, acute surgery, aortic clamp time, extracorporeal circulation time, glomerular filtration rate, left main stenosis, number of diseased vessels, number of CABG, number of distal anastomoses, number of venous CABG, number of free CABG, prior percutaneous coronary intervention (PCI) ≤30 days, prior PCI >30 days, prior MI ≤30 days, and prior MI >30 days. Covariate balancing was evaluated according to calculating standardized mean differences with a value of ≤0.2 considered as sufficient equilibrium of these covariates between the groups (Figure 1). Differences in prevalence of baseline and procedural variables between both groups of patients were evaluated by diagnostic odds ratios according to logistic regression and effect size estimation according to standardized mean differences. The area under the curve (AUC) was calculated from the plasma biomarker concentrations vs. the time after surgery. Data analysis was conducted by SPSS statistical software version 25 (IBM Corp, Armonk, NY, United States). P-values of <0.05 were considered statistically significant.
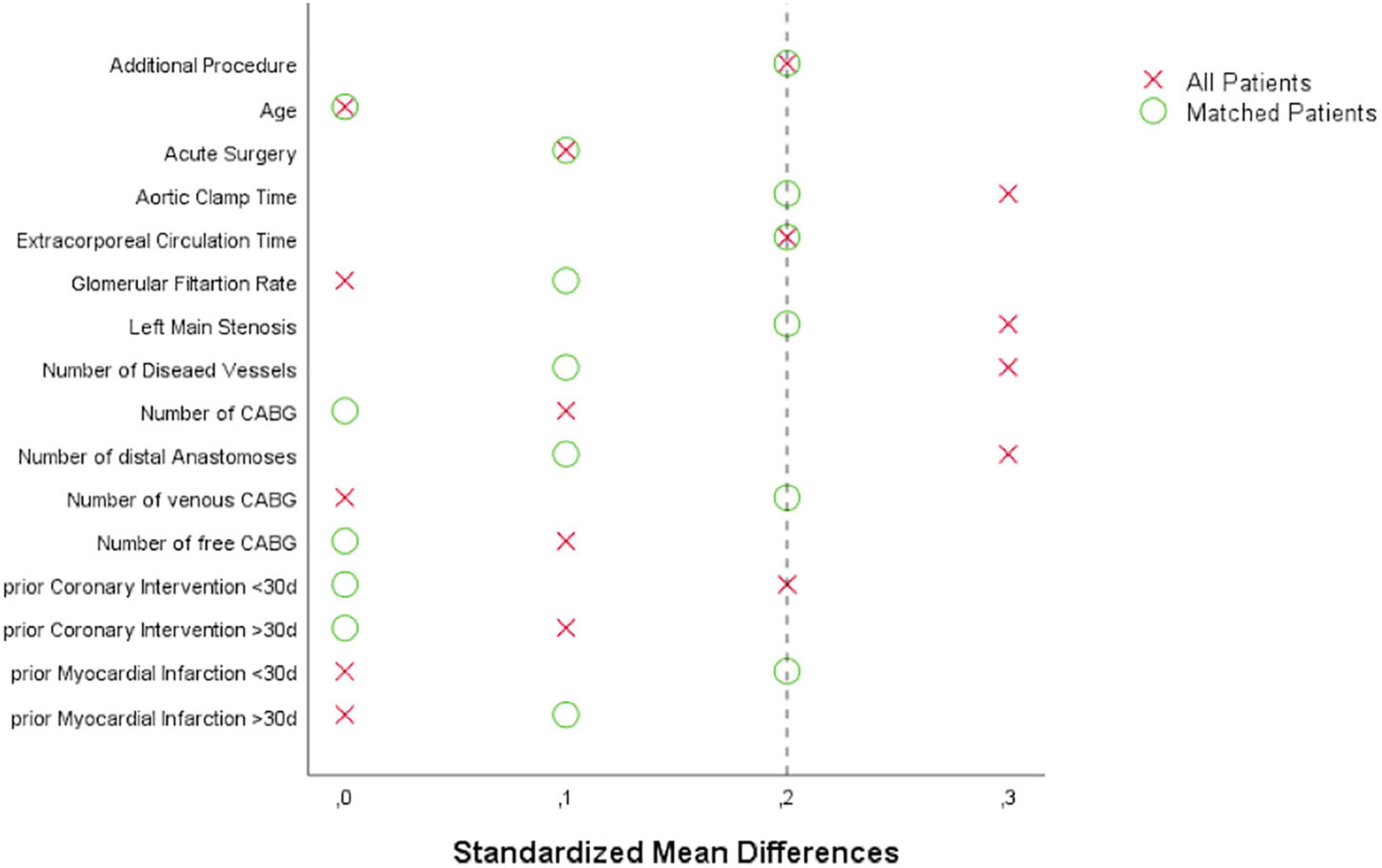
Figure 1. Love plot visualizing covariate balancing according to standardized mean differences before and after propensity score matching: CABG, coronary artery bypass grafting.
Results
Pre- and peri-procedural data
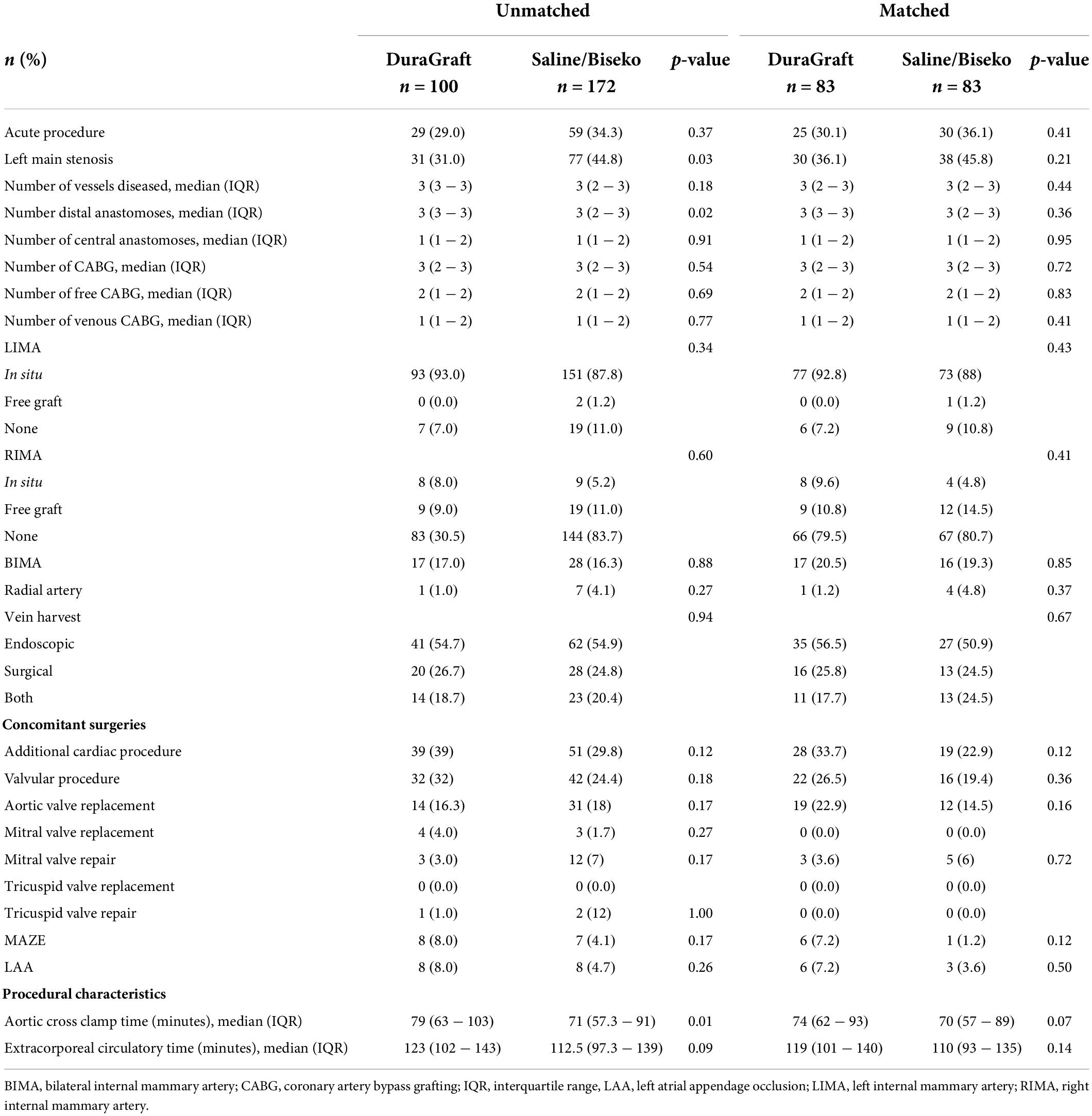
The SVGs of 100 patients were treated with DG and 172 patients with SB. After PSM, 83 patients within each group were selected. The corresponding Love plot (Figure 1) provides visualization of covariate balancing of all included baseline (Table 1) and procedural variables (Table 2) in the propensity model. The included covariates were equally distributed between both groups according to standardized mean differences, which did not exceed rounded maximum values of 0.2. Therefore, the conducted PSM might be considered valid. Moreover, diagnostic odds ratios with associated 95% confidence intervals and effect size according to given standardized mean differences of all baseline and procedural variables of the propensity-matched patient population are presented in a given Forest plot (Figure 2). After PSM, only documented smokers (DG: 17.9 vs. SB: 30.0%; p = 0.03) and those on whom the concomitant MAZE procedure (DG: 7.2 vs. SB: 1.2%; p = 0.12) were conducted were distributed unequally between both groups according to an increased effect size for the given variables, with values of standardized mean differences of 0.265; OR: 1.38; 95% CI: 0.94–2.01, and 0.301; OR: 6.39; 95% CI: 0.75–54.29, respectively. Although both variables revealed a higher prevalence within the DG group, both groups appeared overall homogenized after PSM as the remaining baseline and procedural characteristics were equally distributed within the patient population (Figure 2).
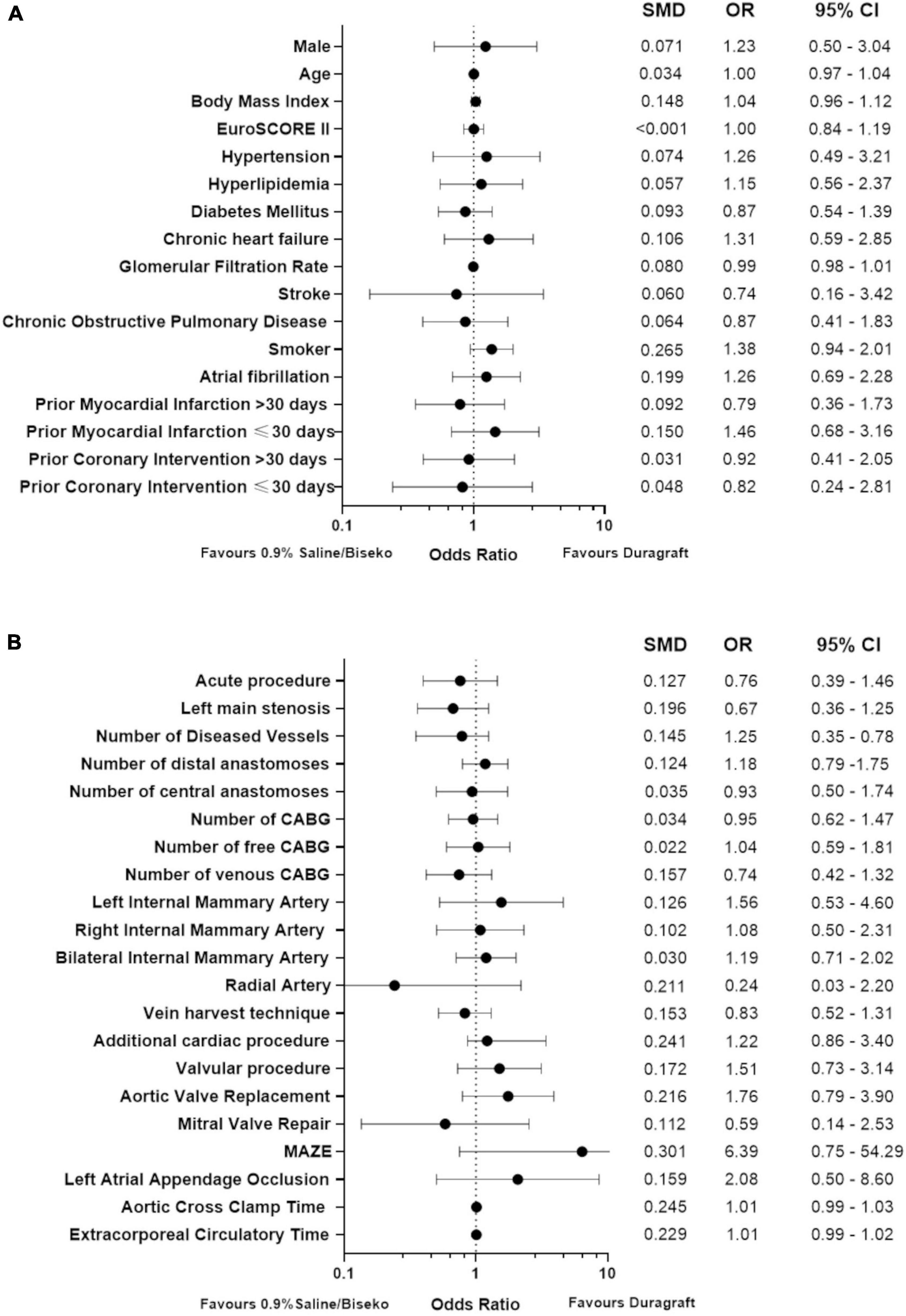
Figure 2. Forest plot presenting diagnostic odds ratios and effect size of baseline (A) and procedural variables (B) after propensity score matching; CABG, coronary artery bypass grafting; SMD, standardized mean differences, OR, odds ratio, 95% CI, 95% confidence interval.
Evaluation of cardiac biomarkers
Data on post-procedural laboratory values (Tables 3, 4) revealed a typical post-operative monophasic course for both hs-TnI and CK (Figure 3). Hs-TnI values were comparable pre-surgery (DG: 11 [5–28] vs. SB: 8 [3–28], p = 0.22). Post-surgery, hs-TnI values were significantly lower from 3 to 6 h until the measurement at 4 days in the DG group: 3–6 h: 4,034 ng/L [IQR 1853–8654] vs. 5,532 ng/L [IQR 3633–8862], p = 0.05; 12–24 h: 2,420 ng/L [IQR 1408–5782] vs. 4166 [IQR 2052–8624], p < 0.01; 2 days: 1,095 ng/L [IQR 479–2311] vs. 1,564 ng/L [IQR 659–5057], p = 0.02, and at 4 days: 488 ng/L [IQR 232–1061] vs. 745 ng/L [IQR 319–1820], p = 0.03. Noteworthy, hs-TnI values only reached borderline significance at 3 days: 709 ng/L [IQR 321–1696] vs. 950 ng/L [IQR 332–2105], p = 0.08. The maximum value and the median AUC were also significantly lower after graft treatment with DG (maximum value: 4,151 ng/L [IQR 2056–8621] vs. 6,349 ng/L [IQR 4061–12664], p < 0.01 and AUC: 6,146 ng/L/24 h [IQR 3121–13248] vs. 10,735 ng/L/24 h [IQR 4859–21484], p = 0.02). CK values in the DG group appeared to be slightly higher at 1–3 h (388 U/L [IQR 286–563) vs. 347 U/L [IQR 267–483], p = 0.05, while lower values in DG patients at 4 days (161 U/L, [IQR 99–276] vs. 208 U/L (136–324), p = 0.07) and at 6 days (85 U/L [IQR 55–169] vs. 108 U/L [IQR 74–160], p = 0.09) did not reach complete statistical significance.
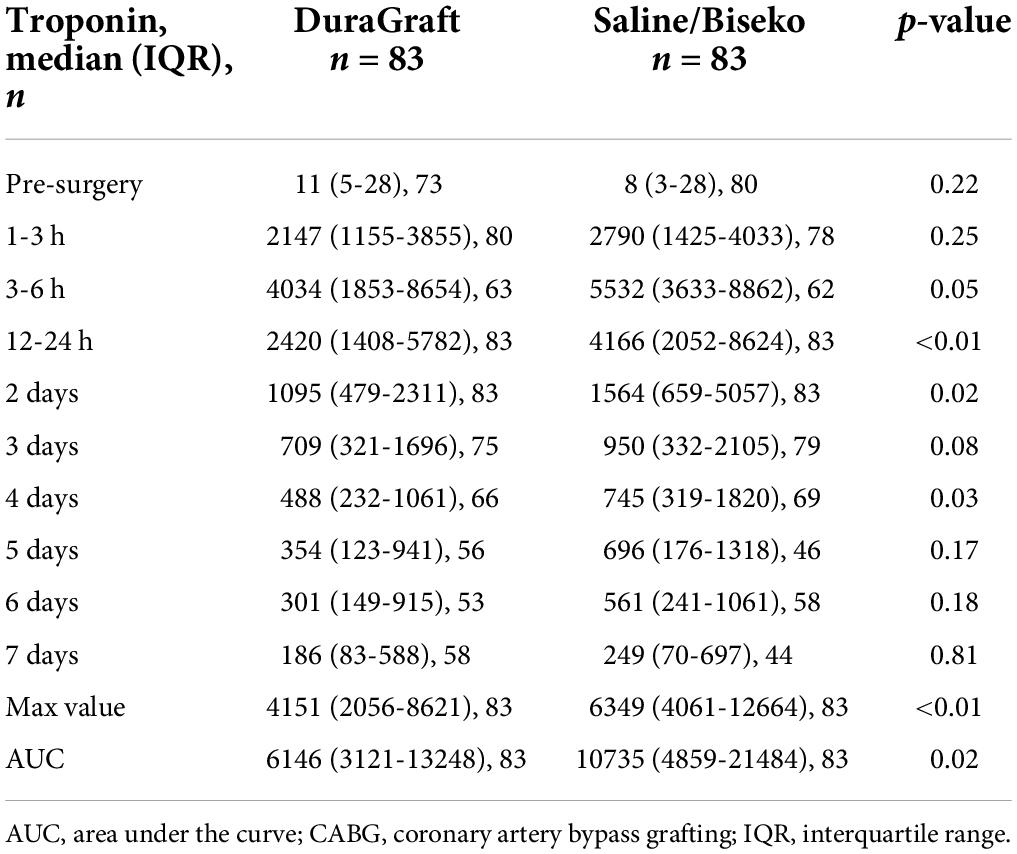
Table 3. Median values and interquartile ranges of high-sensitive Troponin I in nanograms/liter post-CABG after propensity matching.
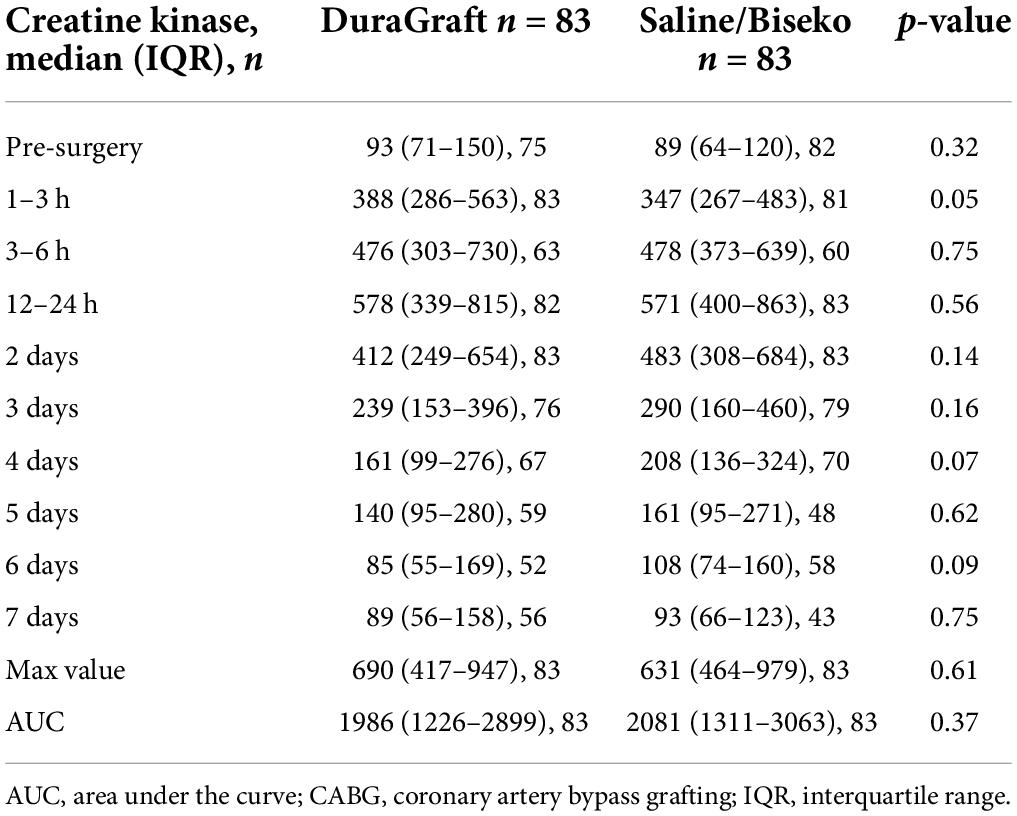
Table 4. Median values and interquartile ranges of creatine kinase in units/liter post-CABG after propensity matching.
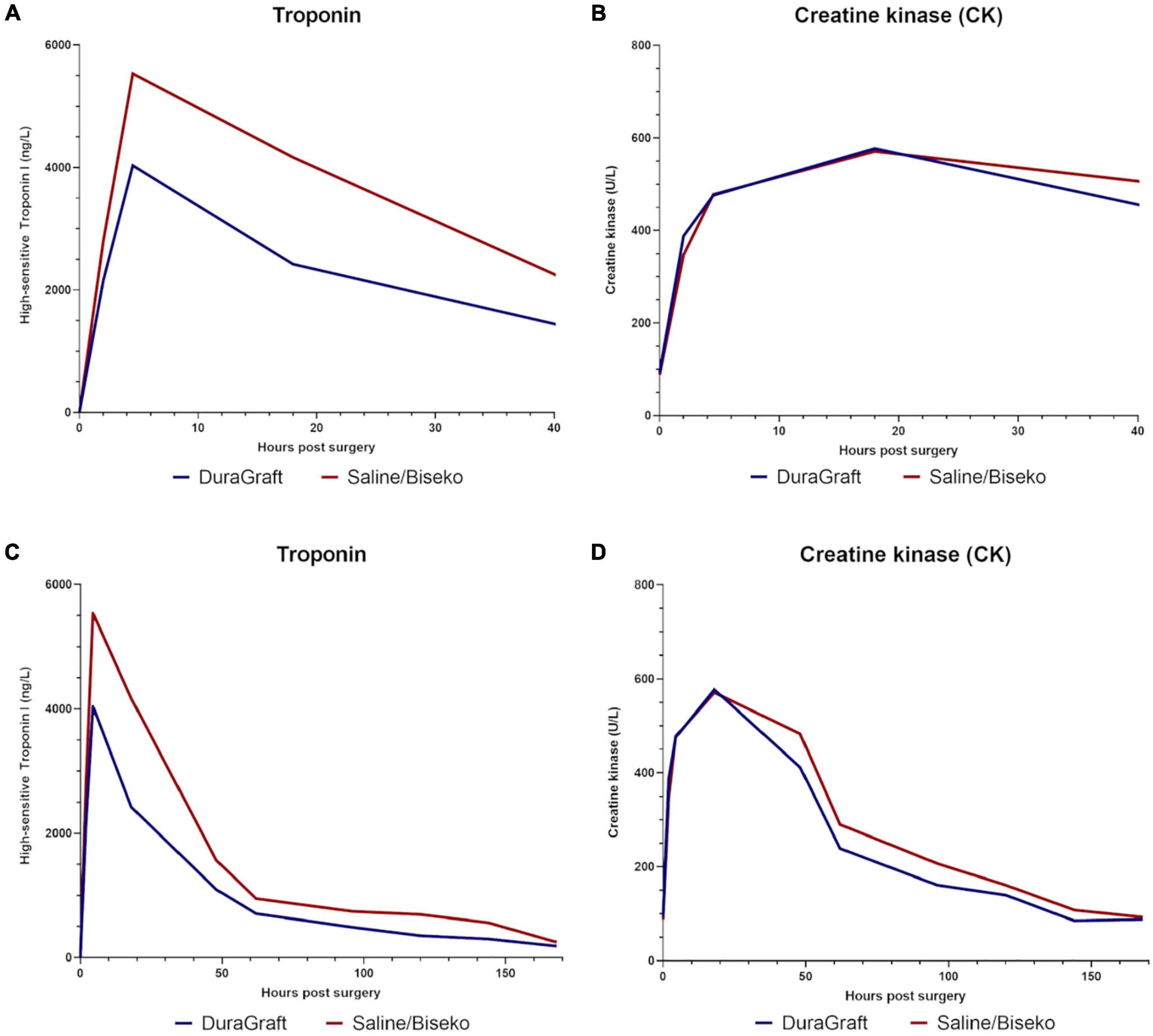
Figure 3. Median values of high-sensitive Troponin I in nanogram/liter and creatine kinase in units/liter within the first hours post-surgery (A,B), and during the overall hospital stay (C,D).
Adverse events
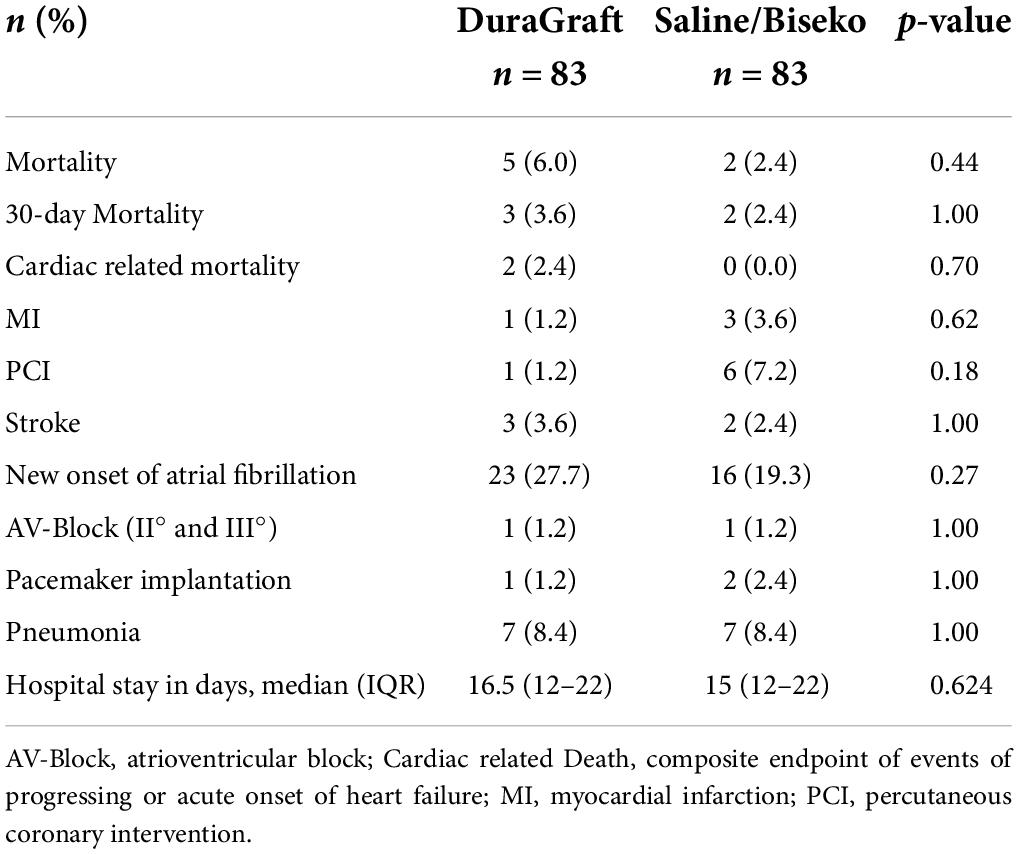
After PSM, the median hospital stay in days (DG: 16.5 [IQR 12–22] vs. SB: 15 [IQR 12–22]; p = 0.62), all-cause mortality (DG: 6.0 vs. SB: 2.4%, p = 0.44), and cardiac-related mortality (DG: 2.4 vs. SB: 0.0%, p = 0.70) did not differ between both groups over the median follow-up of 4 (IQR 0–22) months (Table 5). Noteworthy, all mortality events occurred within 1-year of follow-up. Progression of heart failure was the cause of death in the four patients with cardiac death.
Comment
This study shows that the use of DG for leak testing during distal anastomosis and its subsequent application to the downstream myocardium appears to be associated with improved myocardial protection in patients undergoing ONCAB, identified by significantly lower hs-TnI levels including the maximum value and AUC during the early post-operative phase after CABG when compared to SB. In contrast, values of CK were comparable between both groups within the observation period, which may be due to CK being a non-specific cardiac enzyme, while Troponin I is highly specific for myocardial injury (12).
Several factors influence cardiac marker increase, such as the complexity of the CABG procedure (13, 14) and renal impairment (15). From a clinical perspective, the observed overall lower early post-operative Troponin levels in the DG group might be a predictor of better overall patient outcomes when considering that increased Troponin values have been described to predispose to increased early and long-term mortality and cardiac-related complication rates (13, 14, 16).
In our study, peak values for hs-TnI were reached within temporal proximity of 3–6 h in both groups post-surgery, and the most significant difference in absolute hs-TnI values was seen at 12–24 h after surgery. Afterward, in both groups, values decreased in a logistic linear fashion. Overall, enzyme increase during ONCAB indicates ischemic myocardial damage (17). Sufficient administration of cardioplegia for adequate myocardial protection during on-pump cardiac surgery is crucial. Insufficient protection of the heart during on-pump runs leads to increased local metabolic stress which can be particularly problematic for the right ventricle and in the context of inefficient retrograde application of cardioplegia (18). Since DG has been designed to prevent IRI in vascular conduits, one may hypothesize that additional specific administration of DG into the downstream myocardium via systematic flushing of the distal anastomosis during leak testing may protect the downstream myocardium from IRI as well and enhance myocardial protection, which was reflected by significantly lower post-operative troponin values in our study.
DuraGraft, as well as its precursor GALA solution, on which the basis of DG was developed, has demonstrated superiority in preserving tissue functionality over saline and other GSS in human venous and free arterial conduits by reducing oxidative stress (6, 8, 9) and through the actions of L-arginine (a key component of DG) to sustain NO concentration, thereby maintaining endothelial function and preventing hyperplasia (19). In direct comparison, saline fails to prevent oxidative damage and downstream IRI (6), while its acidic pH (5.5) might induce solution damage as well (3).
On the other hand, Biseko enables superior preservation of graft patency by decreased risk of vasospasm (20) and endothelial pressure damage due to graft flushing (21) when compared to saline; however, Biseko does not protect against ischemic injury. One may speculate that in line with its established protective impact on the endothelium of vascular conduits (6, 8, 9), DG might also have similar beneficial effects on the endothelial integrity and function of the coronary vasculature in the treated territory, which could ultimately explain myocardial protection. This might be particularly driven by L-, which is one of the main components in DG, and which is known (i) to enhance coronary blood flow by enhanced vasodilation following intracoronary application (22) and simultaneously (ii) to reduce IRI (23).
However, if and to what extent the observed positive effects on myocardial protection and its underlying mechanisms are directly comparable to the previously described protective impact of DG on vascular conduits needs further in-depth investigation. Nevertheless, if proven valid, this concept could have great clinical relevance in future on-pump revascularization strategies, especially when considering the frequently seen challenges with inadequate myocardial protection and associated poor recovery of the right ventricle after CABG procedures.
Limitations
This was a retrospective, non-randomized study, hence all established limitations do apply. No additional cardiac imaging for evaluation of myocardial protection and graft patency was conducted. Intraoperative intracoronary flow measurement could not be evaluated retrospectively. Measurement of the myocardial fraction of CK and CK-MB was not done in this study, but may have yielded statistically significant differences, and thus, may be considered for future studies. Finally, this study was not powered for clinical outcome events.
Conclusion
In this study, the administration of DG into the downstream myocardium during graft flushing for leak testing of the distal anastomosis was associated with improved perioperative myocardial protection as measured by significantly lower troponin levels when compared to SB in patients undergoing ONCAB.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethic committee of the city of Vienna. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PS: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing – original draft, and writing – review and editing. ME and BW: conceptualization, investigation, methodology, project administration, supervision, validation, and writing – review and editing. PH, ZA, IC, MM, TA, and MG: conceptualization and writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Department of Cardiac Surgery Floridsdorf and a grant of the Karl Landsteiner Institute Vienna awarded to the PI and corresponding author BW.
Conflict of interest
Author ME was the PI of the European DuraGraft registry and the chair of the registry scientific advisory committee and is a consultant to Somahlution.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, area under the curve; CABG, coronary artery bypass grafting; CK, creatine kinase; DG, duragraft; GSS, graft storage solution; Hs-TnI, high-sensitive Troponin I; IRI, ischemia reperfusion Injury; IQR, interquartile range; MI, myocardial infarction; ONCAB, On-pump coronary artery bypass grafting; PCI, percutaneous coronary intervention; PSM, propensity score matching; SB, saline/biseko; SVG, saphenous vein grafts.
References
1. Caliskan E, de Souza DR, Böning A, Liakopoulos OJ, Choi YH, Pepper J, et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. (2020) 17:155–69.
2. Johansson BL, Souza DS, Bodin L, Filbey D, Loesch A, Geijer H, et al. Slower progression of atherosclerosis in vein grafts harvested with ‘no touch’ technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. (2010) 38:414–9. doi: 10.1016/j.ejcts.2010.02.007
3. Harskamp RE, Alexander JH, Schulte PJ, Brophy CM, Mack MJ, Peterson ED, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg. (2014) 149:798–805. doi: 10.1001/jamasurg.2014.87
4. Wilbring M, Tugtekin SM, Zatschler B, Ebner A, Reichenspurner H, Matschke K, et al. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur J Cardio Thoracic Surg. (2011) 40:811–5. doi: 10.1016/j.ejcts.2011.01.024
5. Wilbring M, Tugtekin SM, Zatschler B, Ebner A, Reichenspurner H, Kappert U, et al. Preservation of endothelial vascular function of saphenous vein grafts after long-time storage with a recently developed potassium-chloride and N-acetylhistidine enriched storage solution. Thorac Cardiovasc Surg. (2013) 61:656–62. doi: 10.1055/s-0032-1311549
6. Pachuk CJ, Rushton-Smith SK, Emmert MY. Intraoperative storage of saphenous vein grafts in coronary artery bypass grafting. Expert Rev Med Devices. (2019) 16:989–97.
7. Thatte HS, Biswas KS, Najjar SF, Birjiniuk V, Crittenden MD, Michel T, et al. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg. (2003) 75:1145–52. doi: 10.1016/s0003-4975(02)04705-7
8. Aschacher T, Baranyi U, Aschacher O, Eichmair E, Messner B, Zimpfer D, et al. A novel endothelial damage inhibitor reduces oxidative stress and improves cellular integrity in radial artery grafts for coronary artery bypass. Front Cardiovasc Med. (2021) 8:736503. doi: 10.3389/fcvm.2021.736503
9. Tekin I, Demir M, Özdem S. Effect of different storage solutions on oxidative stress in human saphenous vein grafts. J Cardiothorac Surg. (2022) 17:7. doi: 10.1186/s13019-022-01752-7
10. Perrault LP, Carrier M, Voisine P, Olsen PS, Noiseux N, Jeanmart H, et al. Sequential multidetector computed tomography assessments after venous graft treatment solution in coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2019). [Online ahead of print]. doi: 10.1016/j.jtcvs.2019.10.115
11. Haime M, McLean RR, Kurgansky KE, Emmert MY, Kosik N, Nelson C, et al. Relationship between intra-operative vein graft treatment with DuraGraft® or saline and clinical outcomes after coronary artery bypass grafting. Expert Rev Cardiovasc Ther. (2018) 16:963–70.
12. de Castro Martínez J, Vázquez Rizaldos S, Velayos Amo C, Herranz Valera J, Almería Varela C, Iloro Mora MI. [Cardiac troponin I in perioperative myocardial infarction after coronary artery bypass surgery]. Rev Esp Cardiol. (2002) 55:245–50.
13. Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. (2006) 114:1468–75.
14. Nesher N, Alghamdi AA, Singh SK, Sever JY, Christakis GT, Goldman BS, et al. Troponin after cardiac surgery: a predictor or a phenomenon? Ann Thorac Surg. (2008) 85:1348–54.
15. Nam K, Shin KW, Kim TK, Kim KH, Kim KB, Jeon Y, et al. Prognostic value of high-sensitivity troponin I after cardiac surgery according to preoperative renal function. Medicine. (2020) 99:e20040. doi: 10.1097/MD.0000000000020040
16. Devereaux PJ, Lamy A, Chan MTV, Allard RV, Lomivorotov VV, Landoni G, et al. High-sensitivity troponin I after cardiac surgery and 30-day mortality. N Engl J Med. (2022) 386:827–36.
17. Chowdhury UK, Malik V, Yadav R, Seth S, Ramakrishnan L, Kalaivani M, et al. Myocardial injury in coronary artery bypass grafting: on-pump versus off-pump comparison by measuring high-sensitivity C-reactive protein, cardiac troponin I, heart-type fatty acid-binding protein, creatine kinase-MB, and myoglobin release. J Thorac Cardiovasc Surg. (2008) 135:1110–9. doi: 10.1016/j.jtcvs.2007.12.029
18. Kaukoranta PK, Lepojärvi MV, Kiviluoma KT, Ylitalo KV, Peuhkurinen KJ. Myocardial protection during antegrade versus retrograde cardioplegia. Ann Thorac Surg. (1998) 66:755–61.
19. Kown MH, Yamaguchi A, Jahncke CL, Miniati D, Murata S, Grunenfelder J, et al. L-arginine polymers inhibit the development of vein graft neointimal hyperplasia. J Thorac Cardiovasc Surg. (2001) 121:971–80.
20. Szolnoky J, Ambrus N, Szabó-Biczók A, Bogáts G, Papp JG, Varró A, et al. Biseko colloidal solution diminishes the vasoreactivity of human isolated radial arteries. Eur J Cardiothorac Surg. (2009) 36:143–7. doi: 10.1016/j.ejcts.2009.03.044
21. Weiss DR, Juchem G, Eblenkamp M, Kemkes BM, Gansera B, Geier M, et al. Search for optimized conditions for sealing and storage of bypass vessels: influence of preservation solution and filling pressure on the degree of endothelialization. Int J Clin Exp Med. (2010) 3:10–27.
22. Hiramatsu T, Forbess JM, Miura T, Mayer JE Jr. Effect of L-arginine cardioplegia on recovery of neonatal lamb hearts after 2 hours of cold ischemia. Ann Thorac Surg. (1995) 60:1187–92. doi: 10.1016/0003-4975(95)00698-K
Keywords: vein graft preservation, storage solutions, myocardium, protection, coronary artery
Citation: Szalkiewicz P, Emmert MY, Heinisch PP, Arnold Z, Crailsheim I, Mach M, Aschacher T, Grabenwöger M and Winkler B (2022) Graft preservation confers myocardial protection during coronary artery bypass grafting. Front. Cardiovasc. Med. 9:922357. doi: 10.3389/fcvm.2022.922357
Received: 17 April 2022; Accepted: 07 July 2022;
Published: 28 July 2022.
Edited by:
Dennis W. T. Nilsen, Stavanger University Hospital, NorwayReviewed by:
Rune Haaverstad, Haukeland University Hospital, NorwayMarcos Lessa, Oswaldo Cruz Institute, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2022 Szalkiewicz, Emmert, Heinisch, Arnold, Crailsheim, Mach, Aschacher, Grabenwöger and Winkler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernhard Winkler, bernhard.winkler@gesundheitsverbund.at
 Philipp Szalkiewicz1
Philipp Szalkiewicz1  Maximilian Y. Emmert
Maximilian Y. Emmert Markus Mach
Markus Mach Bernhard Winkler
Bernhard Winkler