- Department of Head and Neck Surgery, Liaoning Cancer Hospital & Institute, Shenyang, China
Objective: To investigate the relationship between TSH suppression therapy and cardiovascular events in patients with thyroid cancer after surgery.
Methods: Pub Med, Web of Science, and Embase databases were retrieved to collect studies related to the risk of cardiovascular events in patients treated with TSH suppression after thyroid cancer surgery. RevMan statistical software was used for meta-analysis.
Results: A total of 14 studies were included. The mean heart rate of patients after thyroid cancer surgery was higher than that of the control group (SMD=2.59, 95% CI: -0.37,.54), and the risk of atrial fibrillation was increased compared with the control group (RR = 1.52, 95%CI, 1.28-1.81; I = 63%). Ejection fraction and left ventricular end-diastolic diameter were not significantly different between the two groups, ejection fraction SMD = -0.10, 95% CI: -3.73, 3.52, left ventricular end-diastolic diameter SMD = -0.09, 95% CI: - 1.29, 1.11. Patients with TSH suppression after thyroid cancer had higher mean systolic blood pressure than controls (SMD = 1.97, 95% CI: −1.09, 5.03) and mean diastolic blood pressure (SMD = 1.85, 95% CI: -0.15, 3.85).
Conclusion: Meta-analysis concluded that TSH suppression therapy after thyroid cancer surgery increases the risk of atrial fibrillation in patients. In addition, the heart rate, systolic blood pressure and diastolic blood pressure are higher than those in the control group, and there is no significant difference in ejection fraction and left ventricular end-diastolic diameter.
1 Introduction
Differentiated Thyroid Carcinoma (DTC) includes papillary thyroid carcinoma, follicular thyroid carcinoma, and medullary carcinoma. At present, the standard treatment is mainly surgical treatment, supplemented by postoperative endocrine therapy, radioactive iodine therapy and targeted therapy, and the prognosis is good. Long-term thyroid-stimulating hormone (TSH) suppression therapy is the standard of care after surgery for differentiated thyroid cancer (1). In order to inhibit the secretion of thyrotropin, it is necessary to give a certain dose of physiological dose of thyroxine, of which the most widely used is levothyroxine sodium tablets.
However, exogenous subclinical hyperthyroidism characterized by long-term TSH suppression is thought to be associated with adverse cardiovascular effects including tachyarrhythmias, ischemic heart disease, and hypertension (2, 3). It is worth emphasizing, however, that a cross-sectional study of thyroxine-induced heart disease was observed (4), in which higher than recommended doses of the drug were used. In the presence of intrinsic and pre-existing cardiac abnormalities, associated cardiovascular manifestations may be encountered even in milder degrees of thyroxine overdose. However, in a cohort study of up to 28 years of thyroxine treatment (5), no cardiovascular disease-related changes were found. So far, there is no convincing evidence that taking thyrotropin suppression is harmful to the cardiovascular system.
Therefore, this study intends to conduct a Meta-analysis of cardiovascular adverse events and death risk in patients after TSH inhibition for thyroid cancer surgery by systematically reviewing relevant clinical trials published in recent years, in order to provide a reference for clinical treatment plans.
2 Materials and methods
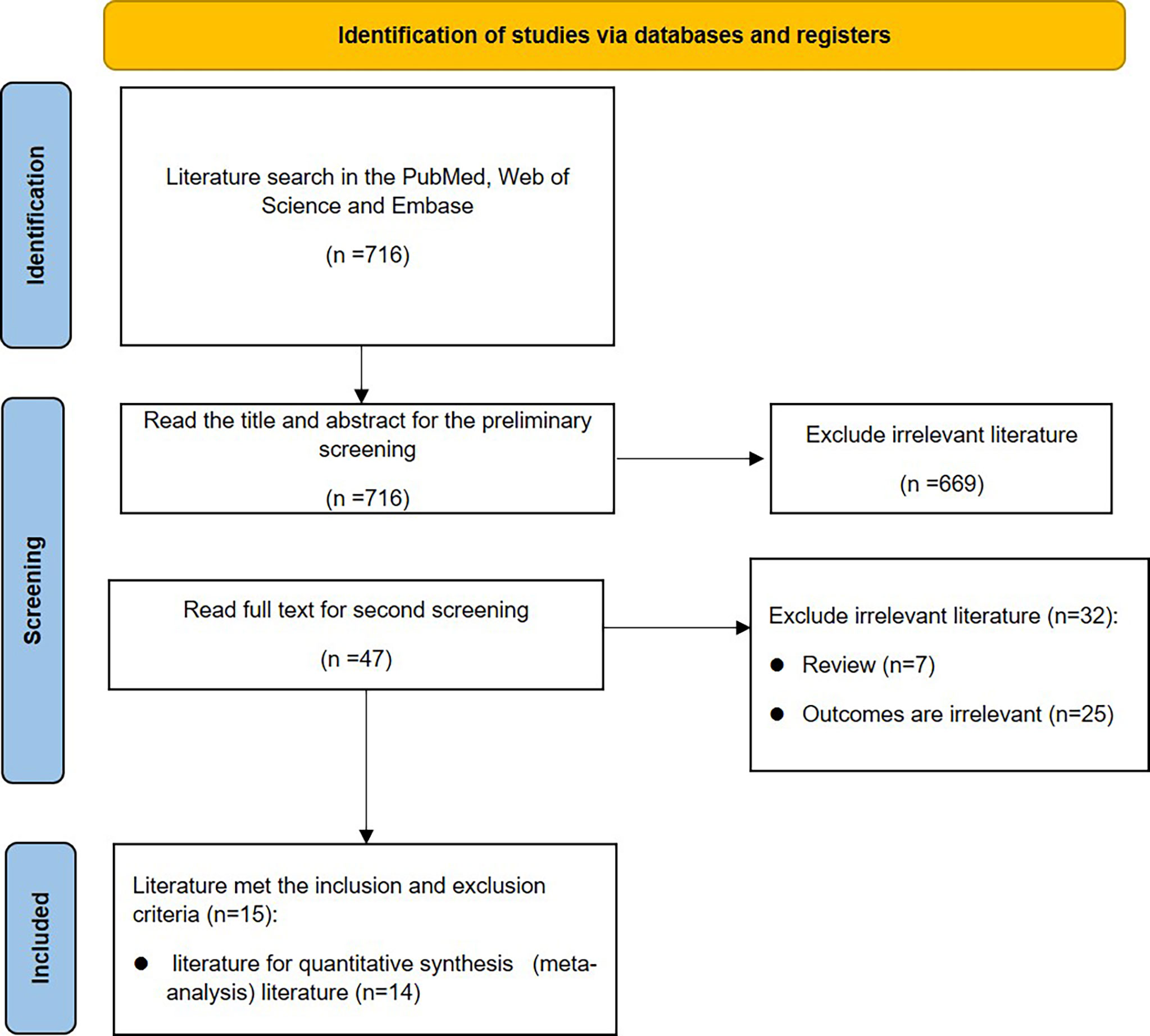
This meta-analysis was conducted in accordance with the PRISMA statement (6). The flowchart is shown in Figure 1.
2.1 Search process
We conducted a systematic literature search through PubMed, Embase and Web of Science, including all relevant literature from the establishment of the database to May 2022. Search by subject heading combined with free words. The search terms were created using the PICO structure. The key terms are as follows: Patient (P) Thyroid Cancer; Intervention (I) Thyroid hormone suppression after thyroidectomy; Outcomes (O) included cardiovascular disease, arrhythmias, myocardial infarction, ventricular dysfunction, heart failure, and cardiac arrest.
2.2 Research options
2.2.1 Inclusion criteria
(1) The original research is a published paper or electronic version; (2) The test method of the type of research should be a case-control study, with complete and reliable data, appropriate statistical methods, and a rigorous control group; (3) Clinical research on the association between TSH suppression after thyroid cancer surgery and the risk of cardiovascular disease; the treatment group was thyroid cancer postoperative patients with cardiovascular disease, the control group is healthy people of the corresponding age group; (4) All patients (case group) have clinical diagnosis of typical cardiovascular disease; (5) Cardiovascular disease is defined as arrhythmia, coronary heart disease, myocardial infarction, essential hypertension, with clear diagnostic and clinical features, such as electrocardiogram, ambulatory blood pressure monitoring, coronary angiography, combined with testing to report abnormal blood lipids, biochemistry, blood pressure or ventricular cardiac function; (6) The included literature provides data on the full TSH inhibitor name and dose.
2.2.2 Exclusion criteria
(1) Case reports, abstracts and reviews, etc.; (2) The experimental design of the original study was not rigorous, or the experimental method did not conform to the case-control study; (3) The diagnostic criteria of the case are not clear; (4) Studies that did not provide the incidence of cardiovascular disease; (5) Documents with incomplete data, and the data could not be obtained after communicating with the author.
2.3 Data extraction and quality assessment
Data sets (XX and XX) were extracted from each eligible study by 2 independent reviewers. Required information includes: author name, year of publication, study design, sample size, mean age of subjects, sex, duration of follow-up, experimental and control group interventions, and outcome measures. Meta-analyses were performed only when 3 or more studies reported the outcome of interest. After the above work is completed, cross-check is carried out, and any disagreements will be discussed and resolved under the arbitration of the third author.
2.4 Evaluation method
The methodological quality of the included SR was assessed using the AMSTAR 2 tool (7). The tool contains a total of 16 items, including seven key items 2, 4, 7, 9, 11, 13, and 15. It is up to the reviewers to judge whether the included literature meets the requirements of the entry, and then divide the credibility level accordingly. If there are 0 or 1 non-critical items that do not meet the requirements, it is rated as “high confidence”; If more than 1 non-critical item does not meet the requirements, it is rated as “medium confidence”; If 1 key item does not meet the requirements, it is rated as “low confidence”; If more than 1 key item does not meet the requirements, with or without non-critical items, it is rated as “very low confidence”.
2.5 Synthesis of evidence
Meta-analysis was performed using RevMan5.4 software to explore the relationship of TSH suppression therapy and the risk of cardiovascular events after thyroid cancer surgery. The continuous data were displayed as mean ± stander division (X̅ ± S) and the categorical variable was displayed as count. First, the heterogeneity test was performed. If I2 ≤ 50%, the fixed effect model was selected; otherwise, the random effect model was used, and subgroup analysis was performed to find the source of heterogeneity. For continuous variable outcome indicators, the mean difference (MD) or standardized mean difference (SMD) was used to judge the effect size; for dichotomous data, the hazard ratio (RR) was used as the combined effect index, and the 95% confidence interval (CI). A funnel plot was used to test for publication bias. Unless otherwise specified, the test level is set at 0.05.
3 Results
3.1 The characteristics of included studies
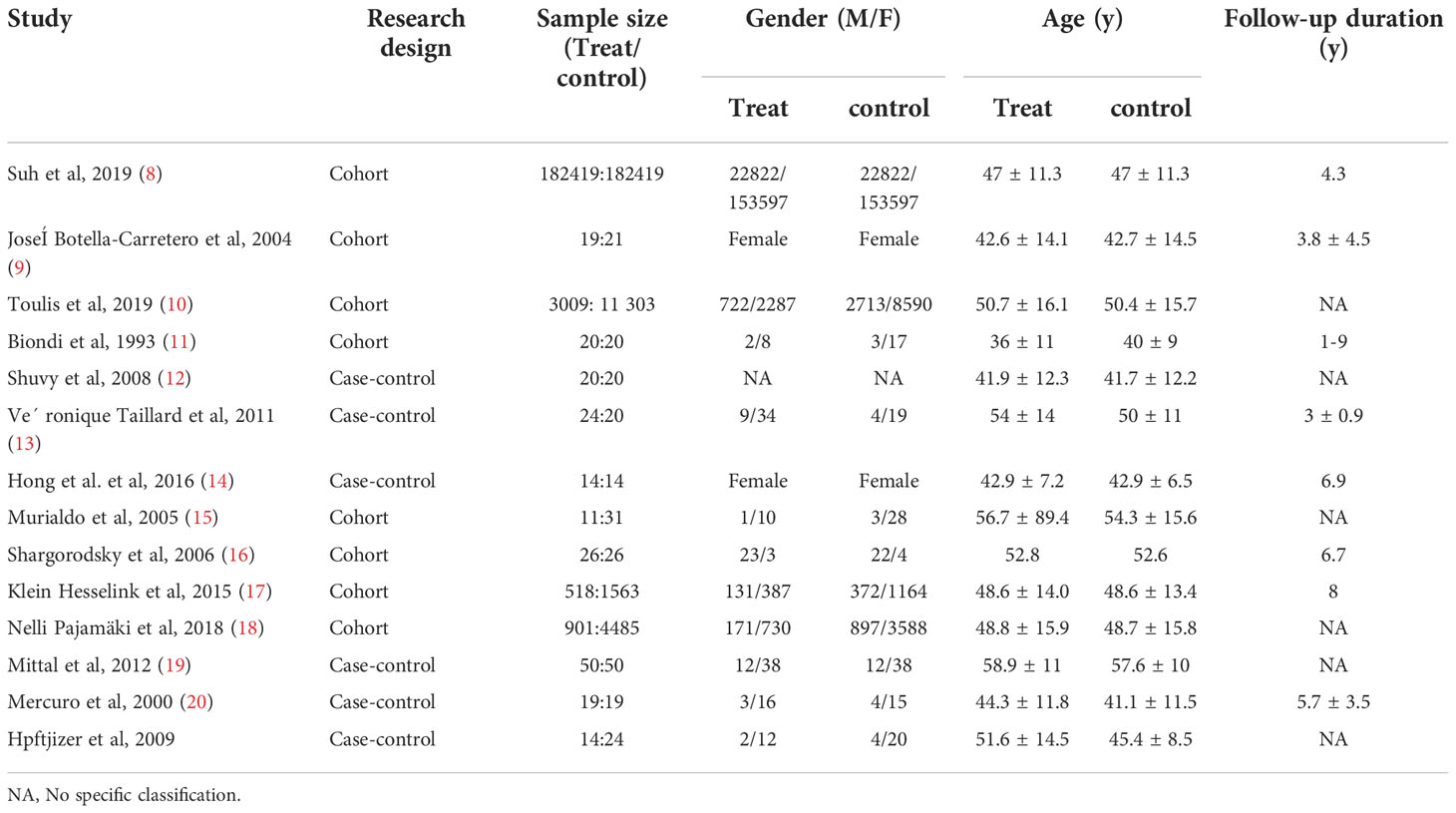
Initially, 714 articles were retrieved. After reading the title abstract, 47 papers were obtained, and the full text was excluded from the review and low-quality literature, and 15 papers were finally included, and 14 studies were performed for quantitative analysis. The search flow chart is shown in Figure 1. The publish year of included studies varied 1993-2019, and involved 187,064 patients. The basic characteristics of the included studies are shown in Table 1.
3.2 The effect of TSH inhibition on the risk of arrhythmia
Nine studies showed the effect of TSH inhibition on the risk of arrhythmia. Compared with control group, patients with DTC had a higher mean hear rate (SMD=2.59, 95% CI: -0.37-5.54), but the difference was not statistically significant (P=0.09), shown in Figure 2A. The results of Figure 2B showed that patients had an increased risk of atrial fibrillation compared with control group (RR = 1.52, 95% CI, 1.28-1.81), and the difference was statistically significant (P<0.00001).
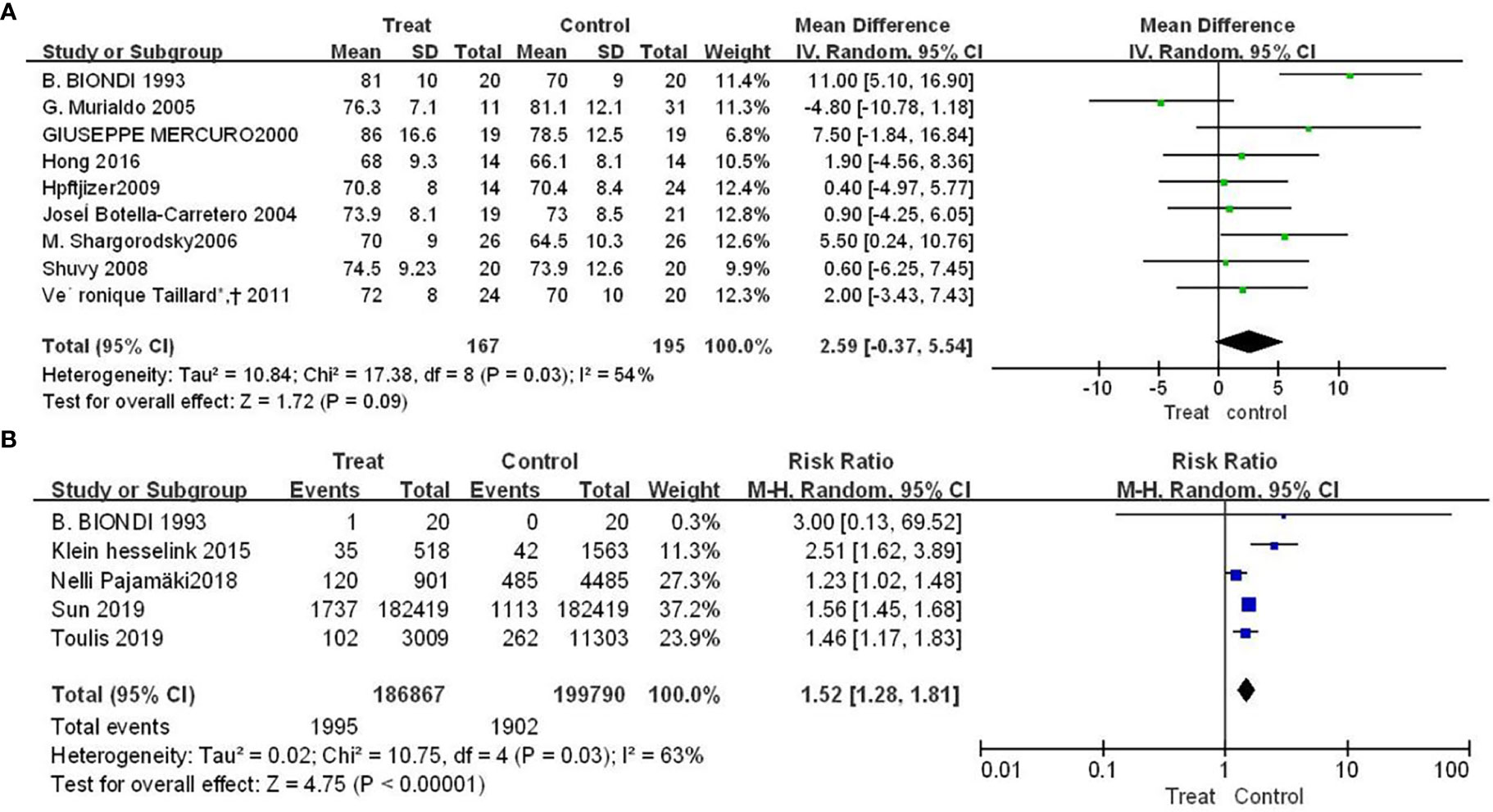
Figure 2 Forest plot of arrhythmia-related adverse events in TSH suppression group and control group, (A) heart rate (B) atrial fibrillation.
3.3 The effect of Euthorac/TSH inhibition on the occurrence of heart failure events
There were three and five studies, respectively, reported the results of Euthorac/TSH inhibition on ejection fraction and left ventricular end-diastolic diameter. The results in Figure 3 showed that the effect of Euthorac/TSH inhibition on the occurrence of heart failure events were not statistically significant. The effect size of ejection fraction was -0.10 (95%CI: -3.73, 3.52; P=0.95; Figure 3A); and the effect size of left ventricular end-diastolic diameter was -0.09 (95% CI: - 1.29, 1.11; P=0.88; Figure 3B).
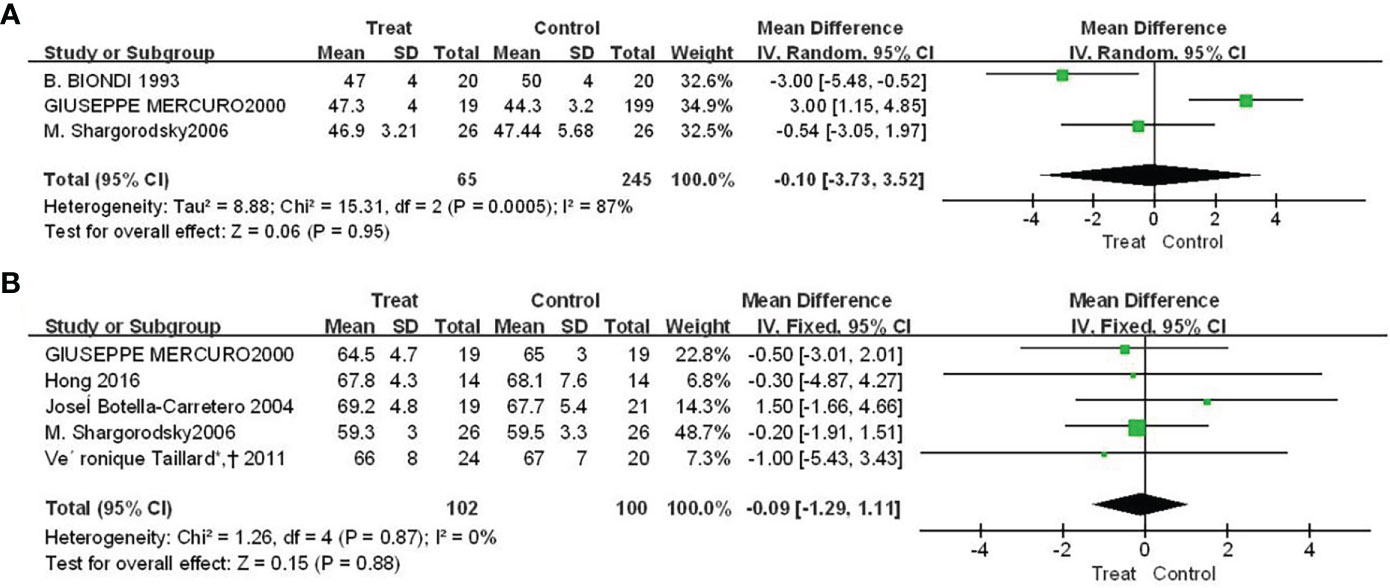
Figure 3 Forest plot comparison of cardiac pumping function between TSH-inhibited group and control group after thyroid cancer surgery. (A) ejection fraction, (B) left ventricular end-diastolic diameter.
3.4 The effect of Euthorac/TSH inhibition on blood pressure
There were four studies reported the results of f Euthorac/TSH inhibition on blood pressure. The heterogeneity evaluation results showed a good homogeneity of the included studies, and the fixed effect model was performed. Patients with TSH suppression after thyroid cancer had higher mean systolic blood pressure than controls (SMD = 1.97, 95% CI: −1.09, 5.03) (Figure 4A), and mean diastolic blood pressure was higher than Control group (SMD = 1.85, 95% CI: -0.15, 3.85) (Figure 4B).
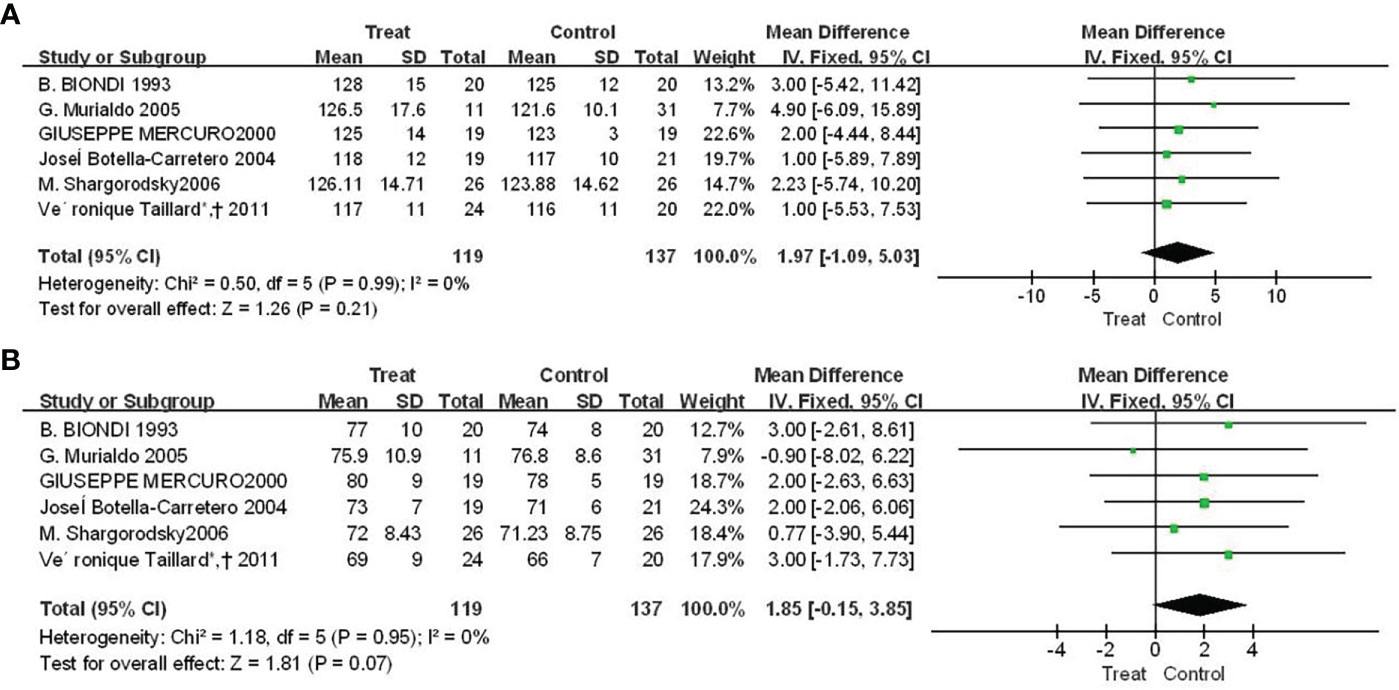
Figure 4 Forest plot of vasoconstriction function between TSH-inhibited group and control group after thyroid cancer surgery. (A) Systolic blood pressure, (B) Diastolic blood pressure.
4 Discussion
The demerits of this meta-analysis showed that, compared with the control group, patients treated with TSH suppression after thyroid cancer had an increased risk of developing atrial fibrillation, but had no significant changes in ejection fraction and left ventricular end-diastolic diameter. Heart rate, systolic and diastolic blood pressure were higher in patients on suppression therapy.
In this study, the patients had a higher risk of developing atrial fibrillation than the control group and had a faster heart rate. Studies have shown that the effects of thyroid hormones on cardiac chronotropy are manifested as increased risk of tachycardia and atrial fibrillation. Thyroid hormones influence cardiac chronotropy through genomic and nongenomic effects on adrenergic-receptor complex components and sodium, potassium, and calcium channels (21). The basic principle behind thyroid hormone suppression therapy is that when patients take supraphysiological doses of thyroid hormone drugs (such as Euthyrox), the thyroid-stimulating hormone (TSH) secreted by the pituitary gland is suppressed at a low level through negative feedback regulation, so as to inhibit the growth and proliferation of thyroid cancer cells and reduce the recurrence rate of thyroid cancer. Thyroid hormone has the effect of exciting the myocardium, which can increase the heart rate and lead to arrhythmia, as well as increase the myocardial contractility, increase the blood pressure, and increase the cardiac load, leading to the side effects of myocardial hypertrophy and even heart failure. There are obvious related side effects in clinical patients with hyperthyroidism. Both hypothyroidism or hyperthyroidism can accelerate the onset of symptomatic cardiovascular disease. Small changes in thyroxine concentrations may have adverse effects on the cardiovascular system. Subclinical thyroid dysfunction is associated with a 20% to 80% increased risk of vascular morbidity and mortality (22). However, TSH suppression is not equivalent to hyperthyroidism, but little attention has been paid to the relationship between TSH suppression and arrhythmia, and there is a lack of randomized, controlled clinical trials in this field. In future research, not only atrial fibrillation and heart rate, but also other arrhythmia-related events such as life-threatening ventricular fibrillation should be addressed.
Ejection fraction and left ventricular end-diastolic diameter did not change significantly. The myocardium is very sensitive to circulating thyroid hormone levels, changes in thyroid hormone status can lead to heart failure, and heart failure itself can alter thyroid hormone status. Individuals with severe long-term hyperthyroidism can increase cardiac output and further lead to symptoms and signs of heart failure. Patients with episodes of hyperthyroidism have higher long-term cardiovascular mortality due to left ventricular hypertrophy, arrhythmias (eg, atrial fibrillation), and increased cardiac preload secondary to fluid overload (23). However, in a small study of levothyroxine treatment, short- and medium-term effects in patients with dilated cardiomyopathy with biochemically reported normal thyroid levels showed that levothyroxine treatment improved left ventricular ejection fraction and cardiac exercise capacity, and decreased diastolic size and systemic vascular resistance, without causing adverse reactions (24). TSH suppression therapy differs from hormone levels in patients with hyperthyroidism. The choice of existing research methods, the size of the sample, and the length of observation time will all affect the analysis of the results. In the absence of mechanistic studies and large randomized controlled trials, the correlation and causality between the two have not been confirmed.
In this study, compared with the control group, the systolic and diastolic blood pressure of the patients increased to varying degrees. The relationship between clinical thyroid hormone levels and hypertension is unclear. Some patients with hyperthyroidism develop systolic hypertension (25). Several observational studies have shown that subclinical hyperthyroidism is not associated with hypertension (26); However, some studies have shown a positive correlation (27), such as the activation of sodium, potassium, and calcium channels in cardiomyocytes and the systemic vasculature via thyroid hormone (21), effects on mitochondrial membranes and mitochondrial production (28), and their relationship to signaling pathways in cardiomyocytes and vascular smooth muscle cells (29). Thyroid hormones activate the PI3K/AKT signaling pathway, induce endothelial nitric oxide production and subsequently reduce systemic vascular resistance (30). Results of a randomized controlled trial showed that levothyroxine treatment improved endothelium-dependent vasodilation in patients with subclinical hypothyroidism (31, 32), although the mechanisms involved are unclear. In addition, the effect of thyroxine on blood pressure is a benefit or a risk, and further research is needed.
Our study has some limitations. First, surgery is the preferred treatment for thyroid and follicular thyroid cancer. However, thyroid and lymph node surgery ranged from conservative to aggressive approaches, which affected the patient’s baseline levels of thyroxine, which in turn affected the effect of TSH suppression on the patient’s hormone levels. In addition, the sample size was small and prospective studies were lacking. Secondly, the data collection of the trial was incomplete, and the trial lacked the records of arrhythmia events of key cardiovascular events. In addition, there were some differences in the collection of some parameters. Ultrasound-related detection was greatly affected by the instrument operator, and many trials lacked this part of the data. Finally, due to differences in baseline and lack of data, TSH levels and cardiovascular adverse events at different doses of TSH inhibitors were not analyzed. Nonetheless, it is necessary to assess the balance of risk for patients with TSH inhibition.
5 Conclusion
Meta-analysis concluded that TSH suppression after thyroid cancer surgery increases the risk of cardiovascular events, but more large-scale long-term prospective studies are needed for further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conception and design of the research: YZ and XY. Acquisition of data: XY, NG, and XG. Analysis and interpretation of the data: XY, JL, and XF. Statistical analysis: XY and YZ. Obtaining financing: None. Writing of the manuscript: XY and NG. Critical revision of the manuscript for intellectual content: YZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Larrad-Jiménez Á., Sánchez-Franco F, Correa R, Vinales K, Corpas E, Harman SM. Endocrinology of aging. Corpas E, editor. Elsevier (2021) p. 267–331.
2. Bin-Hong D, Fu-Man D, Yu L, Xu-Ping W, Bing-Feng B. Effects of levothyroxine therapy on bone mineral density and bone turnover markers in premenopausal women with thyroid cancer after thyroidectomy. Endokrynol Polska (2020) 71:15–20. doi: 10.5603/EP.a2019.0049
3. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New Engl J Med (1994) 331:1249–52. doi: 10.1056/nejm199411103311901
4. Bartalena L, Pinchera A. Levothyroxine suppressive therapy: Harmful and useless or harmless and useful? J endocrinol Invest (1994) 17:675–7. doi: 10.1007/bf03349686
5. Petersen K, Bengtsson C, Lapidus L, Lindstedt G, Nyström E. Morbidity, mortality, and quality of life for patients treated with levothyroxine. Arch Internal Med (1990) 150:2077–81. doi: 10.1001/archinte.1990.00390210063015
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
7. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical Res ed.) (2017) 358:j4008. doi: 10.1136/bmj.j4008
8. Suh B, Shin DW, Park Y, Lim H, Yun JM, Song SO, et al. Increased cardiovascular risk in thyroid cancer patients taking levothyroxine: A nationwide cohort study in Korea. Eur J Endocrinol (2019) 180:11–20. doi: 10.1530/eje-18-0551
9. Botella-Carretero JI, Gómez-Bueno M, Barrios V, Caballero C, García-Robles R, Sancho J, et al. Chronic thyrotropin-suppressive therapy with levothyroxine and short-term overt hypothyroidism after thyroxine withdrawal are associated with undesirable cardiovascular effects in patients with differentiated thyroid carcinoma. Endocrine-related Cancer (2004) 11:345–56. doi: 10.1677/erc.0.0110345
10. Toulis KA, Viola D, Gkoutos G, Keerthy D, Boelaert K, Nirantharakumar K. Risk of incident circulatory disease in patients treated for differentiated thyroid carcinoma with no history of cardiovascular disease. Clin Endocrinol (2019) 91:323–30. doi: 10.1111/cen.13990
11. Biondi B, Fazio S, Carella C, Amato G, Cittadini A, Lupoli G, et al. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J Clin Endocrinol Metab (1993) 77:334–8. doi: 10.1210/jcem.77.2.8345037
12. Shuvy M, Arbelle JE, Grosbard A, Katz A. A simple test of one minute heart rate variability during deep breathing for evaluation of sympatovagal imbalance in hyperthyroidism. Israel Med Assoc J IMAJ (2008) 10:603–6.
13. Taillard V, Sardinoux M, Oudot C, Fesler P, Rugale C, Raingeard I, et al. Early detection of isolated left ventricular diastolic dysfunction in high-risk differentiated thyroid carcinoma patients on TSH-suppressive therapy. Clin Endocrinol (2011) 75:709–14. doi: 10.1111/j.1365-2265.2011.04138.x
14. Hong KS, Son JW, Ryu OH, Choi MG, Hong JY, Lee SJ. Cardiac effects of thyrotropin oversuppression with levothyroxine in young women with differentiated thyroid cancer. Int J Endocrinol (2016) 2016:9846790. doi: 10.1155/2016/9846790
15. Murialdo G, Casu M, Cappi C, Patrone V, Repetto E, Copello F, et al. Effects of recombinant human thyrotropin on heart rate variability and blood pressure in patients on l-thyroxine-suppressive therapy for differentiated thyroid carcinoma. Hormone Res (2005) 64:100–6. doi: 10.1159/000088429
16. Shargorodsky M, Serov S, Gavish D, Leibovitz E, Harpaz D, Zimlichman R. Long-term thyrotropin-suppressive therapy with levothyroxine impairs small and large artery elasticity and increases left ventricular mass in patients with thyroid carcinoma. Thyroid Off J Am Thyroid Assoc (2006) 16:381–6. doi: 10.1089/thy.2006.16.381
17. Klein Hesselink EN, Lefrandt JD, Schuurmans EP, Burgerhof JG, Groen B, Gansevoort RT, et al. Increased risk of atrial fibrillation after treatment for differentiated thyroid carcinoma. J Clin Endocrinol Metab (2015) 100:4563–9. doi: 10.1210/jc.2015-2782
18. Pajamäki N, Metso S, Hakala T, Ebeling T, Huhtala H, Ryödi E, et al. Long-term cardiovascular morbidity and mortality in patients treated for differentiated thyroid cancer. Clin Endocrinol (2018) 88:303–10. doi: 10.1111/cen.13519
19. Mittal A, Poudel B, Pandeya DR, Gupta SP, Sathian B, Yadav SK. Metabolic changes enhance the cardiovascular risk with differentiated thyroid carcinoma–a case control study from manipal teaching hospital of Nepal. Asian Pacific J Cancer Prev APJCP (2012) 13:2335–8. doi: 10.7314/apjcp.2012.13.5.2335
20. Mercuro G, Panzuto MG, Bina A, Leo M, Cabula R, Petrini L, et al. Cardiac function, physical exercise capacity, and quality of life during long-term thyrotropin-suppressive therapy with levothyroxine: effect of individual dose tailoring. J Clin Endocrinol Metab (2000) 85:159–64. doi: 10.1210/jcem.85.1.6298
21. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. New Engl J Med (2001) 344:501–9. doi: 10.1056/nejm200102153440707
22. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Internal Med (2000) 132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004
23. Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol (2012) 167:609–18. doi: 10.1530/eje-12-0627
24. Moruzzi P, Doria E, Agostoni PG, Capacchione V, Sganzerla P. Usefulness of l-thyroxine to improve cardiac and exercise performance in idiopathic dilated cardiomyopathy. Am J Cardiol (1994) 73:374–8. doi: 10.1016/0002-9149(94)90011-6
25. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr hypertension Rep (2003) 5:513–20. doi: 10.1007/s11906-003-0060-7
26. Cai Y, Ren Y, Shi J. Blood pressure levels in patients with subclinical thyroid dysfunction: A meta-analysis of cross-sectional data. Hypertension Res Off J Japanese Soc Hypertension (2011) 34:1098–105. doi: 10.1038/hr.2011.91
27. Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: A matched case-control study. J Am Coll Cardiol (2007) 49:71–81. doi: 10.1016/j.jacc.2006.08.042
28. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocrine Rev (2010) 31:139–70. doi: 10.1210/er.2009-0007
29. Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid Off J Am Thyroid Assoc (1996) 6:505–12. doi: 10.1089/thy.1996.6.505
30. Kuzman JA, Gerdes AM, Kobayashi S, Liang Q. Thyroid hormone activates akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J Mol Cell Cardiol (2005) 39:841–4. doi: 10.1016/j.yjmcc.2005.07.019
31. Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab (2007) 92:1715–23. doi: 10.1210/jc.2006-1869
Keywords: thyroid cancer surgery, TSH suppression, cardiovascular risk, meta-analysis, differentiated thyroid carcinoma
Citation: Yang X, Guo N, Gao X, Liang J, Fan X and Zhao Y (2022) Meta-analysis of TSH suppression therapy and the risk of cardiovascular events after thyroid cancer surgery. Front. Endocrinol. 13:991876. doi: 10.3389/fendo.2022.991876
Received: 12 July 2022; Accepted: 27 October 2022;
Published: 22 December 2022.
Edited by:
Antonino Catalano, University of Messina, ItalyReviewed by:
Elisabetta Morini, Bonino Pulejo Neurology Center (IRCCS), ItalyCristina Martín-Arriscado Arroba, Fundación Investigación Biomédica Hospital 12 de Octubre, Spain
Copyright © 2022 Yang, Guo, Gao, Liang, Fan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuejiao Zhao, zhao_yuejiao@yeah.net
 Xiao Yang
Xiao Yang Yuejiao Zhao
Yuejiao Zhao