- 1Department of Pharmaceutical Regulatory Science and Pharmacoeconomics, School of Pharmacy, Nanjing Medical University, Nanjing, China
- 2Department of Drug Clinical Trial Institution, Fujian Cancer Hospital, Fuzhou, Fujian, China
- 3Department of Research Management, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing, China
- 4Department of Health Policy, School of Health Policy and Management, Nanjing Medical University, Nanjing, China
- 5Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
Background: Toripalimab is the first domestic anti-tumor programmed death 1 antibody marketed in China. The CHOICE-01 trial (identifier: NCT 03856411) demonstrated that toripalimab plus chemotherapy can significantly improve the clinical outcomes of advanced non-small cell lung cancer (NSCLC) patients. However, whether it is cost-effective remains unknown. Given the high cost of combination therapy, a cost-effectiveness analysis of toripalimab plus chemotherapy (TC) versus chemotherapy alone (PC) for the first-line treatment of patients with advanced NSCLC is required.
Methods: A partitioned survival model was adopted to predict the course of disease in advanced NSCLC patients on TC or PC from the perspective of the Chinese healthcare system over a 10-year horizon. The survival data were obtained from the CHOICE-01 clinical trial. Cost and utility values were obtained from local hospitals and kinds of literature. Based on these parameters, the incremental cost-effectiveness ratio (ICER) of TC vs. PC was measured, and one-way sensitivity analyses, probabilistic sensitivity analyses (PSA), and scenario analyses were performed to assess the robustness of the model.
Results: In the base case, TC was associated with an incremental cost of $18510 and an incremental quality-adjusted life year (QALY) of 0.57 compared with PC, resulting in an ICER of $32237/QALY which was lower than the willingness to pay (WTP) threshold ($37654/QALY), TC was cost-effective. The health utility value of progression-free survival, the price of toripalimab, and the cost of best supportive care were factors that significantly influenced the ICER, but no change in any of them could change the model result. TC showed a 90% probability of being a cost-effective option at a WTP threshold of $37,654/QALY. In the 20 and 30-year time horizons, the results remained unchanged and TC remained cost-effective when the second-line treatment was switched to docetaxel.
Conclusion: At a WTP threshold of $37,654 per QALY, TC was cost-effective compared to PC for patients with advanced NSCLC in China.
1 Introduction
People around the world, especially in China, are facing the health issue of lung cancer. According to the latest report published by the Globolan 2020 (1): The incidence and mortality of lung cancer rank first among all malignant tumors in China, with 17.9% and 23.8% respectively, and are both higher than the world average (11.4%, only behind breast cancer (11.7%); 18%). Non-small cell lung cancer (NSCLC) accounts for about 85% of all types of lung cancer (2) and the majority of patients are diagnosed at an advanced stage because of the concealed early symptoms, which results in a dismal prognosis (3). In addition, the burden of disease caused by lung cancer is also considerable. In China, lung cancer accounts for 25.4% of all cancer-related disability-adjusted life years, making it the leading cause of disability-adjusted life year burden (4).
The stage of lung cancer significantly impacts both treatment and prognosis (5). Traditional platinum-based chemotherapy has been the first-line treatment for NSCLC patients (6), but its clinical benefit is modest. The discovery of immune checkpoint inhibitors, represented by programmed death-1(PD-1) and programmed death-ligand 1(PD-L1) antibodies in recent years has opened up new therapy options for advanced NSCLC (7). Numerous clinical trials (8, 9) have shown that chemotherapy plus PD-1/PD-L1 inhibitors can significantly improve the clinical outcomes of patients. Guidelines recommend chemotherapy plus PD-1/PD-L1 inhibitors as the first-line treatment for advanced NSCLC without EGFR or ALK mutations (6, 10). However, high-value antineoplastic medications come at a high cost, thus it is necessary to assess their cost-effectiveness to prevent financial toxicity for patients as a result of high drug prices and to provide the best treatment for patients.
Toripalimab is the first domestic anti-tumor PD-1 antibody marketed in China (11), it is a humanized IgG4K monoclonal antibody special for human PD-1. Clinical studies (12–14) have shown that it has a wide range of anti-tumor activity, including melanoma, lung cancer, gastrointestinal tumors, etc. CHOICE-01 (15) was a randomized, double-blind, placebo-controlled phase III clinical trial conducted in 59 medical centers in China that evaluated the efficacy and safety of toripalimab plus chemotherapy (TC) versus chemotherapy alone (PC) for advanced NSCLC patients. The median follow-up period in this clinical trial was 16.2 months. The findings demonstrated that TC could considerably prolong the progression-free survival (PFS), overall survival (OS), and the anti-tumor response of patients compared to PC, and the toxicity was manageable. The median PFS in the TC group was 2.7 months longer than in the PC group. (8.3 months vs. 5.6 months; HR, 0.49; 95% cl: 0.39-0.61); median OS in the PC group was 17.1 months (95% cl: 14.4 to 22.2) whereas the median OS for the TC arm has not yet reached (95% cl: 21.7 to NE).
Toripalimab for the treatment of melanoma, nasopharyngeal carcinoma, and uroepithelial carcinoma was included in the national reimbursement drug list (NRDL) in 2020 and 2021 (10, 16. 17), respectively. Following the release of its new indication for NSCLC in September 2022 (11), its price may be further reduced after the expiry of the national drug price negotiation (NDPN) on 31 December 2023 (16). However, there is no pharmacoeconomic evaluation of toripalimab for the treatment of advanced NSCLC, so it is necessary to assess the cost-effectiveness of the toripalimab from the perspective of healthcare systems in low-and middle-income countries. In this study, we aimed to evaluate the cost-effectiveness of TC versus PC for the first-line treatment of advanced NSCLC from the perspective of the Chinese healthcare system, to provide evidence for rational drug use in clinical practice and medical decision-making by government departments.
2 Data and methods
We conducted a cost-effectiveness analysis based on the CHOICE-01 clinical trial. The Checklist for Economic Evaluation Reporting Standards Statement (CHEERS) was used to guide this article (Supplementary Material, Table S1) and details of its methodology are given below.
2.1 Population and intervention measure
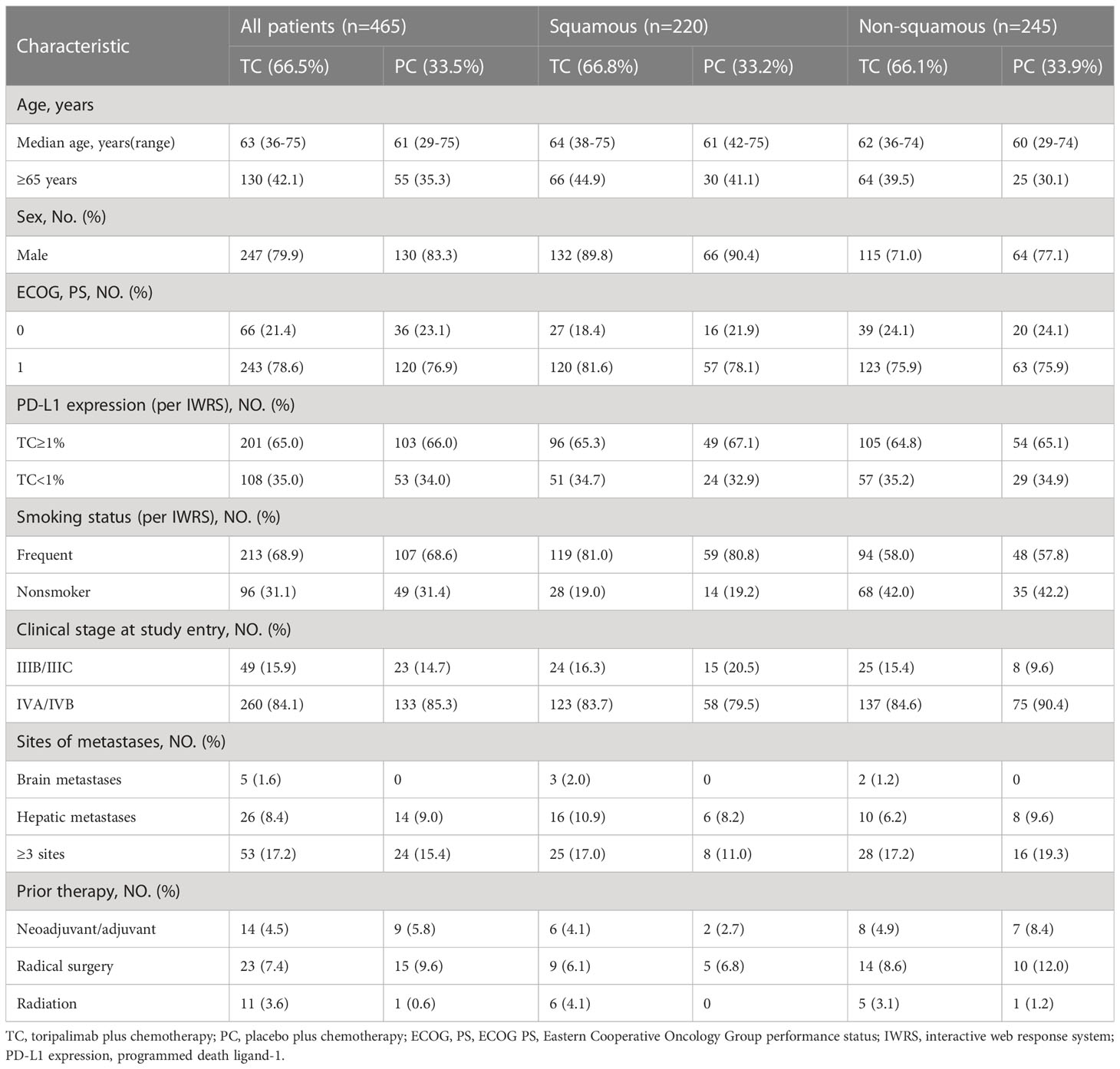
Patients in this study were assumed to be consistent with those in the clinical trial in that all patients were treatment-naive, locally progressed or metastatic NSCLC, or had finished neoadjuvant/adjuvant therapy ≥ 6 months before enrollment. Non-squamous NSCLC patients with EGFR or ALK driver mutations were disqualified. All enrolled patients were stratified according to baseline characteristics such as PD-L1 expression status, histology (squamous v non-squamous), and smoking status, and were randomly assigned to the study group (TC) and control group (PC) in a 2:1 ratio. The baseline information and subgroups of patients are shown in Table 1 (15).
In 3-week cycles, patients in the TC group received toripalimab 240 mg in combination with standard chemotherapy once per cycle and continued for 4-6 cycles, and then followed by maintenance treatment with toripalimab (± pemetrexed*) until disease progression; patients in the PC arm received placebo once per cycle in combination with standard chemotherapy and continued for 4-6 cycles, followed by maintenance treatment with toripalimab (± pemetrexed*) until disease progression. Patients with disease progression were unblinded and patients from the PC group were allowed to crossover to toripalimab monotherapy, with 13.3% and 65.4% of patients in the TC and PC groups with progressive disease receiving second-line treatment with toripalimab, respectively. The clinical trial made no mention of second-line therapy for the remaining patients, who were assumed to receive the best supportive care based on the recommendations of the guideline (6), patients who did not receive second-line treatment with toripalimab were assumed to receive the best supportive care, with 86.7% and 34.6% of patients with progressive disease in the TC and PC groups, respectively, receiving best supportive care as second-line treatment.
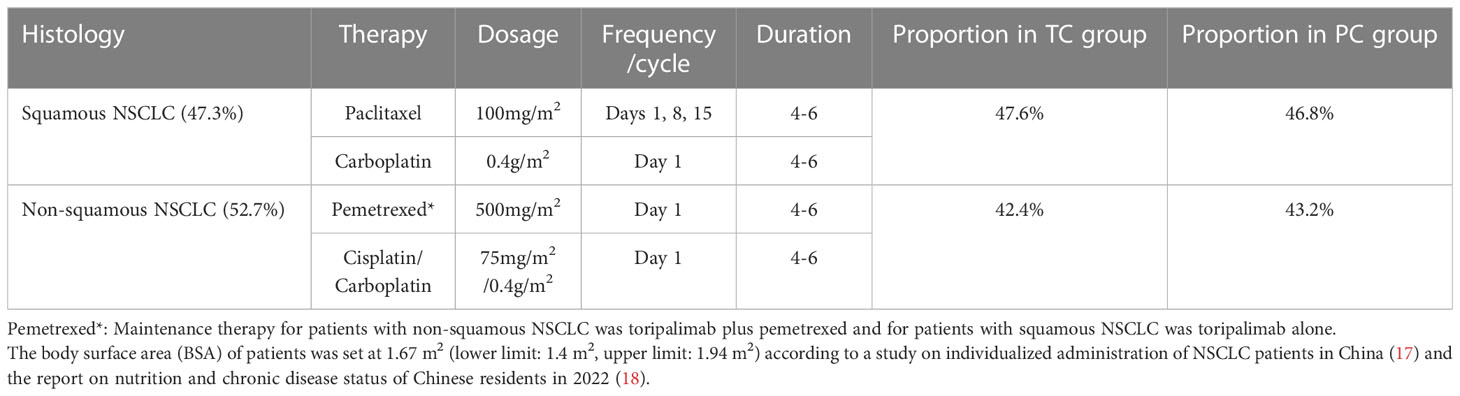
Chemotherapy regimens were based on histological features. Squamous NSCLC patients received paclitaxel plus carboplatin (47.3%) and non-squamous NSCLC patients received pemetrexed plus carboplatin/cisplatin (52.7%) (Table 2). In addition, patients with non-squamous NSCLC received toripalimab along with pemetrexed in maintenance therapy*.
2.2 Model overview
A partitioned survival model was developed in Microsoft Excel® (Microsoft Corp, Redmond, WA, USA) to evaluate the cost-effectiveness of TC versus PC in patients with advanced NSCLC from the perspective of the Chinese healthcare system. In the model, patients were classified into 3 health statuses according to the characteristics of the tumor: progression-free survival (PFS), progressive disease (PD), and death (D). The proportion of patients in different health status at cycle t (3 weeks per cycle) was measured based on the survival curves provided in CHOICE-01 clinical trial. Adverse effects (AEs) were also considered. To simplify the model, we only considered the cost and disutility of grade 3/4 AEs in greater than or equal to 5% of patients in the TC and PC group, the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 (19) was used to identify AEs (Figure 1).
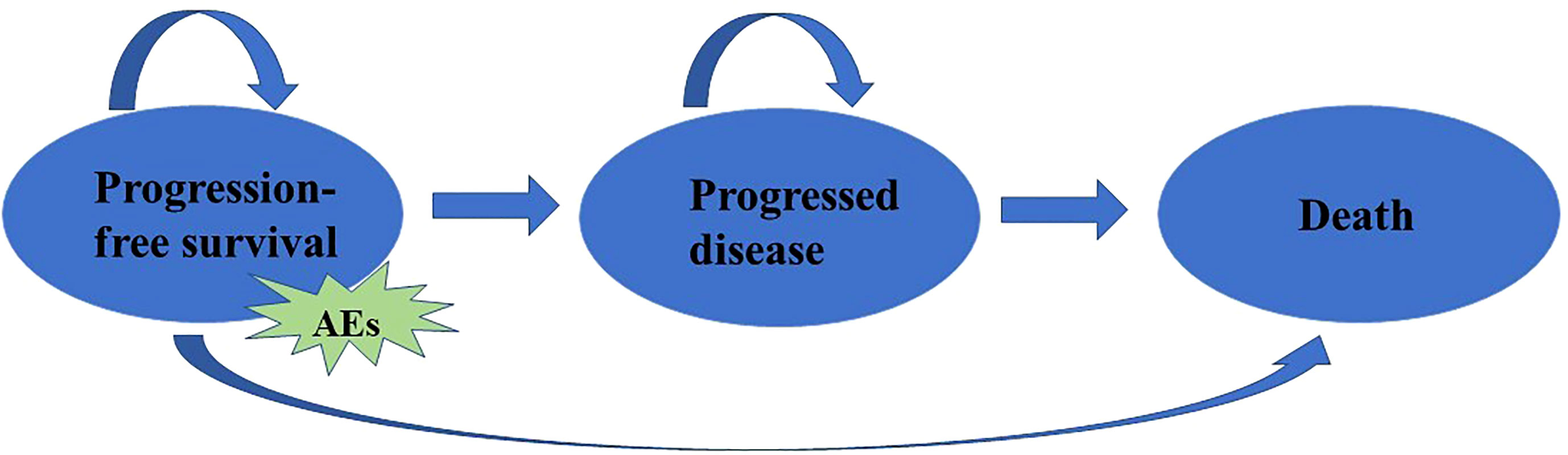
Figure 1 Model structure for advanced NSCLC. AEs: Adverse effects, which were identified by the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE).
The proportion of patients in each healthy state at each cycle was calculated as follows:
a) Proportion of patients in PFS was obtained from the PFS curve.
b) Proportion of patients in PD = Proportion of patients who are alive (obtained from OS curve) - Proportion of patients in PFS (obtained from PFS curve).
c) Proportion of patients in D = 1- Proportion of patients who are alive (obtained from OS curve) (20, 21).
GetData Graph Digitizer (version 2.26 www.getdata.graph.digitizer.com) was used to obtain the individual patient data on PFS and OS by taking points from the Kaplan-Meier survival curves reported in clinical trials. And then R software (22) was used to simulate the long-term survival state of patients by selecting the best-fitting parameter distributions to the survival curve from Weibull, Exponential, Gompertz, Gamma, Log-logistic, and Log-normal distributions.
Based on the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), the Log-normal distribution showed the best fit for both PFS and OS in the TC Group and the PC Group. (Supplementary Material, Table S2, Figure S3). A half-period correction was used to eliminate discretization-related model bias as the survival curve was divided into monthly periods (23).
The primary outcome was the incremental cost-effectiveness ratio (ICER) which was calculated as the cost per quality-adjusted life year (QALY) gained. The ICER was compared with the preset willingness to pay threshold (WTP) to assess the cost-effectiveness of the two regimens. According to the China Guidelines for Pharmacoeconomic Evaluations (2020) (24), the WTP was set as 3 times China’s per capita GDP in 2021. Sensitivity analyses were performed to evaluate the robustness of the model.
2.3 Time horizon and discount rate
Estimates of the number of years of survival for patients with advanced NSCLC were obtained from the National Cancer Institute (NCI) (5), and statistics showed that most patients with advanced NSCLC will die within 10 years (survival rate of 2.7%), so we used a 3-week cycle length and a 10-year lifetime horizon in this study to simulate advanced NSCLC patients’ survival years.
The discount rate for both costs and health outputs was 5% as recommended in the guidelines (24), and sensitivity analysis was performed on a range of 0% to 8%.
2.4 Cost and utility
Since our study perspective was the healthcare system, only direct medical costs were considered including drug costs, adverse reaction (ADR) monitoring costs, laboratory test costs, hospitalization, and nursing costs, best supportive care costs, and hospice care costs. Drug costs include chemotherapy drug costs, toripalimab costs, and ADRs treatment costs which were calculated as a one-time cost within the first treatment cycle; laboratory tests aimed at tumor surveillance, which was performed once at baseline, and then every 6 weeks for 12 months and every 9 weeks after 12 months. The costs of best supportive care and hospice care were obtained from a previous study (25), and the other costs were obtained from Fujian Cancer Hospital, which is a tertiary oncology hospital and participated in the CHOICE-01 clinical trial as one of the sub-centers, so the cost information provided by Fujian Cancer Hospital was consistent with the clinical trial and representative. All costs were translated into USD based on the average exchange rate of CNY to USD in 2021 (1 USD=6.4515 CNY) (26) (Supplementary Material, Table S4).
The utility values for PFS and PD and the disutility values for ADRs were calculated according to the published literature (25, 27), as patients’ quality of life was not examined in the clinical trials. A utility value of 0 for the death state (Supplementary Material, Table S4).
2.5 Sensitivity analysis
Several sensitivity analyses were performed to assess the robustness of the model. One-way sensitivity analysis is to analyse the effect of a variable change in a certain range on the results of the model when other variables remain constant. 95% CI is typically used to restrict the range of variance, if there is no 95% Cl, it may vary by ±10% for non-cost parameters and ±20% for cost parameters based on the base case value (Supplementary Material, Table S4). In addition, toripalimab, which is within the term of the NRDL agreement, may be subject to renegotiation and reimbursement adjustments after the expiry of the agreement on 31 December 2023 due to its new indication of NSCLC. According to the data provided by the Chinese government website, the average cost reduction of updated drugs in 2019 and 2020 were 26.4% and 14.95%, respectively (28), so to fully consider the impact of toripalimab’s price on the robustness of the model, we assumed a 36% cost variability for toripalimab is 36% (20% up or down on the original float). One-way sensitivity analysis was presented as a tornado diagram.
The probability sensitivity analysis (PSA) was performed by Monte Carlo simulations 1000 times, each of which randomly chose samples from the distributions of the model inputs. Costs were described by gamma distributions, utility values by beta distributions, and BSA by the normal distribution (Supplementary Material, Table S4). The PSA was presented by an ICER scatter plot and a cost-effectiveness acceptability curve (CEAC) which reported the probability that TC is more cost-effective at alternative WTP thresholds.
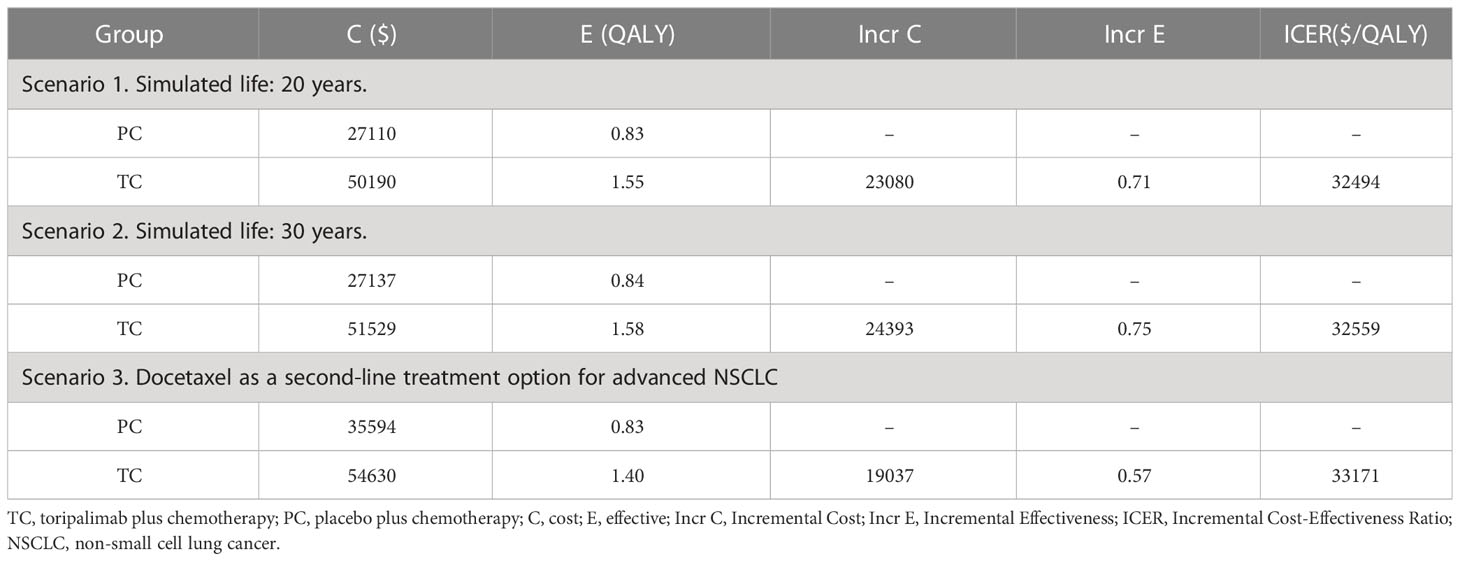
We also conducted scenario analyses of some key assumptions. Firstly, because long-term survival in advanced NSCLC remains uncertain in clinical practice across countries, literature research was conducted and the results showed that the majority of comparable studies in the cost-effectiveness analysis of ICIs for NSCLC were modelled 10 years, 20 years (29), with some model 5 and 30 years of survival (30, 31), but few papers provided a basis for the choice of survival years. In our scenario study, we investigated the effects of 20 and 30 years of time horizon on the model findings to measure the robustness of the model as far as possible. Secondly, as the clinical trial did not mention second-line treatment for patients not treated with toripalimab, we assumed that this population of patients received guideline-based best supportive care, and in the scenario analysis, we analysed the cost-effectiveness of an alternative guideline-recommended second-line treatment: docetaxel, to examine the robustness of the model (6). In summary, we considered 3 scenarios: 20 and 30-year life horizons, and the impact of docetaxel replacing best supportive care as a second-line treatment option for advanced NSCLC on the model outputs. The price of docetaxel was $0.22/mg.
3 Results
3.1 Base-case analysis
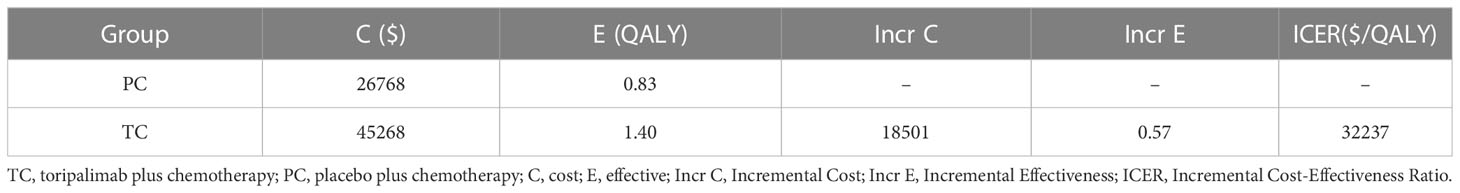
Compared with PC Group, TC Group was associated with an increment cost of $18501 and an increment QALY of 0.57, resulting in an ICER of $32237/QALY (Table 3), which was lower than that of the WTP threshold ($37654) (26). TC was cost-effective.
3.2 One-way sensitivity analysis
The one-way sensitivity analyses revealed that the factors that had the greatest impact on the model were the health utility value of PFS, the cost of toripalimab, and the cost of best supportive care, respectively, but no change in any of these parameters could make the ICER higher than the WTP threshold. The model was robust (Figure 2).
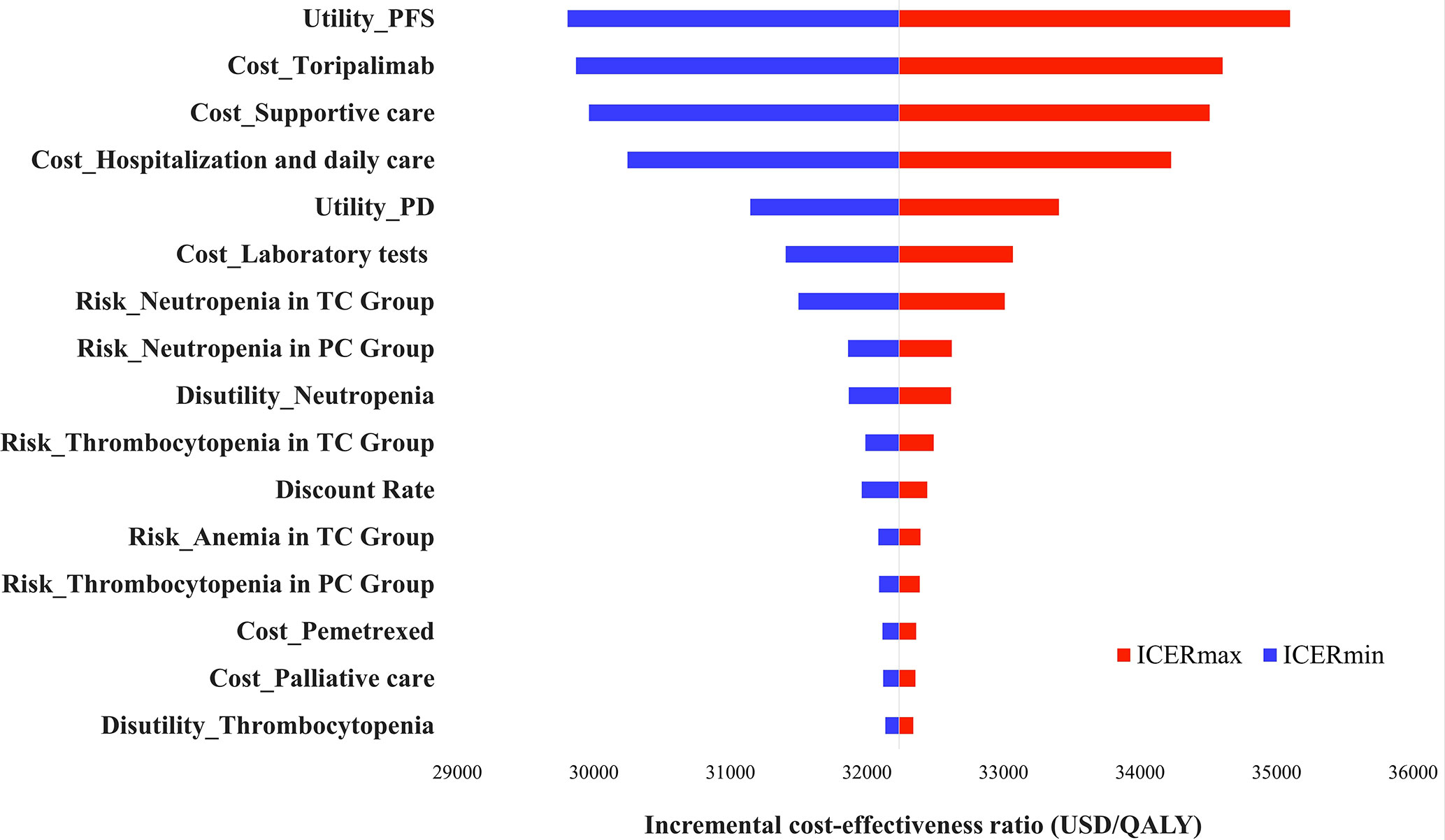
Figure 2 Tornado diagram of one-way sensitivity analyses. TC, toripalimab plus chemotherapy; PC, placebo plus chemotherapy; ICER, Incremental Cost-Effectiveness Ratio.
3.3 Probabilistic sensitivity analysis
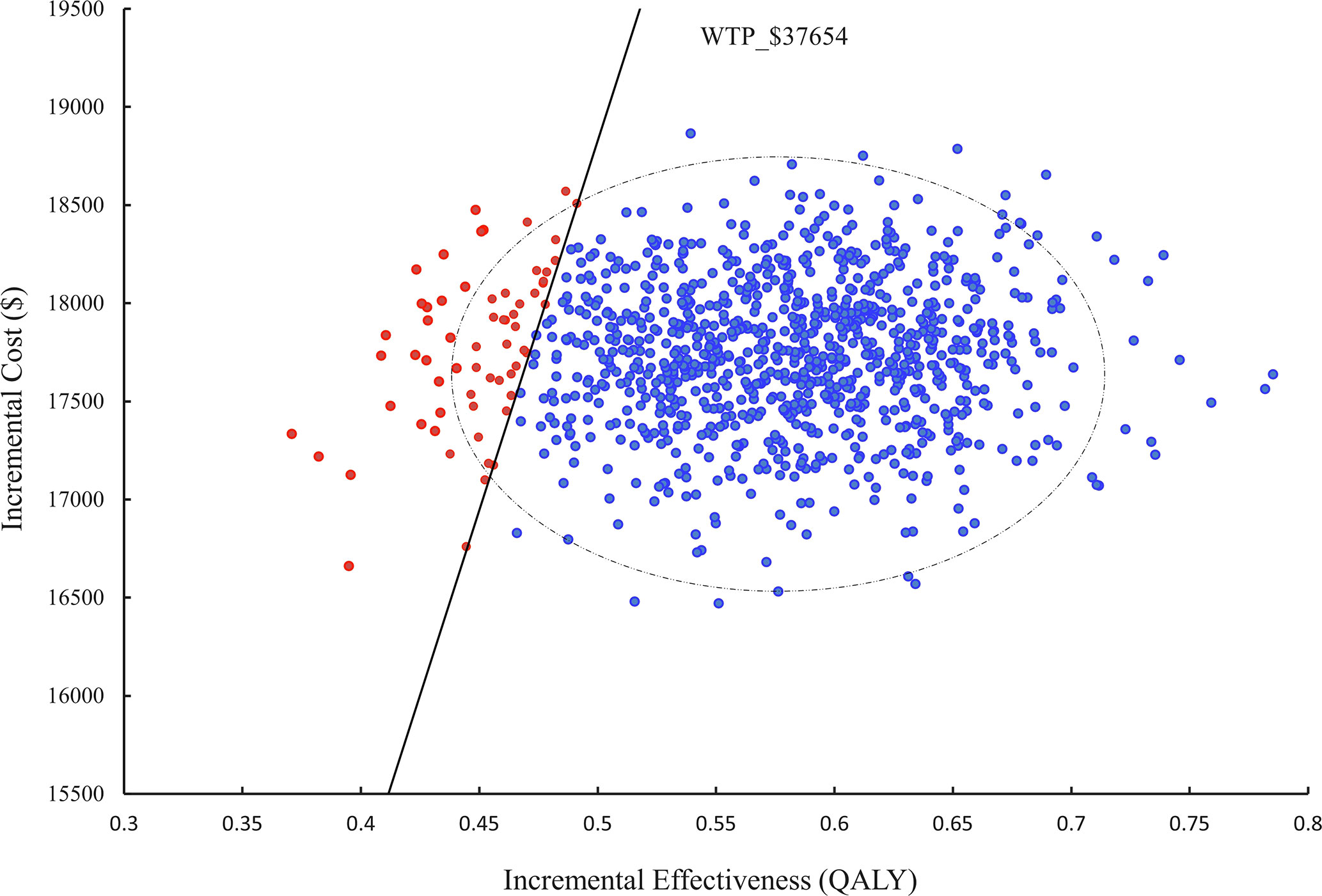
In a probabilistic sensitivity analysis (PSA), 1000 iterations of Monte Carlo simulations were performed and the results were presented as an ICER scatter plot (Figure 3) and a CEAC (Figure 4), which showed a 90% probability of TC being a cost-effective option at a WTP threshold of $37,654.
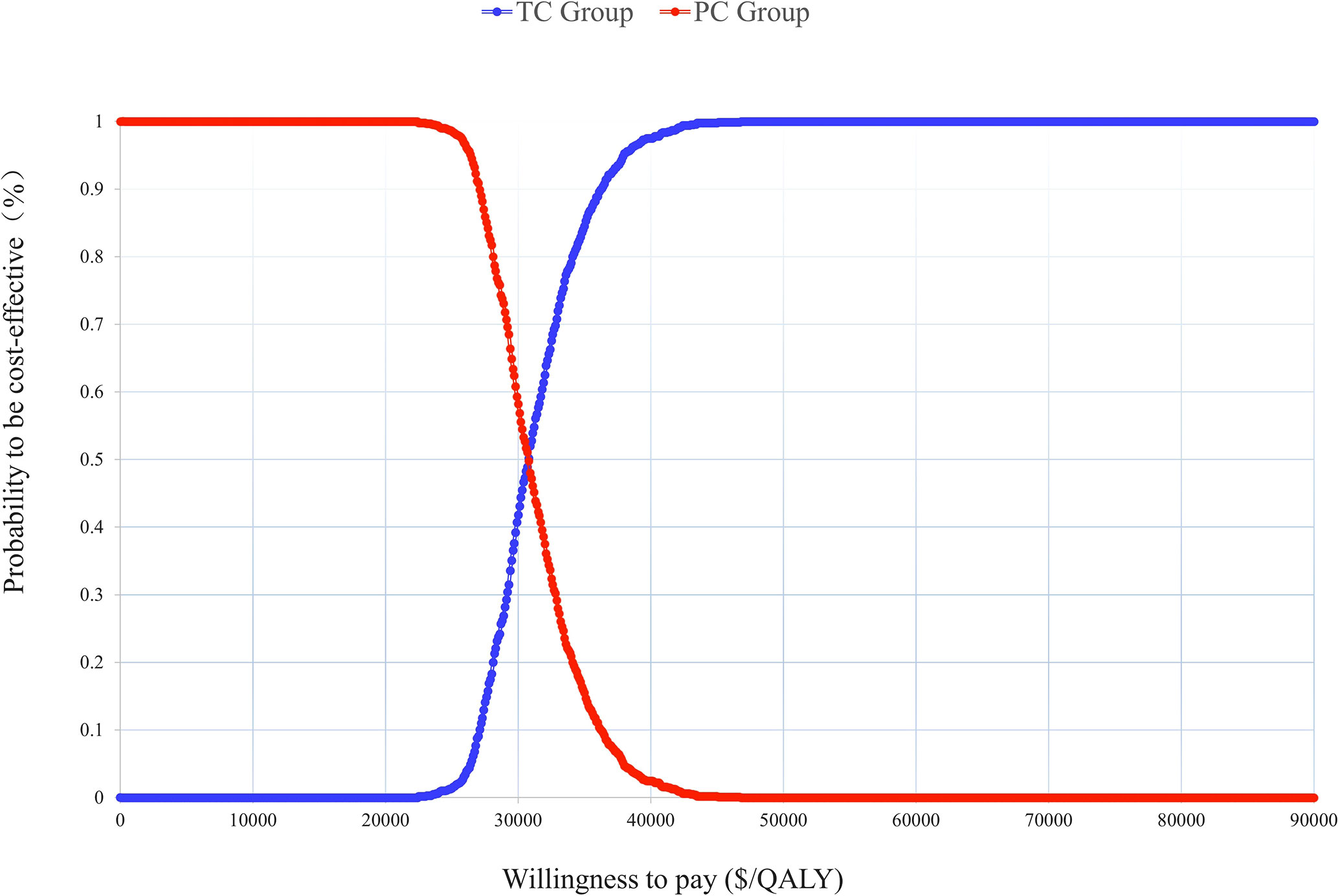
Figure 4 Cost-effectiveness acceptable curve (CEAC). TC, toripalimab plus chemotherapy; PC, placebo plus chemotherapy.
3.4 Scenario analysis
The results showed that the ICERs were all lower than the WTP threshold at simulated 20,30 years and docetaxel as a second-line treatment ($32494, $32559, $33171 vs. $37654), TC was still cost-effective (Table 4).
4 Discussion
Immune checkpoint inhibitors (ICIs) have drawn a lot of attention since their launch. Although it is a promising field of cancer treatment, it was of considerable significance to the influence of its high cost, especially for low- and middle-income countries. Since 2016, the Chinese government has been implementing an NDPN policy, aiming to reduce the price of innovative medicines with high clinical value (32, 33). High-value innovative medicines, represented by anticancer drugs, have gradually established a value-oriented negotiation access model since 2017 (34), and pharmacoeconomic evaluation has started to play an important role in drug market evaluation. Worth mentioning, innovative drugs that are already in the NRDL do not have to go through negotiations when new medical insurance indications are added, but the drugs also face pressure to reduce prices future (16).
Toripalimab, the first domestic PD-1 antibody with broad antitumor activity marketed in China (11), was approved for marketing by National Medical Products Administration in September 2022 for first-line treatment of advanced non-squamous NSCLC without EGFR/ALK driver mutations. This is the sixth indication for which toripalimab has been approved in China with three indications having previously passed the NDPN and entered the NRDL. In this study, we measured the cost-effectiveness of toripalimab plus chemotherapy for first-line treatment for patients with advanced NSCLC using the price after health insurance reimbursement. The perspective of this research was the Chinese healthcare system, aiming to provide an evidence-based basis for rational clinical use and health insurance decisions. To our knowledge, this is the first analysis to evaluate the cost-effectiveness of toripalimab for advanced NSCLC.
According to our model, TC was cost-effective compared to PC at a WTP threshold of $37,654 per QALY. The utility value of PFS, the price of toripalimab, and the cost of supportive care had the greatest influence on the ICER which were similar to previous studies, but none of them could change the model result. The PSA showed a 90% probability that the ICER was below the WTP threshold. Different survival years and second-line treatment options were also discussed in the scenario analysis and the results remained unchanged, TC was a more cost-effective option for Chinese advanced NSCLC patients.
As a novel treatment option, both imported and domestic ICIs are widely used in China, but the high costs of ICIs can also place a heavy burden on society. Previous studies have demonstrated the cost-effectiveness of imported and domestic ICIs for advanced NSCLC. For imported ICIs, the ICERs for atezolizumab plus chemotherapy (35) and pembrolizumab plus chemotherapy (36) versus chemotherapy alone from the Chinese perspective were $78936/QALY and $107846/QALY, $448414/QALY (pembrolizumab, 2 and 10 mg/kg) showed poor cost-effectiveness. which may be due to the high price of the drugs. A systematic analysis of Pembrolizumab for NSCLC also revealed that it was cost-effective in the US and Switzerland but not in China, France, the UK, or Singapore when combined with chemotherapy (37). For domestic ICIs, the ICERs of sintilimab plus chemotherapy (38) and camrelizumab plus chemotherapy (39) versus chemotherapy alone were $34249/QALY and $63080/QALY, respectively. Compared to other ICIs, toripalimab offers a more cost-effective option with an ICER of $32237/QALY. Additionally, this study was conducted from a Chinese healthcare perspective. Toripalimab combination regimens for the treatment of advanced NSCLC may be more cost-effective from the perspective of the developed country payer. Previously, Junshi Biosciences Co., Ltd has submitted marketing authorization applications to the US FDA, the UK Medicines and Healthcare Products Regulatory Agency, and the European Medicines Agency (11) for two other indications.
Subgroup analyses were not conducted because there are still gaps in the survival data for subgroup participants. Among patients with different PD-L1 expression status and histological features, patients in the PD-L1 tumor cell proportion score ≥50% and non-squamous NSCLC subgroup showed better mPFS and mOS in the clinical trial which may translate into longer QALYs, and resulting in higher ICERs under nearly the same cost of medication. As the clinical experiment moves forward, hopefully, this issue will be looked into more thoroughly.
The study was subjected to several limitations. First, the survival data in this study were obtained from the CHOICE-01 clinical trial rather than real-world data, which may lead to an overestimation of the cost-effectiveness of the combination regimen, as we know that real-world patients’ survival data are typically inferior to those from phase 3 clinical trials which may lead to longer QALYs at the same cost. In addition, as the clinical trials only included Chinese populations, the lack of real-world, global, multi-center survival data and the fact that we discuss the cost-effectiveness of the combination regimen only from the perspective of the Chinese healthcare system may lead to an underestimation of the cost-effectiveness of toripalimab. To our knowledge, China is a developing country, whereas developed countries such as Europe and the US have a much higher GDP per capita and toripalimab may be more cost-effective; Second, the utility and disutility values used in the article were derived from previous studies because there was no information available in the trial on patients’ quality of life. Third, since no second-line treatment regimen was mentioned in the trial, we assumed that all patients received the best supportive care based on guidelines which may not be realistic. Fourth, not all adverse reactions were included in the model. Clinical trials showed a higher incidence of thyroid disease, diarrhea, and edema in the TC group, which may be related to the use of immunosuppressants, but none of the incidences exceeded 5%, so they were not included in the model. In addition, a significant number of patients in the CHOICE-01 trial were switched from the PC group to the TC group after progression due to ethical issues, which may have diluted the survival advantage of the TC group and increased the cost of PC.
Despite these limitations, our study has a high level of confidence. A partitioned survival model was used to simulate the survival of patients with tumors, unlike the markov model, the partitioned survival model does not require the calculation of transfer probabilities between model states, the proportion of patients in each state was directly obtained from the survival curve provided by the clinical trial, avoiding assumptions such as memorylessness and natural mortality and allowing for more accurate modeling of disease events (21). The simulation results showed that the mPFS and mOS of the simulated survival curves were within the 95% confidence interval. In addition, the utility values were derived from previous studies on the quality of life in Chinese NSCLC patients. Last but not least, the incidence and cost of ADRs were analyzed in sensitivity analyses, which showed that these factors had little effect on the model results.
5 Conclusions
In conclusion, TC was cost-effective compared to PC for patients with advanced NSCLC at a WTP threshold of $37,654 per QALY in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
MZ contributed to data analysis and writing; KX, MZ and YB contributed to modelling and design this study. YL collected the research data. CZ performed the statistical analysis. LZ and XL contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 72074123 and 71673147) and the China Medical Board (Grant No. 17-277).
Acknowledgments
We express our appreciation to all the hospital pharmacists in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1169752/full#supplementary-material
References
1. Global Cancer Observatory. Population Fact sheets. (2023). Available at: https://gco.iarc.ctsheets/populations/160-china-fact-sheets.pdf (Accessed January 11, 2023).
2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553(7689):446–54. doi: 10.1038/nature25183
3. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-small-cell lung cancer. Nat Rev Dis Primers (2015) 1:15009. doi: 10.1038/nrdp.2015.9
4. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the united states and united kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) (2021) 1(10):1037–48. doi: 10.1002/cac2.12197
5. Surveillance, epidemiology, and end results programm. cancer stat facts: lung and bronchus cancer (2022). Available at: https://seer.cancer.gov/statfacts/html/lungb.html (Accessed December 31, 2022).
6. Chinese Society of Medical Oncology, Journal of the Chinese Medical Association. The Chinese medical association clinical guidelines for the treatment of lung cancer. Chin Med J (2022) 102(23):1706–40. doi: 10.3760/cma.j.cn112137-20220413-00795
7. Califano R, Kerr K, Morgan RD, Lo Russo G, Garassino M, Morgillo F, et al. Immune checkpoint blockade: a new era for non-small cell lung cancer. Curr Oncol Rep (2016) 18(9):59. doi: 10.1007/s11912-016-0544-7
8. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/S2213-2600(20)30365-9
9. Nosaki K, Saka H, Hosomi Y, Baas P, de Castro G Jr, Reck M, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer (2019) 135:188–95. doi: 10.1016/j.lungcan.2019.07.004
10. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw (2021) 19(3):254–66. doi: 10.6004/jnccn.2021.0013
11. Available at: https://www.junshipharma.com (Accessed January 2, 2023).
12. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol (2021) 39(7):704–12. doi: 10.1200/JCO.20.02712
13. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell (2022) 40(3):277–288.e3. doi: 10.1016/j.ccell.2022.02.007
14. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med (2021) 27(9):1536–43. doi: 10.1038/s41591-021-01444-0
15. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-Small-Cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J Clin Oncol (2023) 41(3):651–663. doi: 10.1200/JCO.22.00727.
16. Lin Z. New drug indications into health insurance rules change from price negotiation to easy renewal. First Financial Daily (2022), 2022-10-17(A10). doi: 10.28207/n.cnki.ndycj.2022.004030
17. Zhang J, Zhou F, Qi H, Ni H, Hu Q, Zhou C, et al. Randomized study of individualized pharmacokinetically-guided dosing of paclitaxel compared with body-surface area dosing in Chinese patients with advanced non-small cell lung cancer. Br J Clin Pharmacol (2019) 85(10):2292–301. doi: 10.1111/bcp.13982
18. Report on the state of nutrition and chronic diseases in China (2020). Available at: http://journal.crnews.net/ncpsczk/2021n/d2q/lsxf/934382_20210205101914.html (Accessed December 30, 2022).
19. Cancer therapy evaluation program. common terminology criteria for adverse events (CTCAE) version 5.0 (2017). Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5.0.xlsx (Accessed 31 December 2022).
20. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Burke T. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ (2018) 21(12):1191–205. doi: 10.1080/13696998.2018.1521416
21. Accelerating access to new drugs to encourage innovation in the industry - interpreting the new version of the national health insurance drug list . Available at: http://www.gov.cn/zhengce/2023-01/21/content_5738317.htm (Accessed April 21, 2023).
22. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2022). Available at: https://www.R-project.org/.
23. Wo T, Chen L, Xi XY. Discussion on the intra period correction method of Markov model in pharmacoeconomic evaluation [J]. China Pharm (2020) 31(08):980–4. Available at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7i8oRR1PAr7RxjuAJk4dHXohtVYUVexZV2gCRZExotWskqRaLyXfSO6Tjo-JmkYMBh&uniplatform=NZKPT
24. Liu GE. Guidelines for pharmacoeconomic evaluation in China (2020). Beijing: China Market Publishing House (2020).
25. Liu G, Kang S, Wang X, Shang F. Cost-effectiveness analysis of atezolizumab versus chemotherapy as first-line treatment for metastatic non-Small-Cell lung cancer with different PD-L1 expression status. Front Oncol (202) 11:669195. doi: 10.3389/fonc.2021.669195
26. Statistical bulletin on national economic and social development of the people's republic of China 2021 . Available at: http://www.gov.cn/shuju/2022-02/28/content_5676015.htm (Accessed 2 January 2023).
27. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol (2017) 13(5):e195–203. doi: 10.1111/ajco.12477
28. Which drugs are expected to be included in the health insurance? how to renew the contract of negotiated drugs? –the national health insurance catalogue adjustment in 2022 - "Highlights" interpretation Chinese government website (2022). Available at: http://www.gov.cn (Accessed January 4, 2023).
29. Ding H, Xin W, Tong Y, Sun J, Xu G, Ye Z, et al. Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: a systematic review. PloS One (2020) 15(9):e0238536. doi: 10.1371/journal.pone.0238536
30. Zhou D, Luo X, Zhou Z, Zeng X, Wan X, Tan C, et al. Cost-effectiveness analysis of tislelizumab, nivolumab and docetaxel as second- and third-line for advanced or metastatic non-small cell lung cancer in China. Front Pharmacol (2022) 13:880280. doi: 10.3389/fphar.2022.880280
31. Kuznik A, Smare C, Chen CI, Venkatachalam M, Keeping S, Atsou K, et al. Cost-effectiveness of cemiplimab versus standard of care in the united states for first-line treatment of advanced non-small cell lung cancer with programmed death-ligand 1 expression ≥50. Value Health (2022) 25(2):203–14. doi: 10.1016/j.jval.2021.08.009
32. Cai L, Tao T, Li H, Zhang Z, Zhang L, Li X. Impact of the national drug price negotiation policy on the utilization, cost, and accessibility of anticancer medicines in China: a controlled interrupted time series study. J Glob Health (2022) 12:11016. doi: 10.7189/jogh.12.11016
33. Zhu H, Zhu J, Zhou Y, Shan L, Li C, Cui Y, et al. Impact of the national reimbursement drug list negotiation policy on accessibility of anticancer drugs in China: an interrupted time series study. Front Public Health (2022) 10:921093. doi: 10.3389/fpubh.2022.921093
34. Kong FC. Comparison of international experience and enlightenment in the value assessment of innovative drugs. Chin J Health Policy (2022) 15(6):17–23. Available at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKibYlV5Vjs7iJTKGjg9uTdeTsOI_ra5_XebAZGNa7haPYT0mrHvFzpJ2xy0NaMFK0vsurW931X4t&uniplatform=NZKPT
35. Cheng S, Pei R, Li J, Li B, Tang L, Yin T, et al. Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high PD-L1 expression: a cost-effectiveness analysis from US and Chinese perspectives. Ann Transl Med (2021) 9(18):1481. doi: 10.21037/atm-21-4294
36. Shi Y, Chen W, Zhang Y, Bo M, Li C, Zhang M, et al. Cost-effectiveness of pembrolizumab versus docetaxel as second-line treatment of non-small cell lung cancer in China. Ann Transl Med (2021) 9(18):1480. doi: 10.21037/atm-21-4178
37. Zhang C, Zhang J, Tan J, Tian P, Li W. Cost-effectiveness of pembrolizumab for the treatment of non-Small-Cell lung cancer: a systematic review. Front Oncol (2022) 12:815587. doi: 10.3389/fonc.2022.815587
38. Liu H, Wang Y, He Q. Cost-effectiveness analysis of sintilimab plus pemetrexed and platinum versus chemotherapy alone as first-line treatment in metastatic non-squamous non-small cell lung cancer in China. Health Econ Rev (2022) 12(1):66. doi: 10.1186/s13561-022-00410-x
39. Xiang G, Gu L, Chen X, Wang F, Chen B, Zhao J, et al. Economic evaluation of first-line camrelizumab for advanced non-small-cell lung cancer in China. Front Public Health (2021) 9:743558. doi: 10.3389/fpubh.2021.743558
40. National health insurance bureau ministry of human resources and social security issues the 2022 edition of the national health insurance drug list (2022). Available at: http://www.gov.cn/zhengce/zhengceku/2023-01/18/content_5737840.htm (Accessed January 2, 2023).
41. Policy interpretation of the notice of the national health insurance bureau, ministry of human resources and social security on the issuance of the national drug catalogue for basic medical insurance, work injury insurance and maternity insurance (2021). Available at: https://www.gov.cn/zhengce/zhengceku/202112/03/5655651/files/3f779fd3949b4c3ca266b49c7a51689c.pdf (Accessed January 2, 2023).
Keywords: toripalimab, PD-1, cost-effectiveness, partitioned survival model, advanced non-small cell lung cancer (advanced NSCLC), chemothearpy, China
Citation: Zhang M, Xu K, Lin Y, Zhou C, Bao Y, Zhang L and Li X (2023) Cost-effectiveness analysis of toripalimab plus chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer in China. Front. Immunol. 14:1169752. doi: 10.3389/fimmu.2023.1169752
Received: 20 February 2023; Accepted: 19 May 2023;
Published: 29 May 2023.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Shuo Kang, Second Hospital of Hebei Medical University, ChinaDongzhe Hong, Brigham and Women’s Hospital and Harvard Medical School, United States
Shiwei Gong, Huazhong University of Science and Technology, China
Copyright © 2023 Zhang, Xu, Lin, Zhou, Bao, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, xinli@njmu.edu.cn; Lingli Zhang, zhanglingli@njmu.edu.cn
 Mengdie Zhang
Mengdie Zhang Kai Xu1
Kai Xu1 Yingtao Lin
Yingtao Lin Yuwen Bao
Yuwen Bao Xin Li
Xin Li